Clinical Applications of Anterior Segment Optical Coherence Tomography: An Updated Review
Abstract
1. Introduction
2. Technical Aspects of AS-OCT
| Characteristic | Time-Domain OCT | Fourier-Domain OCT | Ultra-High-Resolution OCT | |
|---|---|---|---|---|
| Spectral-Domain OCT | Swept-Source OCT | |||
| Examples in clinical use | 1. Visante OCT (Carl Zeiss Meditec, Jena, Germany) 2. Heidelberg slit lamp OCT (Heidelberg Engineering, Heidelberg, Germany) | 1. Spectralis (Heidelberg Engineering, Heidelberg, Germany) 2. iVue80 (Optovue, Fremont, CA, USA) 3. Cirrus OCT (Carl Zeiss Meditec, Jena, Germany) | 1. Casia SS-1000 OCT (Tomey, Nagoya, Japan) 2. Triton OCT (Topcon Corporation, Tokyo, Japan) 3. Anterion OCT (Heidelberg Engineering, Heidelberg, Germany) *** | 1. SOCT Copernicus HR (Optopol Technologies SA, Zawiercie, Poland) |
| Optical source | Superluminescent diode [5] | Superluminescent diode [5] | Swept-source laser [5] | Superluminescent diode [5] |
| Wavelength | 1 = 1310 2 = 1310 nm [5] | 1 = 820 nm 2 = 840 nm 3 = 840 nm [5] | 1 = 1310 nm 2 = 1310 nm [5] 3 = 1300 nm [15] | 1 = 850 nm [16] |
| Scan width | 1 = 16 mm 2 = 15 mm [17,18,19] | 1 = 6 mm 2 = 13 mm 3 = 6 mm [5] | 1 = 16 mm 2 = 16 mm [5,20] 3 = 9 mm [15] | 1 = 10 mm [16] |
| Scan depth | 1 = 6 mm 2 = 7 mm [17,18,19] | 1 = 2 mm 2 = 2–2.3 mm (retina) 3 = 2 mm [5,21] | 1 = 6 mm 2 = 6 mm [5] 3 = 11 mm [15] | N/A ** |
| Axial resolution * | 1 = 18 μm 2 = >25 μm [17,18,19] | 1 = 7 μm 2 = 5 μm 3 = 5 μm [5,21] | 1 = 10 μm 2 = 8 μm [5] 3 = <10 μm [15] | 1 = 3 μm [16] |
| Transverse resolution * | 1 = 60 μm 2 = 20–100 μm [17,18,19] | 1 = 20 μm 2 = 15 μm 3 = 15 μm [5,21] | 1 = 30 μm 2 = 30 μm [5] 3 = <45 μm [22] | 1 = 12–18 μm [16] |
| A-scan rate (scanning speed in scans per second) | 1 = 2000 scans/s 2 = 200 scans/s [5] | 1 = 40,000 scans/s 2 = 80,000 scans/s 3 = 27,000 scans/s [5,21] | 1 = 30,000 scans/s 2 = 100,000 scans/s [5] 3 = 16,640 scans/s [15] | 1 = 52,000 scans/s [16] |
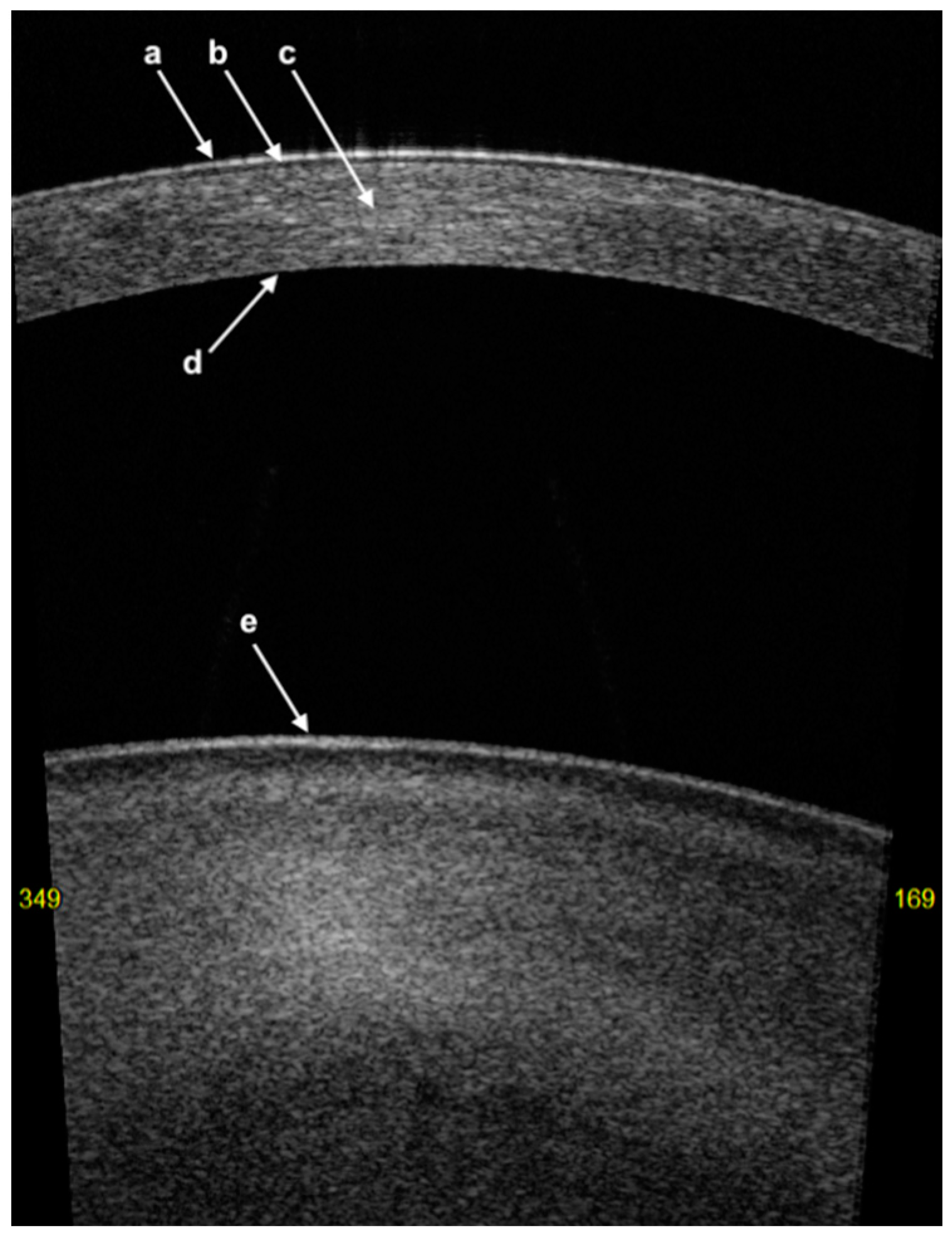
3. Corneal Anatomy on AS-OCT Imaging
- The first hyper-reflective band represents the tear film with a thickness of less than 5 µm [24];
- The first hyporeflective band represents the cornea epithelium with a thickness of between 50 and 70 µm;
- Bowman’s layer (BL) is represented as a linear structure with similar hyper-reflectivity to that of the stroma;
- The stroma has variable hyper-reflectivity with a thickness of around 500 µm;
- The pre-Descemet’s layer (or Dua’s layer (PDL)) and Descemet membrane (DM) are shown as a hyper-reflective band of tissue. In the context of DM detachment (type 2 or mixed), PDL and DM may show as two separate hyper-reflective bands [25].
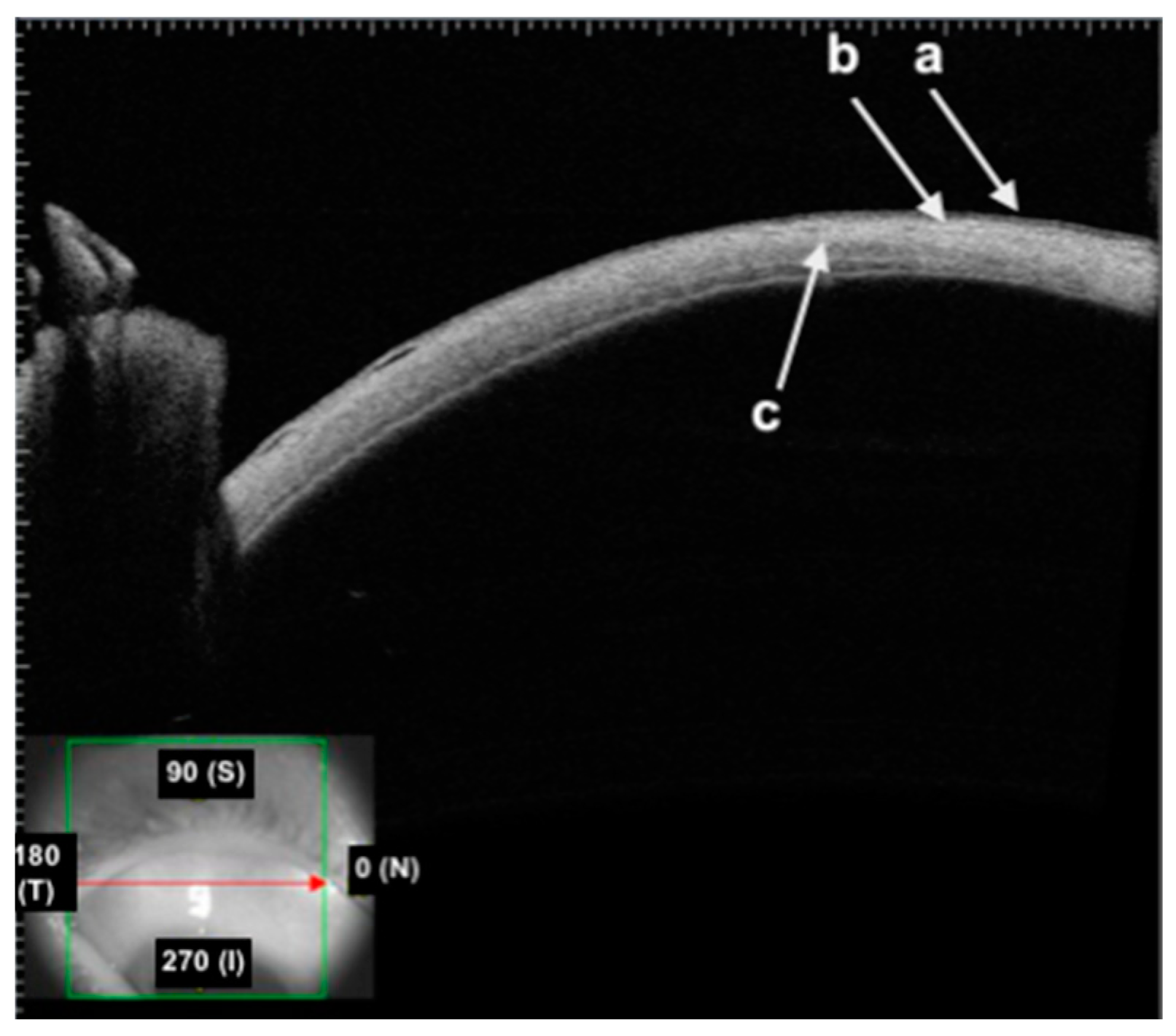
- The first hyper-reflective and hyporeflective band both represent the conjunctival epithelium;
- The second hyper-reflective band that underlies the conjunctival epithelium is the substantia propria;
- The thick hyper-reflective band represents the sclera.
4. Clinical Applications for Conjunctival Diseases
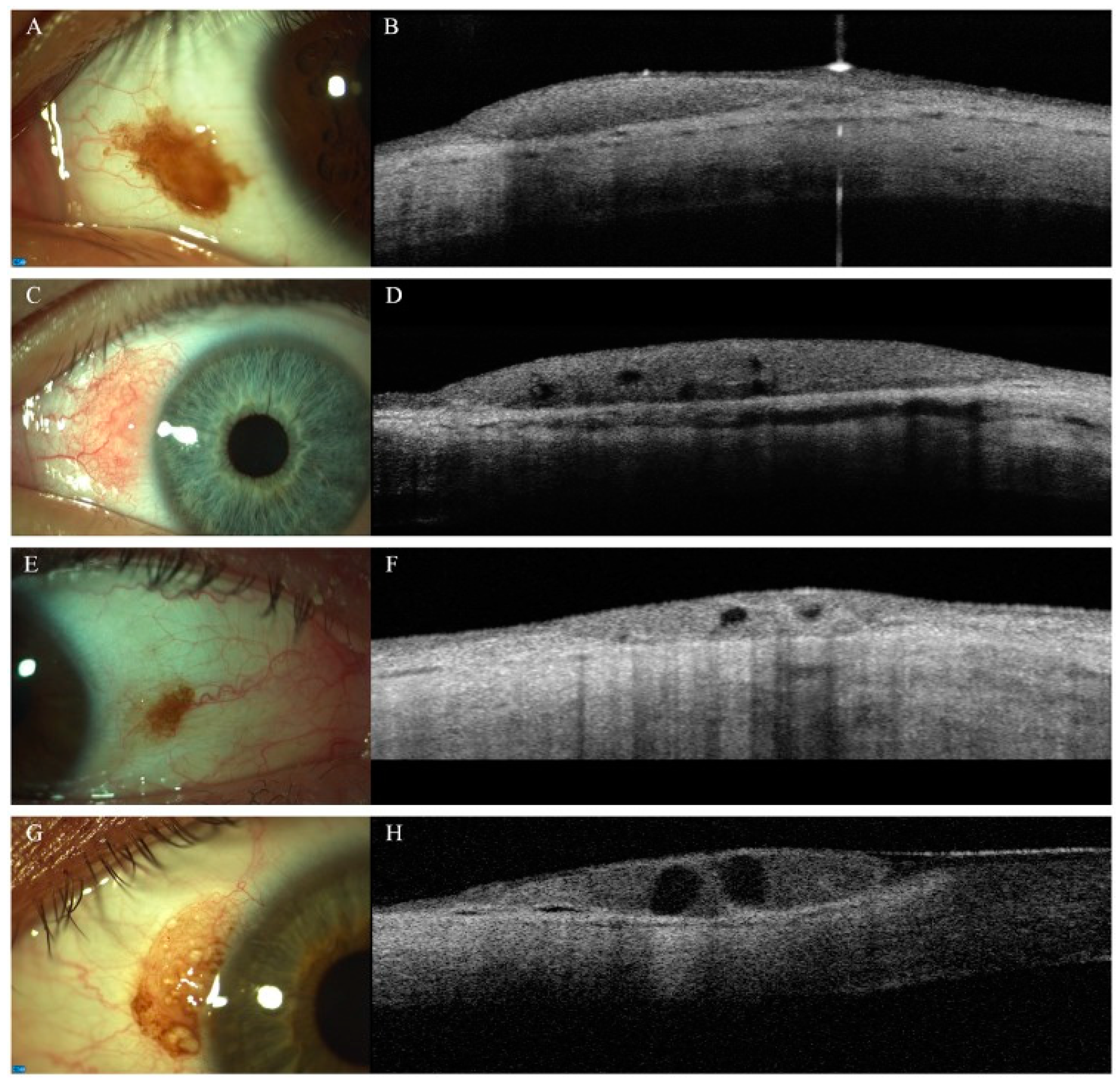
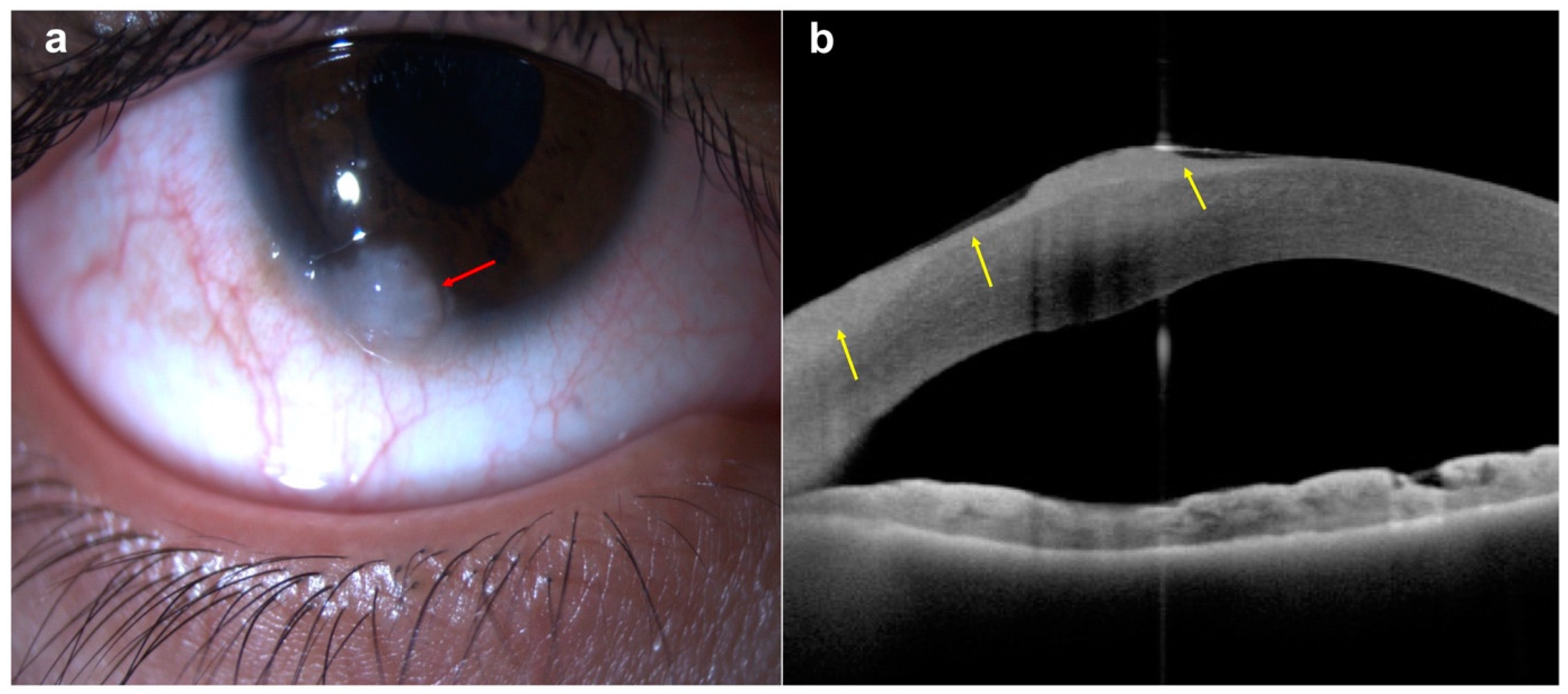
4.1. Conjunctival Benign and Malignant Tumors
4.2. Pterygium, Pinguecula, Pseudopterygium, and Salzmann Nodular Degeneration
5. Clinical Applications for Scleral Diseases
Episcleritis and Scleritis
6. Clinical Applications for Corneal Diseases
6.1. Keratoconus
6.2. Corneal Dystrophy
6.3. Infectious and Noninfectious Keratitis
6.4. Corneal Foreign Bodies
6.5. Epithelial Thickness Mapping in Corneal Disease
7. Clinical Applications in Corneal and Refractive Surgeries
7.1. Keratoplasty
7.2. Refractive Surgery
7.3. Epithelial Thickness Mapping and Refractive Surgery
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabriele, M.L.; Wollstein, G.; Ishikawa, H.; Kagemann, L.; Xu, J.; Folio, L.S.; Schuman, J.S. Optical Coherence Tomography: History, Current Status, and Laboratory Work. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Wagner, S.K.; Keane, P.A. Optical Coherence Tomography in the 2020s—Outside the Eye Clinic. Eye 2021, 35, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.L.; Wolf, S.; Dolz-Marco, R.; Tafreshi, A.; Schmitz-Valckenberg, S.; Holz, F.G. Ophthalmic Diagnostic Imaging: Retina. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Springer: Cham, Switzerland, 2019; pp. 87–106. [Google Scholar]
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-Scale Resolution Imaging of the Anterior Eye In Vivo with Optical Coherence Tomography. Arch. Ophthalmol. 1994, 112, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Dos Santos, V.A.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior Segment Optical Coherence Tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Triolo, G.; Barboni, P.; Savini, G.; De Gaetano, F.; Monaco, G.; David, A.; Scialdone, A. The Use of Anterior-Segment Optical-Coherence Tomography for the Assessment of the Iridocorneal Angle and Its Alterations: Update and Current Evidence. J. Clin. Med. 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.D.; Devarajan, K.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. Optical Coherence Tomography Angiography for the Anterior Segment. Eye Vis. 2019, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Alió, J.L.; Del Barrio, J.L.A. (Eds.) Atlas of Anterior Segment Optical Coherence Tomography; Essentials in Ophthalmology; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-53373-1. [Google Scholar]
- Ramos, J.L.B.; Li, Y.; Huang, D. Clinical and Research Applications of Anterior Segment Optical Coherence Tomography—A Review. Clin. Exp. Ophthalmol. 2009, 37, 81–89. [Google Scholar] [CrossRef]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical Coherence Tomography Today: Speed, Contrast, and Multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef]
- Keane, P.A.; Ruiz-Garcia, H.; Sadda, S.R. Clinical Applications of Long-Wavelength (1000-Nm) Optical Coherence Tomography. Ophthalmic Surg. Lasers Imaging 2011, 42, S67–S74. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Mercado, C.; Wall, S.C.; Galor, A.; Wang, J.; Karp, C.L. High Resolution Anterior Segment Optical Coherence Tomography of Ocular Surface Lesions: A Review and Handbook. Expert. Rev. Ophthalmol. 2021, 16, 81–95. [Google Scholar] [CrossRef]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-Resolution Ophthalmic Optical Coherence Tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Springer: Cham, Switzerland, 2019; pp. 59–85. [Google Scholar]
- Bille, J.F. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Optopol Technology. SOCT Copernicus HR; Optopol Technology: Zawiercie, Poland, 2014. [Google Scholar]
- Bald, M.; Li, Y.; Huang, D. Anterior Chamber Angle Evaluation with Fourier-Domain Optical Coherence Tomography. J. Ophthalmol. 2012, 2012, 103704. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Rollins, A.M.; Roth, J.E.; Yazdanfar, S.; Westphal, V.; Bardenstein, D.S.; Izatt, J.A. Real-Time Optical Coherence Tomography of the Anterior Segment at 1310 Nm. Arch. Ophthalmol. 2001, 119, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Rio-Cristobal, A.; Martin, R. Corneal Assessment Technologies: Current Status. Surv. Ophthalmol. 2014, 59, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Topcon Healthcare. DRI OCT Triton Series—A Multimodal Swept Source OCT; Topcon Healthcare: Oakland, NJ, USA, 2022. [Google Scholar]
- Optovue Inc. Optovue Iseries; Optovue Inc.: Fremont, CA, USA, 2022. [Google Scholar]
- Dua, H.S.; Darren, S.J.; Al-Aqaba, T.M.; Said, D.G. Pathophysiology of Keratoconus. In Keratoconus; Elsevier: Amsterdam, The Netherlands, 2023; pp. 51–64. [Google Scholar]
- Azzopardi, M.; Chong, Y.J.; Ng, B.; Recchioni, A.; Logeswaran, A.; Ting, D.S.J. Diagnosis of Acanthamoeba Keratitis: Past, Present and Future. Diagnostics 2023, 13, 2655. [Google Scholar] [CrossRef] [PubMed]
- Werkmeister, R.M.; Alex, A.; Kaya, S.; Unterhuber, A.; Hofer, B.; Riedl, J.; Bronhagl, M.; Vietauer, M.; Schmidl, D.; Schmoll, T.; et al. Measurement of Tear Film Thickness Using Ultrahigh-Resolution Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5578–5583. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Sinha, R.; D’Souza, S.; Potgieter, F.; Ross, A.; Kenawy, M.; Scott, I.; Said, D.G. “Descemet Membrane Detachment”: A Novel Concept in Diagnosis and Classification. Am. J. Ophthalmol. 2020, 218, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Demirci, H.; Karatza, E.; Shields, J.A. Clinical Survey of 1643 Melanocytic and Nonmelanocytic Conjunctival Tumors. Ophthalmology 2004, 111, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Fasiudden, A.; Mashayekhi, A.; Shields, J.A. Conjunctival Nevi: Clinical Features and Natural Course in 410 Consecutive Patients. Arch. Ophthalmol. 2004, 122, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L. Eyelid, Conjunctival and Orbital Tumors. An Atlas and Textbook; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Vizvári, E.; Skribek, Á.; Polgár, N.; Vörös, A.; Sziklai, P.; Tóth-Molnár, E. Conjunctival Melanocytic Naevus: Diagnostic Value of Anterior Segment Optical Coherence Tomography and Ultrasound Biomicroscopy. PLoS ONE 2018, 13, e0192908. [Google Scholar] [CrossRef]
- Shields, C.L.; Belinsky, I.; Romanelli-Gobbi, M.; Guzman, J.M.; Mazzuca, D.; Green, W.R.; Bianciotto, C.; Shields, J.A. Anterior Segment Optical Coherence Tomography of Conjunctival Nevus. Ophthalmology 2011, 118, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Höllhumer, R.; Williams, S.; Michelow, P. Ocular Surface Squamous Neoplasia: Management and Outcomes. Eye 2021, 35, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L.; Scartozzi, R. Survey of 1264 Patients with Orbital Tumors and Simulating Lesions: The 2002 Montgomery Lecture, Part 1. Ophthalmology 2004, 111, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.S.; Vora, G.K.; Gupta, P.K. Anterior Segment Imaging in Ocular Surface Squamous Neoplasia. J. Ophthalmol. 2016, 2016, 5435092. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, N.; Lake, S.L.; Coupland, S.E.; Damato, B.E. Conjunctival Melanoma and Melanocytic Intra-Epithelial Neoplasia. Eye 2013, 27, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.R.; Nanji, A.A.; Galor, A.; Karp, C.L. Management of Conjunctival Malignant Melanoma: A Review and Update. Expert. Rev. Ophthalmol. 2014, 9, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Shousha, M.A.; Karp, C.L.; Canto, A.P.; Hodson, K.; Oellers, P.; Kao, A.A.; Bielory, B.; Matthews, J.; Dubovy, S.R.; Perez, V.L.; et al. Diagnosis of Ocular Surface Lesions Using Ultra-High-Resolution Optical Coherence Tomography. Ophthalmology 2013, 120, 883–891. [Google Scholar] [CrossRef]
- Shields, C.L.; Chien, J.L.; Surakiatchanukul, T.; Sioufi, K.; Lally, S.E.; Shields, J.A. Conjunctival Tumors: Review of Clinical Features, Risks, Biomarkers, and Outcomes—The 2017 J. Donald M. Gass Lecture. Asia Pac. J. Ophthalmol. 2017, 6, 109–120. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Mercado, C.; Tran, A.Q.; Garcia, A.; Diaz, P.F.M.; Dubovy, S.R.; Galor, A.; Karp, C.L. The Use of High Resolution Anterior Segment Optical Coherence Tomography for the Characterization of Conjunctival Lymphoma, Conjunctival Amyloidosis and Benign Reactive Lymphoid Hyperplasia. Eye Vis. 2019, 6, 17. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Galor, A.; Wang, J.; Karp, C.L. Optical Coherence Tomography for Ocular Surface and Corneal Diseases: A Review. Eye Vis. 2018, 5, 13. [Google Scholar] [CrossRef]
- Bradley, J.C.; Yang, W.; Bradley, R.H.; Reid, T.W.; Schwab, I.R. The Science of Pterygia. Br. J. Ophthalmol. 2010, 94, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Liu, Y.-C.; Patil, M.; Ji, A.J.S.; Fang, X.L.; Tham, Y.C.; Lee, Y.-F.; Htoon, H.M.; Mehta, J.S. Proposal and Validation of a New Grading System for Pterygium (SLIT2). Br. J. Ophthalmol. 2021, 105, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, J.; Geng, J.; Yuan, Z.; Huang, D. Geographical Prevalence and Risk Factors for Pterygium: A Systematic Review and Meta-Analysis. BMJ Open 2013, 3, e003787. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F.; Pearlman, E. The Eye E-Book: Basic Sciences in Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Norn, M.S. Prevalence of Pinguecula in Greenland and in Copenhagen, and Its Relation to Pterygium and Spheroid Degeneration. Acta Ophthalmol. 1979, 57, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Panchapakesan, J.; Hourihan, F.; Mitchell, P. Prevalence of Pterygium and Pinguecula: The Blue Mountains Eye Study. Aust. N. Z. J. Ophthalmol. 1998, 26 (Suppl. S1), S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, A.; Hashemi, H.; Khabazkhoob, M.; Mohammad, K. Prevalence and Risk Factors of Pterygium and Pinguecula: The Tehran Eye Study. Eye 2009, 23, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Viso, E.; Gude, F.; Rodríguez-Ares, M.T. Prevalence of Pinguecula and Pterygium in a General Population in Spain. Eye 2011, 25, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.; Mohamed, T.A. Spectral Domain Anterior Segment Optical Coherence Tomography Assessment of Pterygium and Pinguecula. Acta Ophthalmol. 2012, 90, 461–465. [Google Scholar] [CrossRef]
- Urbinati, F.; Borroni, D.; Rodríguez-Calvo-de-Mora, M.; Sánchez-González, J.-M.; García-Lorente, M.; Zamorano-Martín, F.; Rachwani-Anil, R.; Ortiz-Pérez, S.; Romano, V.; Rocha-de-Lossada, C. Pseudopterygium: An Algorithm Approach Based on the Current Evidence. Diagnostics 2022, 12, 1843. [Google Scholar] [CrossRef]
- Whitcup, S.M.; Sen, H.N. Whitcup and Nussenblatt’s Uveitis: Fundamentals and Clinical Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Okhravi, N.; Odufuwa, B.; McCluskey, P.; Lightman, S. Scleritis. Surv. Ophthalmol. 2005, 50, 351–363. [Google Scholar] [CrossRef]
- Preetam Peraka, R.; Murthy, S.I. Role of Anterior Segment Optical Coherence Tomography in Scleral Diseases: A Review. Semin. Ophthalmol. 2023, 38, 238–247. [Google Scholar] [CrossRef]
- Levison, A.L.; Lowder, C.Y.; Baynes, K.M.; Kaiser, P.K.; Srivastava, S.K. Anterior Segment Spectral Domain Optical Coherence Tomography Imaging of Patients with Anterior Scleritis. Int. Ophthalmol. 2016, 36, 499–508. [Google Scholar] [CrossRef] [PubMed]
- McGhee, C.N. Keratoconus: The arc of past, present and future. Clin. Exp. Optom. 2013, 96, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An Updated Review. Cont. Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Cresta, F.B.; Orlandin, L.F.; Lucas, M.B. Clinical Presentation and Evolution of Keratoconus. In Keratoconus: A Comprehensive Guide to Diagnosis and Treatment; Almodin, E., Nassaralla, B.A., Sandes, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–12. ISBN 978-3-030-85361-7. [Google Scholar]
- Pérez, J.F.; Marcos, A.V.; Peña, F.J.M. Early Diagnosis of Keratoconus: What Difference Is It Making? Br. J. Ophthalmol. 2014, 98, 1465–1466. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Rana-Rahman, R.; Chen, Y.; Bell, D.; Danjoux, J.-P.; Morgan, S.J.; Ghosh, S.; Baylis, O. Effectiveness and Safety of Accelerated (9 mW/cm2) Corneal Collagen Cross-Linking for Progressive Keratoconus: A 24-Month Follow-Up. Eye 2019, 33, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Ong, Z.Z.; Rampat, R.; Alió Del Barrio, J.L.; Barua, A.; Ang, M.; Mehta, J.S.; Said, D.G.; Dua, H.S.; Ambrósio, R.; et al. Management of Keratoconus: An Updated Review. Front. Med. 2023, 10, 1212314. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lim, D.K.-A.; Lim, B.X.H.; Wong, N.; Hafezi, F.; Manotosh, R.; Lim, C.H.L. Corneal Cross-Linking: The Evolution of Treatment for Corneal Diseases. Front. Pharmacol. 2021, 12, 686630. [Google Scholar] [CrossRef]
- Li, Y.; Meisler, D.M.; Tang, M.; Lu, A.T.H.; Thakrar, V.; Reiser, B.J.; Huang, D. Keratoconus Diagnosis with Optical Coherence Tomography Pachymetry Mapping. Ophthalmology 2008, 115, 2159–2166. [Google Scholar] [CrossRef]
- Kim, J.S.; Rho, C.R.; Cho, Y.W.; Shin, J. Comparison of Corneal Thickness Measurements Using Ultrasound Pachymetry, Noncontact Tonopachy, Pentacam HR, and Fourier-Domain OCT. Medicine 2021, 100, e25638. [Google Scholar] [CrossRef]
- Yücekul, B.; Dick, H.B.; Taneri, S. Systematic Detection of Keratoconus in OCT: Corneal and Epithelial Thickness Maps. J. Cataract. Refract. Surg. 2022, 48, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Herber, R.; Lenk, J.; Pillunat, L.E.; Raiskup, F. Comparison of Corneal Tomography Using a Novel Swept-Source Optical Coherence Tomographer and Rotating Scheimpflug System in Normal and Keratoconus Eyes: Repeatability and Agreement Analysis. Eye Vis. 2022, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pavlatos, E.; Chamberlain, W.; Huang, D.; Li, Y. Keratoconus Detection Using OCT Corneal and Epithelial Thickness Map Parameters and Patterns. J. Cataract. Refract. Surg. 2021, 47, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Wolter, J.R.; Henderson, J.W.; Clahassey, E.G. Ruptures of Descemet’s Membrane in Keratoconus Causing Acute Hydrops and Posterior Keratoconus. Am. J. Ophthalmol. 1967, 63, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.L. Pathophysiology and Treatment of Corneal Hydrops. Ophthalmic Surg. 1976, 7, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Srinivasan, S. Pneumodescemetopexy with Perfluoroethane (C2F6) for the Treatment of Acute Hydrops Secondary to Keratoconus. Eye 2014, 28, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Said, D.G.; Dua, H.S. Are Descemet Membrane Ruptures the Root Cause of Corneal Hydrops in Keratoconic Eyes? Am. J. Ophthalmol. 2019, 205, 204. [Google Scholar] [CrossRef]
- Fan Gaskin, J.C.; Patel, D.V.; McGhee, C.N.J. Acute Corneal Hydrops in Keratoconus—New Perspectives. Am. J. Ophthalmol. 2014, 157, 921–928. [Google Scholar] [CrossRef]
- Kreps, E.O.; Claerhout, I.; Koppen, C. The Outcome of Scleral Lens Fitting for Keratoconus with Resolved Corneal Hydrops. Cornea 2019, 38, 855–858. [Google Scholar] [CrossRef]
- Basu, S.; Vaddavalli, P.K.; Vemuganti, G.K.; Ali, M.H.; Murthy, S.I. Anterior Segment Optical Coherence Tomography Features of Acute Corneal Hydrops. Cornea 2012, 31, 479–485. [Google Scholar] [CrossRef]
- Dua, H.S.; Freitas, R.; Mohammed, I.; Ting, D.S.; Said, D.G. The Pre-Descemet’s Layer (Dua’s Layer, Also Known as the Dua-Fine Layer and the Pre-Posterior Limiting Lamina Layer): Discovery, Characterisation, Clinical and Surgical Applications, and the Controversy. Prog. Retin. Eye Res. 2023, 97, 101161. [Google Scholar] [CrossRef] [PubMed]
- Gokul, A.; Krishnan, T.; Emanuel, P.O.; Saunders, M.; McGhee, C.N. Persisting Extreme Acute Corneal Hydrops with a Giant Intrastromal Cleft Secondary to Keratoconus. Clin. Exp. Optom. 2015, 98, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.Q.; Kocaba, V.; Weiss, J.S.; Jurkunas, U.V.; Kinoshita, S.; Aldave, A.J.; Mehta, J.S. Corneal Dystrophies. Nat. Rev. Dis. Primers 2020, 6, 46. [Google Scholar] [CrossRef]
- Weiss, J.S.; Møller, H.U.; Lisch, W.; Kinoshita, S.; Aldave, A.J.; Belin, M.W.; Kivelä, T.; Busin, M.; Munier, F.L.; Seitz, B.; et al. The IC3D Classification of the Corneal Dystrophies. Cornea 2008, 27, S1–S83. [Google Scholar] [CrossRef] [PubMed]
- Siebelmann, S.; Scholz, P.; Sonnenschein, S.; Bachmann, B.; Matthaei, M.; Cursiefen, C.; Heindl, L.M. Anterior Segment Optical Coherence Tomography for the Diagnosis of Corneal Dystrophies According to the IC3D Classification. Surv. Ophthalmol. 2018, 63, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H. Global Causes of Blindness and Distance Vision Impairment 1990–2020: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious Keratitis: An Update on Epidemiology, Causative Microorganisms, Risk Factors, and Antimicrobial Resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef] [PubMed]
- Ung, L.; Bispo, P.J.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The Persistent Dilemma of Microbial Keratitis: Global Burden, Diagnosis, and Antimicrobial Resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef]
- Stapleton, F. The Epidemiology of Infectious Keratitis. Ocul. Surf. 2023, 28, 351–363. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. 12-Year Analysis of Incidence, Microbiological Profiles and In Vitro Antimicrobial Susceptibility of Infectious Keratitis: The Nottingham Infectious Keratitis Study. Br. J. Ophthalmol. 2021, 105, 328–333. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Cairns, J.; Gopal, B.P.; Ho, C.S.; Krstic, L.; Elsahn, A.; Lister, M.; Said, D.G.; Dua, H.S. Risk Factors, Clinical Outcomes, and Prognostic Factors of Bacterial Keratitis: The Nottingham Infectious Keratitis Study. Front. Med. 2021, 8, 715118. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, J.; Chen, K.; Chen, Q.; Zheng, Q.; Liu, X.; Weng, H.; Wu, S.; Chen, W. Preventing Corneal Blindness Caused by Keratitis Using Artificial Intelligence. Nat. Commun. 2021, 12, 3738. [Google Scholar] [CrossRef] [PubMed]
- Rampat, R.; Deshmukh, R.; Chen, X.; Ting, D.S.W.; Said, D.G.; Dua, H.S.; Ting, D.S.J. Artificial Intelligence in Cornea, Refractive Surgery, and Cataract: Basic Principles, Clinical Applications, and Future Directions. Asia Pac. J. Ophthalmol. 2021, 10, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Gopal, B.P.; Deshmukh, R.; Seitzman, G.D.; Said, D.G.; Dua, H.S. Diagnostic Armamentarium of Infectious Keratitis: A Comprehensive Review. Ocul. Surf. 2022, 23, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, A.; Kuo, J.; Anderson, D.; Hossain, P. Assessment of the Use of Anterior Segment Optical Coherence Tomography in Microbial Keratitis. Am. J. Ophthalmol. 2008, 146, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, A.; Yadegarfar, G.; Fievez, M.; Anderson, D.F.; Hossain, P. In Vivo Quantification of Bacterial Keratitis with Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Singhal, D.; Maharana, P.K.; Agarwal, T.; Sinha, R.; Satpathy, G.; Bageshwar, L.M.S.; Titiyal, J.S. Spectral Domain Anterior Segment Optical Coherence Tomography in Fungal Keratitis. Cornea 2018, 37, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Ghosh, S. Central Toxic Keratopathy after Contact Lens Wear and Mechanical Debridement: Clinical Characteristics, and Visual and Corneal Tomographic Outcomes. Eye Contact Lens 2019, 45, e15–e23. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Danjoux, J.-P. Late-Onset Traumatic Dislocation of Laser in Situ Keratomileusis Corneal Flaps: A Case Series with Many Clinical Lessons. Int. Ophthalmol. 2019, 39, 1397–1403. [Google Scholar] [CrossRef]
- Takezawa, Y.; Suzuki, T.; Shiraishi, A. Observation of Retrocorneal Plaques in Patients with Infectious Keratitis Using Anterior Segment Optical Coherence Tomography. Cornea 2017, 36, 1237–1242. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Galal, M.; Kulkarni, B.; Elalfy, M.S.; Lake, D.; Hamada, S.; Said, D.G.; Dua, H.S. Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011–2020: A 10-Year Study. J. Fungi 2021, 7, 966. [Google Scholar] [CrossRef]
- Soliman, W.; Nassr, M.A.; Abdelazeem, K.; Al-Hussaini, A.K. Appearance of Herpes Simplex Keratitis on Anterior Segment Optical Coherence Tomography. Int. Ophthalmol. 2019, 39, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.; Fathalla, A.M.; El-Sebaity, D.M.; Al-Hussaini, A.K. Spectral Domain Anterior Segment Optical Coherence Tomography in Microbial Keratitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Kobayashi, A.; Yokogawa, H.; Ishibashi, Y.; Oikawa, Y.; Tokoro, M.; Sugiyama, K. In Vivo Imaging of Radial Keratoneuritis in Patients with Acanthamoeba Keratitis by Anterior-Segment Optical Coherence Tomography. Ophthalmology 2014, 121, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Lee, J.S.; Yoo, J.-M.; Park, J.M.; Seo, S.-W.; Chung, I.-Y.; Kim, S.J. Comparison of Anterior Segment Optical Coherence Tomography Findings in Acanthamoeba Keratitis and Herpetic Epithelial Keratitis. Int. J. Ophthalmol. 2018, 11, 1416. [Google Scholar] [PubMed]
- Sridhar, M.S.; Shaik, B. Anterior Segment Optical Coherence Tomography of Microsporidial Keratoconjunctivitis. Indian. J. Ophthalmol. 2018, 66, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Thanathanee, O.; Laohapitakvorn, S.; Anutarapongpan, O.; Suwan-Apichon, O.; Bhoomibunchoo, C. Anterior Segment Optical Coherence Tomography Images in Microsporidial Keratoconjunctivitis. Cornea 2019, 38, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, H.; Kobayashi, A.; Yamazaki, N.; Sugiyama, K. In Vivo Imaging of Coin-Shaped Lesions in Cytomegalovirus Corneal Endotheliitis by Anterior Segment Optical Coherence Tomography. Cornea 2014, 33, 1332–1335. [Google Scholar] [CrossRef]
- Kobayashi, R.; Hashida, N.; Soma, T.; Koh, S.; Miki, A.; Usui, S.; Maeda, N.; Nishida, K. Clinical Findings of Anterior Segment Spectral Domain Optical Coherence Tomography Images in Cytomegalovirus Corneal Endotheliitis. Cornea 2016, 36, 411–414. [Google Scholar] [CrossRef]
- Pinto, R.D.P.; Lira, R.P.C.; Arieta, C.E.L.; de Castro, R.S.; Bonon, S.H.A. The Prevalence of Adenoviral Conjunctivitis at the Clinical Hospital of the State University of Campinas, Brazil. Clinics 2015, 70, 748–750. [Google Scholar] [CrossRef]
- Sambursky, R.P.; Fram, N.; Cohen, E.J. The Prevalence of Adenoviral Conjunctivitis at the Wills Eye Hospital Emergency Room. Optom.-J. Am. Optom. Assoc. 2007, 78, 236–239. [Google Scholar] [CrossRef]
- Hoffman, J. Adenovirus: Ocular Manifestations. Community Eye Health 2020, 33, 73–75. [Google Scholar] [PubMed]
- Pennington, M.R.; Saha, A.; Painter, D.F.; Gavazzi, C.; Ismail, A.M.; Zhou, X.; Chodosh, J.; Rajaiya, J. Disparate Entry of Adenoviruses Dictates Differential Innate Immune Responses on the Ocular Surface. Microorganisms 2019, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Arici, C.; Mergen, B. Late-Term Topical Tacrolimus for Subepithelial Infiltrates Resistant to Topical Steroids and Ciclosporin Secondary to Adenoviral Keratoconjunctivitis. Br. J. Ophthalmol. 2021, 105, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Gouider, D.; Khallouli, A.; Maalej, A.; Khochtali, S.; Khairallah, M. Role of Anterior Segment Optical Coherence Tomography in Monitoring Epidemic Keratoconjunctivitis. J. Curr. Ophthalmol. 2022, 33, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Royal College of General Practitioners. Royal College of Ophthalmologists (2001) Ophthalmology for General Practice Trainees; Medical Protection Society: London, UK, 2001. [Google Scholar]
- Wong, T.Y.; Lincoln, A.; Tielsch, J.M.; Baker, S.P. The Epidemiology of Ocular Injury in a Major US Automobile Corporation. Eye 1998, 12 Pt 5, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Celebi, A.R.C.; Kilavuzoglu, A.E.; Altiparmak, U.E.; Cosar, C.B.; Ozkiris, A. The Role of Anterior Segment Optical Coherence Tomography in the Management of an Intra-Corneal Foreign Body. Springerplus 2016, 5, 1559. [Google Scholar] [CrossRef] [PubMed]
- Armarnik, S.; Mimouni, M.; Goldenberg, D.; Segev, F.; Meshi, A.; Segal, O.; Geffen, N. Characterization of Deeply Embedded Corneal Foreign Bodies with Anterior Segment Optical Coherence Tomography. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1247–1252. [Google Scholar] [CrossRef]
- Akbaş, E.; Barut Selver, Ö.; Palamar, M. Retrospective Evaluation of Corneal Foreign Bodies with Anterior Segment Optical Coherence Tomography. Turk. J. Ophthalmol. 2021, 51, 265–268. [Google Scholar] [CrossRef]
- Simon, G.; Ren, Q.; Kervick, G.N.; Parel, J.-M. Optics of the Corneal Epithelium. J. Refract. Surg. 1993, 9, 42–50. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Silverman, R.H.; Coleman, D.J. High-Frequency Ultrasound Measurement of the Thickness of the Corneal Epithelium. J. Refract. Surg. 1993, 9, 385–387. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Vida, R.S. Applications of Epithelial Thickness Mapping in Corneal Refractive Surgery. Saudi J. Ophthalmol. 2022, 36, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Urs, R.; Lloyd, H.O.; Reinstein, D.Z.; Silverman, R.H. Comparison of Very-High-Frequency Ultrasound and Spectral-Domain Optical Coherence Tomography Corneal and Epithelial Thickness Maps. J. Cataract. Refract. Surg. 2016, 42, 95–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.F.; Petroll, W.M.; Møller-Pedersen, T.; Maurer, J.K.; Cavanagh, H.D.; Jester, J.V. Epithelial and Corneal Thickness Measurements by In Vivo Confocal Microscopy through Focusing (CMTF). Curr. Eye Res. 1997, 16, 214–221. [Google Scholar] [CrossRef]
- Sella, R.; Zangwill, L.M.; Weinreb, R.N.; Afshari, N.A. Repeatability and Reproducibility of Corneal Epithelial Thickness Mapping with Spectral-Domain Optical Coherence Tomography in Normal and Diseased Cornea Eyes. Am. J. Ophthalmol. 2019, 197, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.X.; Wang, L.; Weikert, M.P.; Montes de Oca, I.; Koch, D.D. Evaluation of the Repeatability and Reproducibility of Corneal Epithelial Thickness Mapping for a 9-Mm Zone Using Optical Coherence Tomography. Cornea 2019, 38, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Khamar, P.; Rao, K.; Wadia, K.; Dalal, R.; Grover, T.; Versaci, F.; Gupta, K. Advanced Epithelial Mapping for Refractive Surgery. Indian J. Ophthalmol. 2020, 68, 2819. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Simpson, T.; Jones, L. Corneal and Epithelial Thickness in Keratoconus: A Comparison of Ultrasonic Pachymetry, Orbscan II, and Optical Coherence Tomography. J. Refract. Surg. 2006, 22, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Gobbe, M.; Archer, T.J.; Couch, D. Epithelial Thickness Profile as a Method to Evaluate the Effectiveness of Collagen Cross-Linking Treatment After Corneal Ectasia. J. Refract. Surg. 2011, 27, 356–363. [Google Scholar] [CrossRef]
- Kanellopoulos, A.J.; Asimellis, G. In Vivo 3-Dimensional Corneal Epithelial Thickness Mapping as an Indicator of Dry Eye: Preliminary Clinical Assessment. Am. J. Ophthalmol. 2014, 157, 63–68. [Google Scholar] [CrossRef]
- Ondas, O.; Keles, S. Central Corneal Thickness in Patients with Atopic Keratoconjunctivitis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 1687. [Google Scholar]
- Buffault, J.; Zéboulon, P.; Liang, H.; Chiche, A.; Luzu, J.; Robin, M.; Rabut, G.; Labetoulle, M.; Labbé, A.; Baudouin, C. Assessment of Corneal Epithelial Thickness Mapping in Epithelial Basement Membrane Dystrophy. PLoS ONE 2020, 15, e0239124. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Georgeon, C.; Knoeri, J.; Tourabaly, M.; Leveziel, L.; Bouheraoua, N.; Borderie, V.M. Corneal Epithelial Thickness Mapping in the Diagnosis of Ocular Surface Disorders Involving the Corneal Epithelium: A Comparative Study. Cornea 2022, 41, 1353. [Google Scholar] [CrossRef] [PubMed]
- Seitz, B.; Asi, F.; Mäurer, S.; Hamon, L.; Quintin, A.; Langenbucher, A. Anterior Segment OCT: Application to Improve Graft Selection for Corneal Transplantation. In Atlas of Anterior Segment Optical Coherence Tomography; Springer: Cham, Switzerland, 2021; pp. 223–236. [Google Scholar]
- Ting, D.S.J.; Sau, C.Y.; Srinivasan, S.; Ramaesh, K.; Mantry, S.; Roberts, F. Changing Trends in Keratoplasty in the West of Scotland: A 10-Year Review. Br. J. Ophthalmol. 2012, 96, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Dunker, S.L.; Veldman, M.H.J.; Winkens, B.; van den Biggelaar, F.J.H.M.; Nuijts, R.M.M.A.; Kruit, P.J.; Dickman, M.M.; Dutch Cornea Consortium. Real-World Outcomes of DMEK: A Prospective Dutch Registry Study. Am. J. Ophthalmol. 2021, 222, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Q.; Rubenstein, D.; Price, A.J.; Côté, E.; Levitt, M.; Sharpen, L.; Slomovic, A. Evolving Surgical Techniques of and Indications for Corneal Transplantation in Ontario: 2000–2012. Can. J. Ophthalmol. 2013, 48, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Deshmukh, R.; Ting, D.S.W.; Ang, M. Big Data in Corneal Diseases and Cataract: Current Applications and Future Directions. Front. Big Data 2023, 6, 1017420. [Google Scholar] [CrossRef]
- Liu, S.; Wong, Y.L.; Walkden, A. Current Perspectives on Corneal Transplantation. Clin. Ophthalmol. 2022, 16, 631–646. [Google Scholar] [CrossRef]
- Kaiserman, I.; Bahar, I.; Rootman, D.S. Corneal Wound Malapposition after Penetrating Keratoplasty: An Optical Coherence Tomography Study. Br. J. Ophthalmol. 2008, 92, 1103–1107. [Google Scholar] [CrossRef]
- Weller, J.M.; Hübner, L.; Kruse, F.E.; Tourtas, T. Characterisation of Ectasia after Penetrating Keratoplasty in Keratoconus Eyes Using Anterior Segment Optical Coherence Tomography. Br. J. Ophthalmol. 2023, bjo-2022-322859. [Google Scholar] [CrossRef]
- Niederkorn, J.Y.; Larkin, D.F.P. Immune Privilege of Corneal Allografts. Ocul. Immunol. Inflamm. 2010, 18, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Z.; Wong, T.L.; Suresh, L.; Hammoudeh, Y.; Lister, M.; Said, D.G.; Dua, H.S.; Ting, D.S.J. A 7-Year Review of Clinical Characteristics, Predisposing Factors and Outcomes of Post-Keratoplasty Infectious Keratitis: The Nottingham Infectious Keratitis Study. Front. Cell Infect. Microbiol. 2023, 13, 1250599. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Benlloch, A.; Montesel, A.; Pareja-Aricò, L.; Mingo-Botín, D.; Michael, R.; Barraquer, R.I.; Alió, J. Causes of Corneal Transplant Failure: A Multicentric Study. Acta Ophthalmol. 2021, 99, e922–e928. [Google Scholar] [CrossRef] [PubMed]
- Larkin, D.F. Corneal Allograft Rejection. Br. J. Ophthalmol. 1994, 78, 649–652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yenerel, N.M.; Kucumen, R.B.; Gorgun, E. The Complementary Benefit of Anterior Segment Optical Coherence Tomography in Penetrating Keratoplasty. Clin. Ophthalmol. 2013, 7, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Teichmann, K.D. Big-Bubble Technique to Bare Descemet’s Membrane in Anterior Lamellar Keratoplasty. J. Cataract. Refract. Surg. 2002, 28, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Wylegała, E.; Nowińska, A. Usefulness of Anterior Segment Optical Coherence Tomography in Descemet Membrane Detachment. Eur. J. Ophthalmol. 2009, 19, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, W.; Austin, A.; Terry, M.; Jeng, B.H.; Rose-Nussbaumer, J. Survey of Experts on Current Endothelial Keratoplasty Techniques. J. Clin. Exp. Ophthalmol. 2016, 7, 608. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Giebel, A.W.; Fairchild, K.M.; Price, F.W., Jr. Descemet’s Membrane Endothelial Keratoplasty: Prospective Multicenter Study of Visual and Refractive Outcomes and Endothelial Survival. Ophthalmology 2009, 116, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Ham, L.; Dapena, I.; van Luijk, C.; van der Wees, J.; Melles, G.R.J. Descemet Membrane Endothelial Keratoplasty (DMEK) for Fuchs Endothelial Dystrophy: Review of the First 50 Consecutive Cases. Eye 2009, 23, 1990–1998. [Google Scholar] [CrossRef]
- Vasiliauskaite, I.; Kocaba, V.; van Dijk, K.; Baydoun, L.; Lanser, C.; Lee, D.; Jager, M.J.; Melles, G.R.J.; Oellerich, S. Long-Term Outcomes of Descemet Membrane Endothelial Keratoplasty: Effect of Surgical Indication and Disease Severity. Cornea 2023, 42, 1229–1239. [Google Scholar] [CrossRef]
- Ang, M.; Ting, D.S.J.; Kumar, A.; May, K.O.; Htoon, H.M.; Mehta, J.S. Descemet Membrane Endothelial Keratoplasty in Asian Eyes: Intraoperative and Postoperative Complications. Cornea 2020, 39, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.B.; Jacobs, D.S.; Musch, D.C.; Kaufman, S.C.; Reinhart, W.J.; Shtein, R.M. Descemet’s Stripping Endothelial Keratoplasty: Safety and Outcomes: A Report by the American Academy of Ophthalmology. Ophthalmology 2009, 116, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Dirisamer, M.; van Dijk, K.; Dapena, I.; Ham, L.; Oganes, O.; Frank, L.E.; Melles, G.R.J. Prevention and Management of Graft Detachment in Descemet Membrane Endothelial Keratoplasty. Arch. Ophthalmol. 2012, 130, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Nair, S.; Ting, D.S.J.; Agarwal, T.; Beltz, J.; Vajpayee, R.B. Graft Detachments in Endothelial Keratoplasty. Br. J. Ophthalmol. 2022, 106, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tey, K.Y.; Tan, S.Y.; Ting, D.S.J.; Mehta, J.S.; Ang, M. Effects of Combined Cataract Surgery on Outcomes of Descemet’s Membrane Endothelial Keratoplasty: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 857200. [Google Scholar] [CrossRef] [PubMed]
- Coco, G.; Levis, H.J.; Borgia, A.; Romano, D.; Pagano, L.; Virgili, G.; Kaye, S.B.; Romano, V. Posterior Stromal Ripples Increase Risk of Descemet’s Membrane Endothelial Keratoplasty Graft Detachment Worsening over Time. Acta Ophthalmol. 2023, 101, e205–e214. [Google Scholar] [CrossRef] [PubMed]
- Fernández López, E.; Baydoun, L.; Gerber-Hollbach, N.; Dapena, I.; Liarakos, V.S.; Ham, L.; Melles, G.R.J. Rebubbling Techniques for Graft Detachment after Descemet Membrane Endothelial Keratoplasty. Cornea 2016, 35, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.-Y.; Quilendrino, R.; Musa, F.U.; Liarakos, V.S.; Dapena, I.; Melles, G.R.J. Predictive Value of Optical Coherence Tomography in Graft Attachment after Descemet’s Membrane Endothelial Keratoplasty. Ophthalmology 2013, 120, 240–245. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Said, D.G.; Dua, H.S. Interface Haze after Descemet Stripping Automated Endothelial Keratoplasty. JAMA Ophthalmol. 2019, 137, 1201–1202. [Google Scholar] [CrossRef]
- Song, A.; Deshmukh, R.; Lin, H.; Ang, M.; Mehta, J.S.; Chodosh, J.; Said, D.G.; Dua, H.S.; Ting, D.S.J. Post-Keratoplasty Infectious Keratitis: Epidemiology, Risk Factors, Management, and Outcomes. Front. Med. 2021, 8, 707242. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kaur, M.; Titiyal, J.S.; Aldave, A. Infectious Keratitis after Lamellar Keratoplasty. Surv. Ophthalmol. 2021, 66, 623–643. [Google Scholar] [CrossRef]
- Market Scope: Refractive Surgery to Grow 9.6% a Year through 2025, Despite COVID-19. Available online: https://eyewire.news/news/market-scope-refractive-surgery-to-grow-9-6-a-year-through-2025-despite-covid-19 (accessed on 28 September 2023).
- Sutton, G.L.; Kim, P. Laser in Situ Keratomileusis in 2010—A Review. Clin Exp Ophthalmol. 2010, 38, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, H.P.; Donnenfeld, E.D.; Kohnen, T.; Lindstrom, R.L.; Potvin, R.; Tremblay, D.M.; Solomon, K.D. Modern Laser in Situ Keratomileusis Outcomes. J. Cataract. Refract. Surg. 2016, 42, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Pallikaris, I.G.; Papatzanaki, M.E.; Stathi, E.Z.; Frenschock, O.; Georgiadis, A. Laser in Situ Keratomileusis. Lasers Surg. Med. 1990, 10, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Buratto, L.; Ferrari, M. Excimer Laser Intrastromal Keratomileusis: Case Reports. J. Cataract. Refract. Surg. 1992, 18, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.G.; Schmack, I.; Holley, G.P.; Waring, G.O.; Grossniklaus, H.E.; Edelhauser, H.F. Interface Fluid Syndrome in Human Eye Bank Corneas after LASIK: Causes and Pathogenesis. Ophthalmology 2007, 114, 1848–1859.e1. [Google Scholar] [CrossRef] [PubMed]
- Lyle, W.A.; Jin, G.J. Interface Fluid Associated with Diffuse Lamellar Keratitis and Epithelial Ingrowth after Laser in Situ Keratomileusis. J. Cataract. Refract. Surg. 1999, 25, 1009–1012. [Google Scholar] [CrossRef]
- Galvis, V.; Berrospi, R.D.; Tello, A.; Santaella, G. Interface Fluid Syndrome (IFS) Following Toxic Anterior Segment Syndrome (TASS): Not Related to High Intraocular Pressure but to Endothelial Failure. Saudi J. Ophthalmol. 2019, 33, 88–93. [Google Scholar] [CrossRef]
- Rosas Salaroli, C.H.; Li, Y.; Huang, D. High-Resolution Optical Coherence Tomography Visualization of LASIK Flap Displacement. J. Cataract. Refract. Surg. 2009, 35, 1640–1642. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Srinivasan, S.; Danjoux, J.-P. Epithelial Ingrowth Following Laser in Situ Keratomileusis (LASIK): Prevalence, Risk Factors, Management and Visual Outcomes. BMJ Open Ophthalmol. 2018, 3, e000133. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, M.C.; Mannis, M.J.; Kim, E.S. Epithelial Downgrowth after Femtosecond Laser-Assisted Cataract Surgery. Am. J. Ophthalmol. Case Rep. 2019, 15, 100507. [Google Scholar] [CrossRef] [PubMed]
- Maumenee, A.E.; Paton, D.; Morse, P.H.; Butner, R. Review of 40 Histologically Proven Cases of Epithelial Downgrowth Following Cataract Extraction and Suggested Surgical Management. Am. J. Ophthalmol. 1970, 69, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.; Meyer, R.F.; Hood, C.I. Epithelial Downgrowth Following Penetrating Keratoplasty in the Aphake. Arch. Ophthalmol. 1977, 95, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.J.; Trentacoste, J.; Pon, D.M.; Albert, D.M. Epithelial Downgrowth: A 30-Year Clinicopathological Review. Br. J. Ophthalmol. 1989, 73, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Randleman, J.B.; Russell, B.; Ward, M.A.; Thompson, K.P.; Stulting, R.D. Risk Factors and Prognosis for Corneal Ectasia after LASIK. Ophthalmology 2003, 110, 267–275. [Google Scholar] [CrossRef]
- Binder, P.S.; Lindstrom, R.L.; Stulting, R.D.; Donnenfeld, E.; Wu, H.; McDonnell, P.; Rabinowitz, Y. Keratoconus and Corneal Ectasia after LASIK. J. Cataract. Refract. Surg. 2005, 31, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Goebels, S.; Eppig, T.; Wagenpfeil, S.; Cayless, A.; Seitz, B.; Langenbucher, A. Complementary Keratoconus Indices Based on Topographical Interpretation of Biomechanical Waveform Parameters: A Supplement to Established Keratoconus Indices. Comput. Math. Methods Med. 2017, 2017, 5293573. [Google Scholar] [CrossRef]
- Ambrósio, R.; Dawson, D.G.; Salomão, M.; Guerra, F.P.; Caiado, A.L.C.; Belin, M.W. Corneal Ectasia after LASIK despite Low Preoperative Risk: Tomographic and Biomechanical Findings in the Unoperated, Stable, Fellow Eye. J. Refract. Surg. 2010, 26, 906–911. [Google Scholar] [CrossRef]
- Klein, S.R.; Epstein, R.J.; Randleman, J.B.; Stulting, R.D. Corneal Ectasia after Laser in Situ Keratomileusis in Patients without Apparent Preoperative Risk Factors. Cornea 2006, 25, 388–403. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Gobbe, M.; Archer, T.J.; Youssefi, G.; Sutton, H.F.S. Stromal Surface Topography-Guided Custom Ablation as a Repair Tool for Corneal Irregular Astigmatism. J. Refract. Surg. 2015, 31, 54–59. [Google Scholar] [CrossRef]
- Huang, D.; Tang, M.; Shekhar, R. Mathematical Model of Corneal Surface Smoothing after Laser Refractive Surgery. Am. J. Ophthalmol. 2003, 135, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H.; Urs, R.; RoyChoudhury, A.; Archer, T.J.; Gobbe, M.; Reinstein, D.Z. Epithelial Remodeling as Basis for Machine-Based Identification of Keratoconus. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M.; Silverman, R.H.; Coleman, D.J. Epithelial Thickness in the Normal Cornea: Three-Dimensional Display With Very High Frequency Ultrasound. J. Refract. Surg. 2008, 24, 571–581. [Google Scholar] [PubMed]
- Gaster, R.N.; Canedo, A.L.C.; Rabinowitz, Y.S. Corneal Collagen Cross-Linking for Keratoconus and Post-LASIK Ectasia. Int. Ophthalmol. Clin. 2013, 53, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, O.; Brass, R.; Weiss, J.L.; Huang, D. Corneal Epithelial Thickness Mapping by Fourier-Domain Optical Coherence Tomography in Normal and Keratoconic Eyes. Ophthalmology 2012, 119, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Schallhorn, J.M.; Randleman, J.B. Utility of Regional Epithelial Thickness Measurements in Corneal Evaluations. Surv. Ophthalmol. 2020, 65, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Maslin, J.S.; Barkana, Y.; Dorairaj, S.K. Anterior Segment Imaging in Glaucoma: An Updated Review. Indian. J. Ophthalmol. 2015, 63, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Foo, V.H.; Yang, L.W.Y.; Sia, J.T.; Ang, M.; Lin, H.; Chodosh, J.; Mehta, J.S.; Ting, D.S.W. Artificial Intelligence for Anterior Segment Diseases: Emerging Applications in Ophthalmology. Br. J. Ophthalmol. 2021, 105, 158–168. [Google Scholar] [CrossRef]
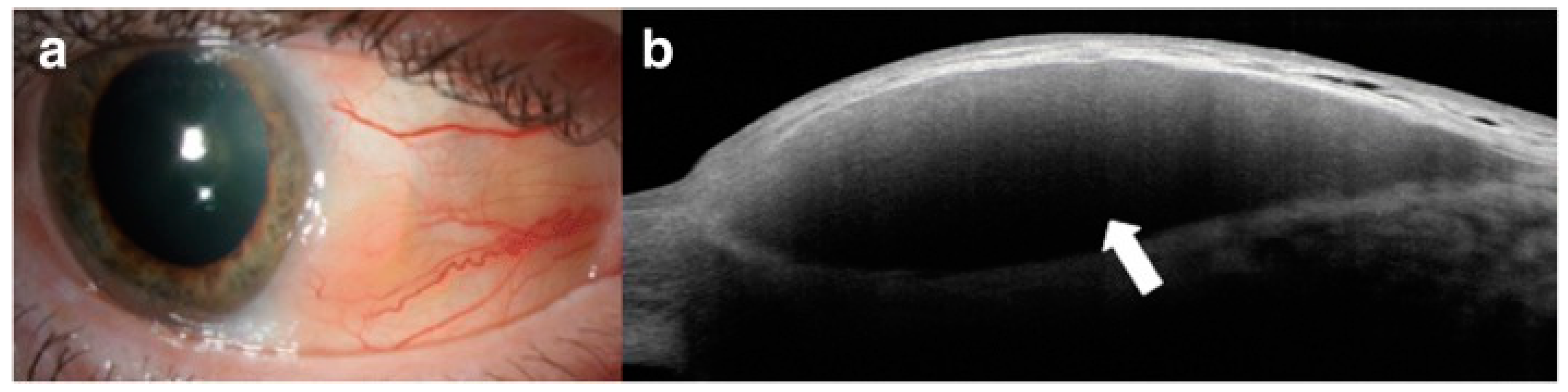
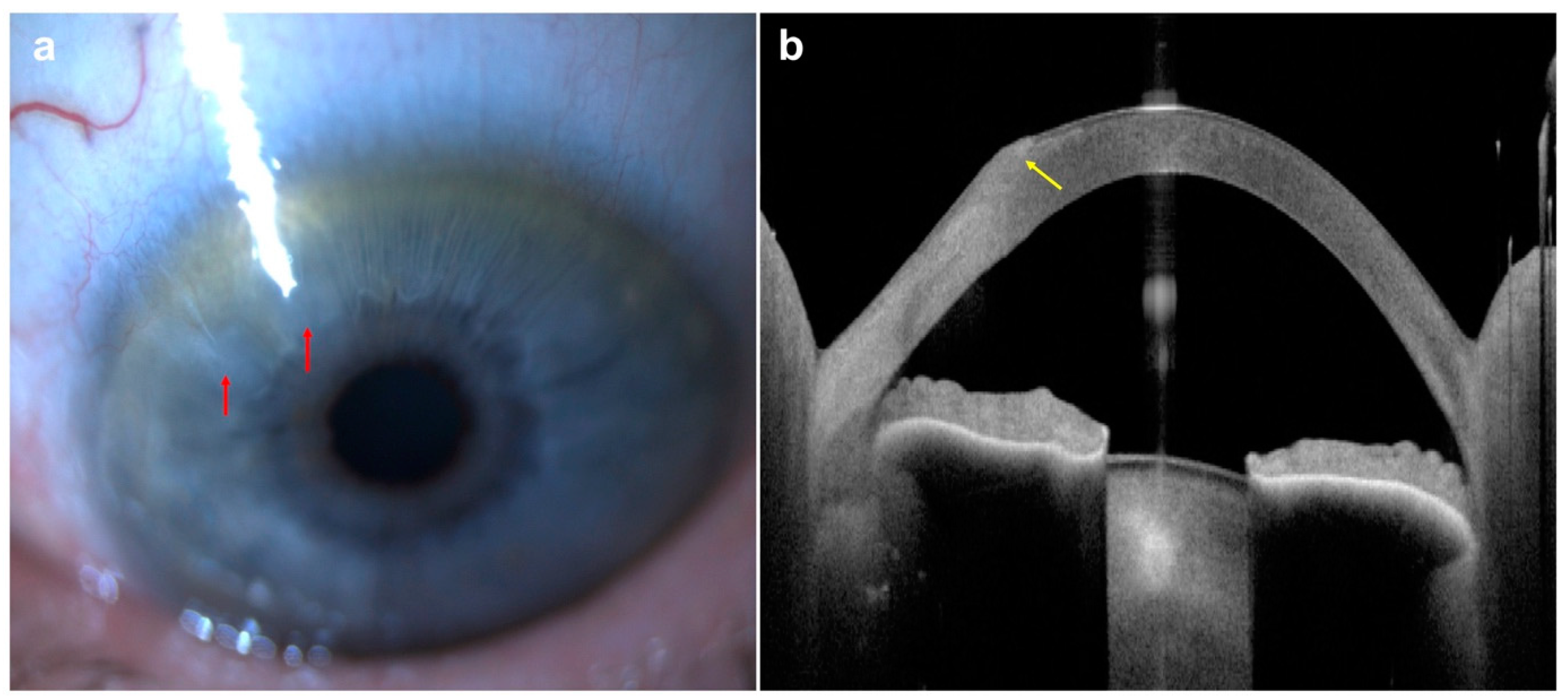
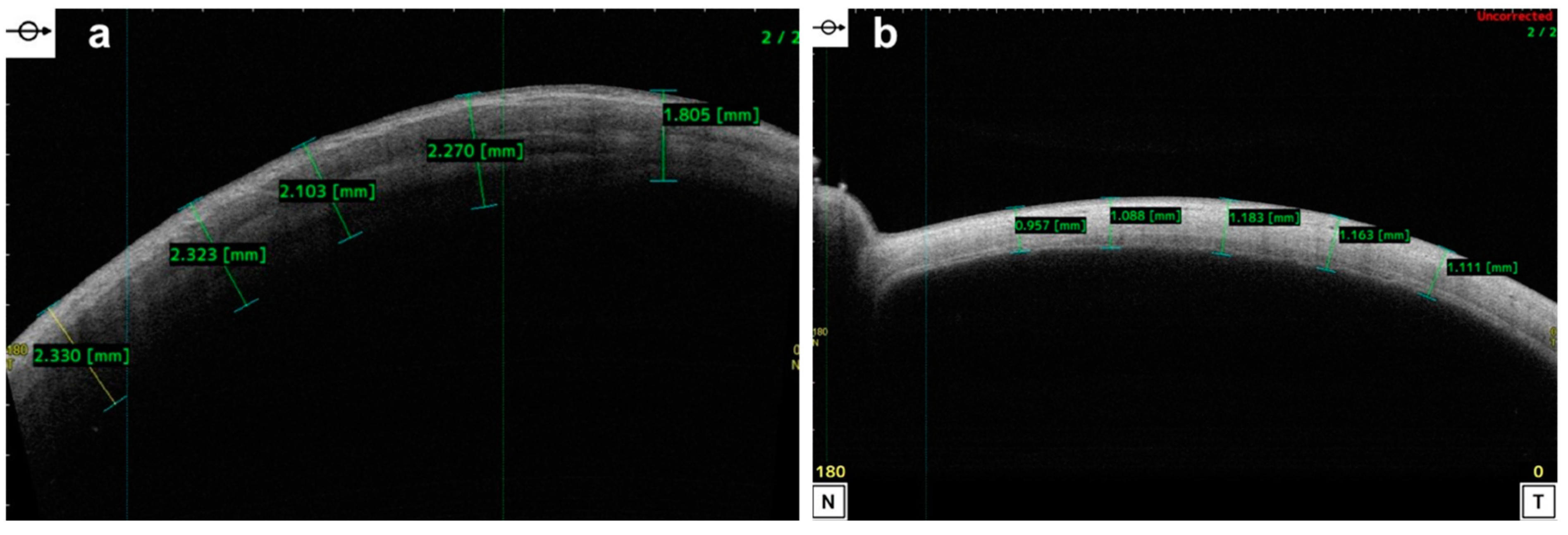
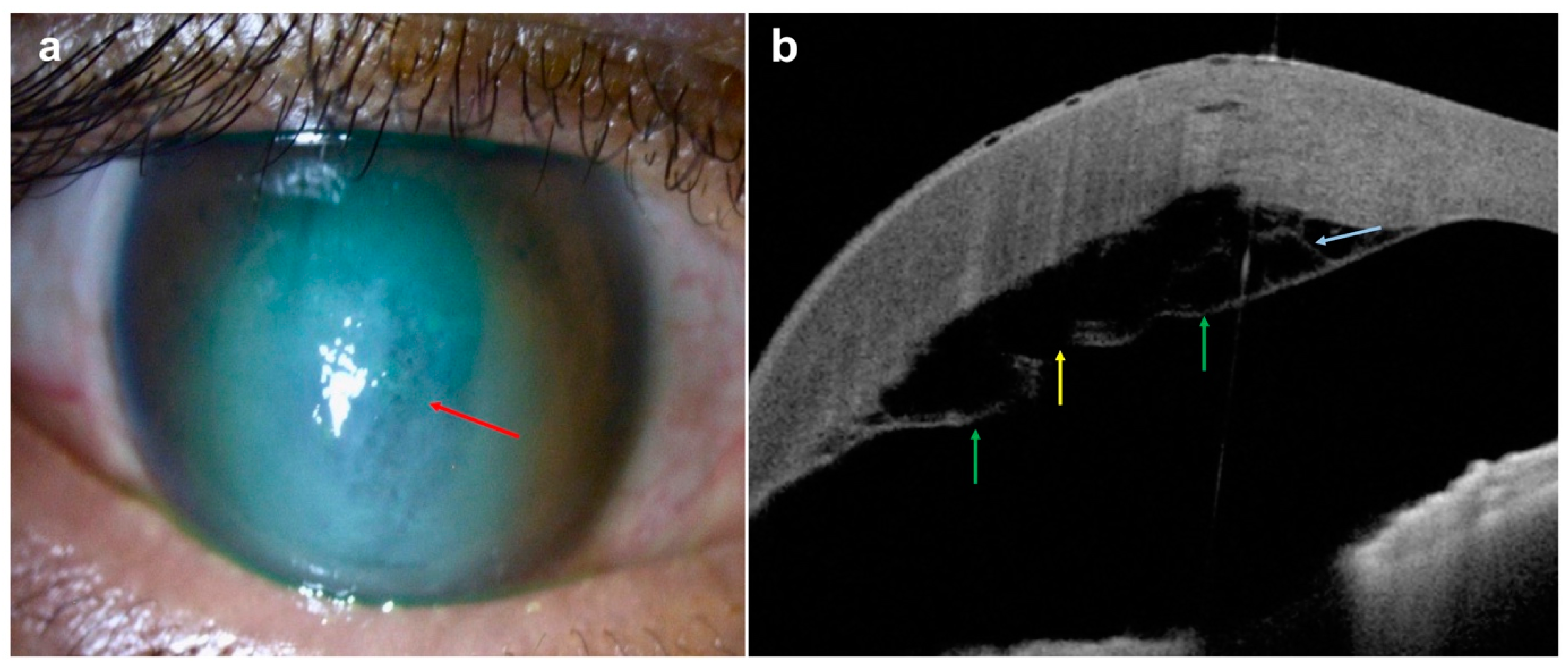
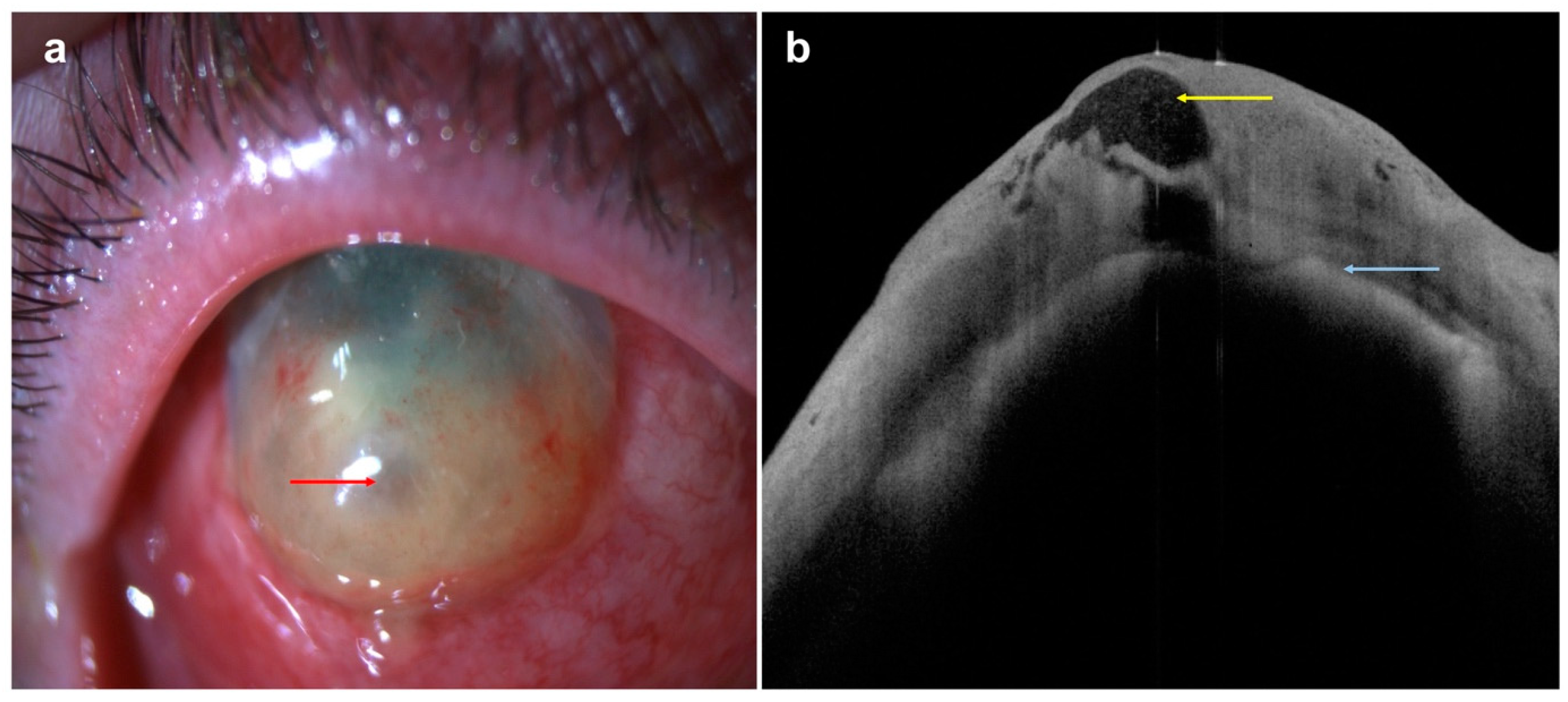


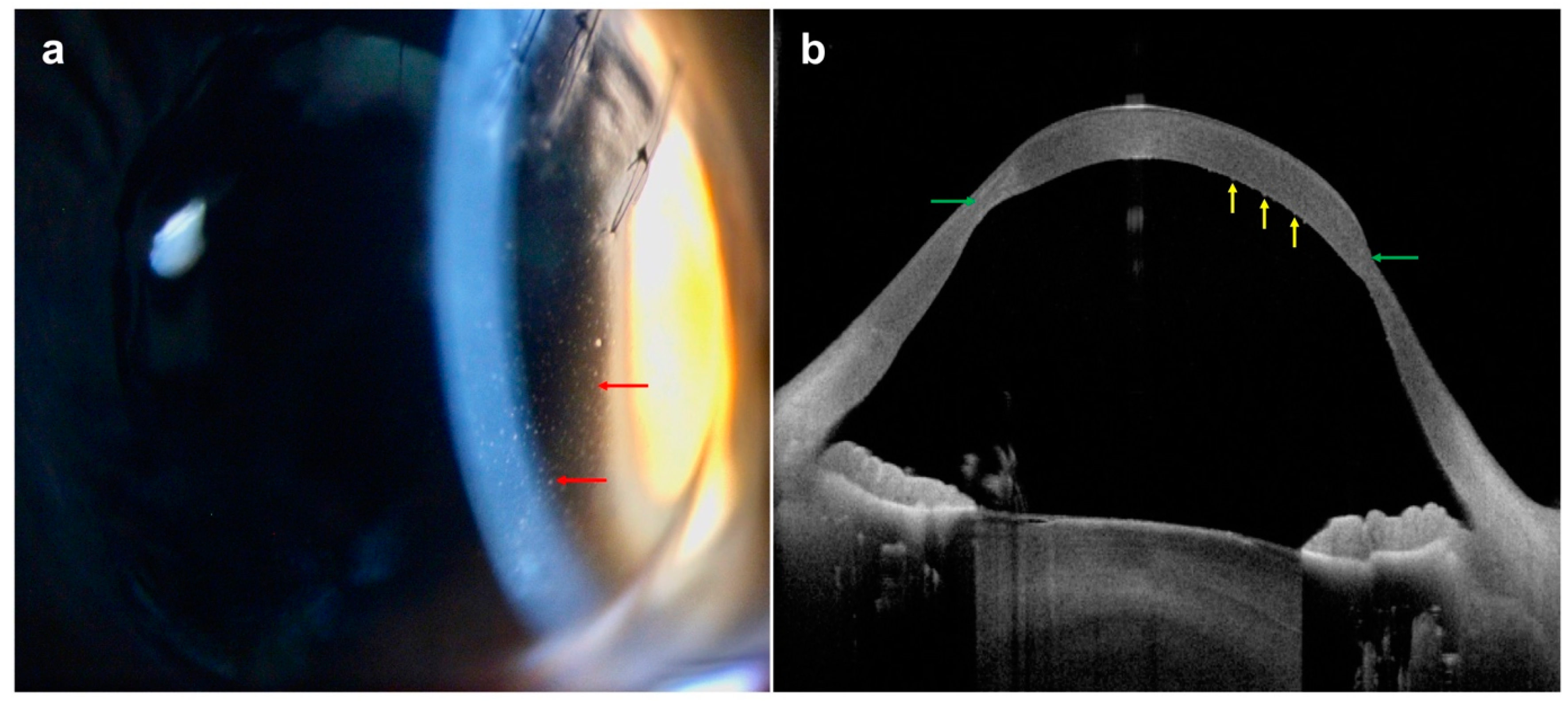
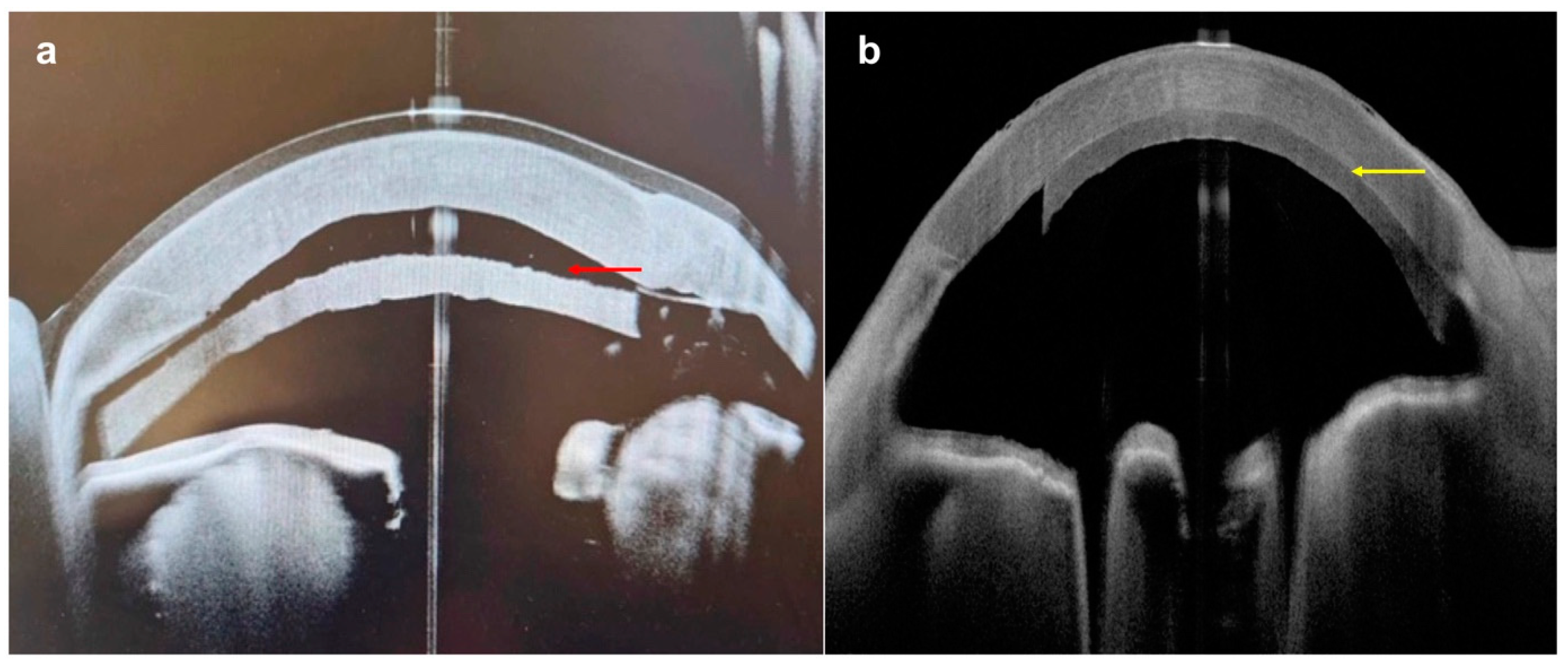
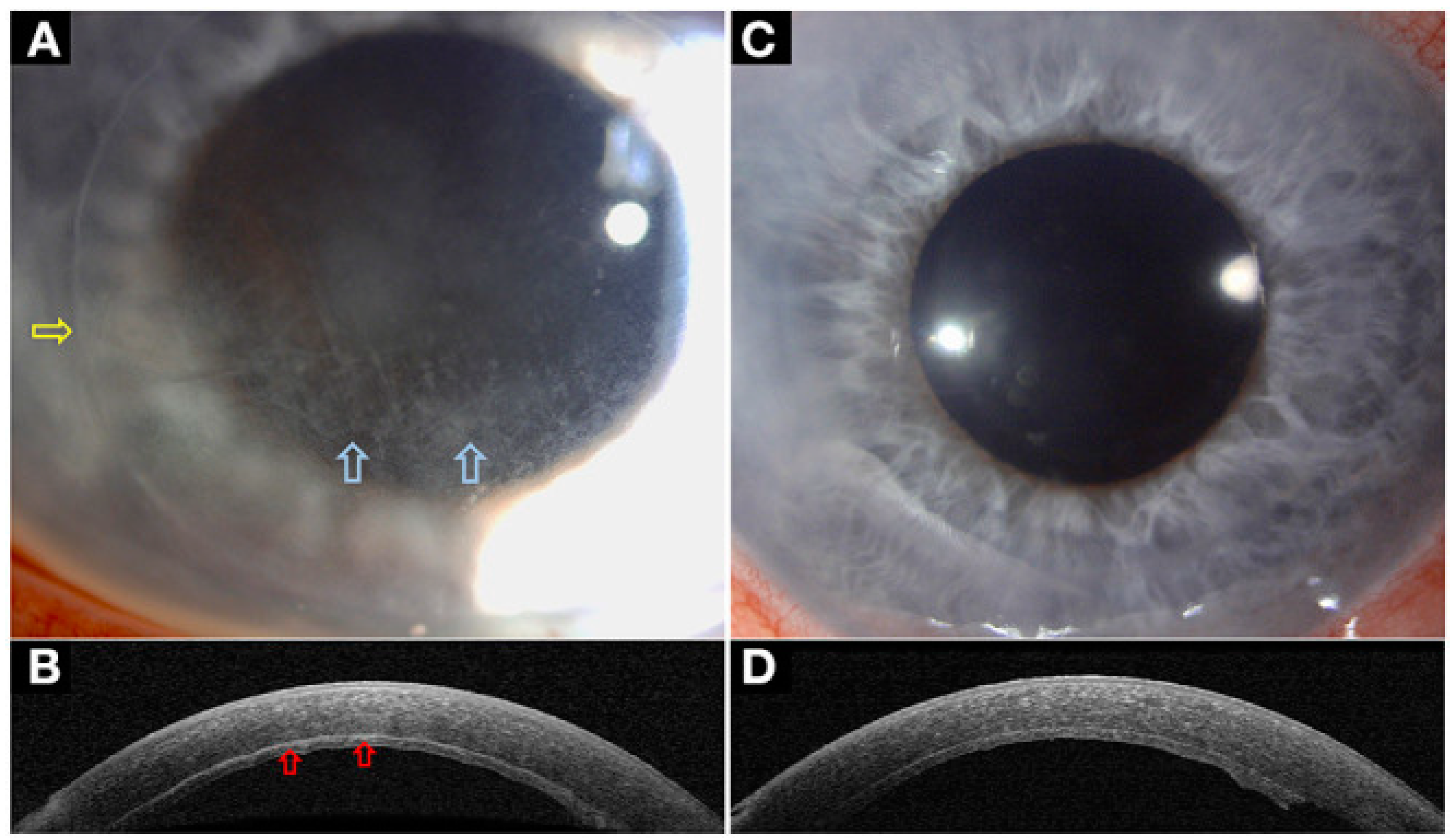
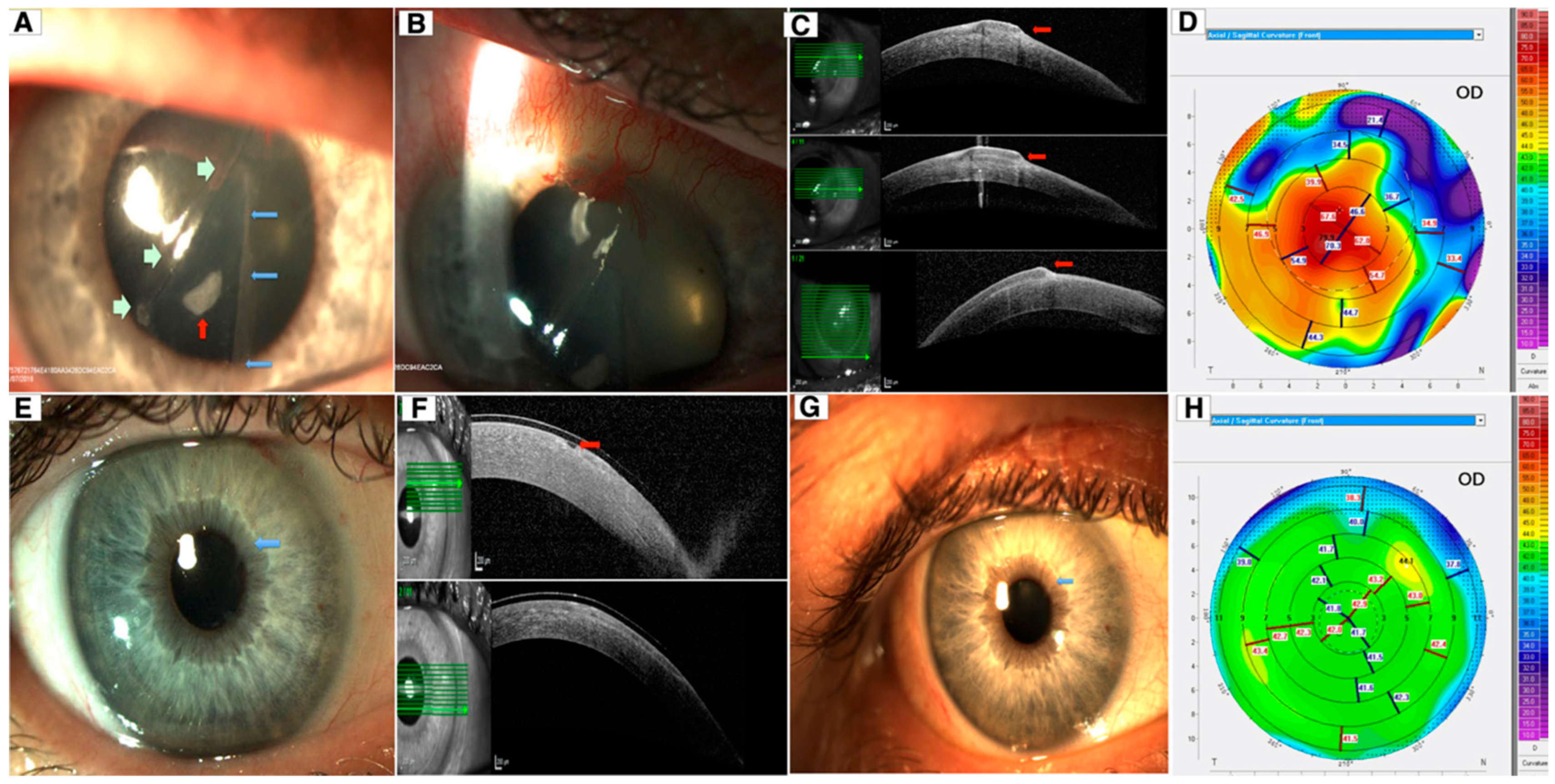

| Disease | AS-OCT Features |
|---|---|
| Conjunctival melanocytic naevus | |
| Ocular surface squamous neoplasia (OSSN) |
|
| Conjunctival melanoma | |
| Conjunctival lymphoma |
|
| Pterygium |
|
| Pinguecula |
|
| Salzmann nodular degeneration |
|
| Pseudopterygium |
|
| Episcleritis |
|
| Scleritis |
|
| Keratoconus | |
| Acute corneal hydrops |
|
| Infectious keratitis |
Differentiation of different form of infectious keratitis:
|
| Corneal foreign body |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, Y.J.; Azzopardi, M.; Hussain, G.; Recchioni, A.; Gandhewar, J.; Loizou, C.; Giachos, I.; Barua, A.; Ting, D.S.J. Clinical Applications of Anterior Segment Optical Coherence Tomography: An Updated Review. Diagnostics 2024, 14, 122. https://doi.org/10.3390/diagnostics14020122
Chong YJ, Azzopardi M, Hussain G, Recchioni A, Gandhewar J, Loizou C, Giachos I, Barua A, Ting DSJ. Clinical Applications of Anterior Segment Optical Coherence Tomography: An Updated Review. Diagnostics. 2024; 14(2):122. https://doi.org/10.3390/diagnostics14020122
Chicago/Turabian StyleChong, Yu Jeat, Matthew Azzopardi, Gulmeena Hussain, Alberto Recchioni, Jaishree Gandhewar, Constantinos Loizou, Ioannis Giachos, Ankur Barua, and Darren S. J. Ting. 2024. "Clinical Applications of Anterior Segment Optical Coherence Tomography: An Updated Review" Diagnostics 14, no. 2: 122. https://doi.org/10.3390/diagnostics14020122
APA StyleChong, Y. J., Azzopardi, M., Hussain, G., Recchioni, A., Gandhewar, J., Loizou, C., Giachos, I., Barua, A., & Ting, D. S. J. (2024). Clinical Applications of Anterior Segment Optical Coherence Tomography: An Updated Review. Diagnostics, 14(2), 122. https://doi.org/10.3390/diagnostics14020122






