Diagnostic Performance of a Deep Learning-Powered Application for Aortic Dissection Triage Prioritization and Classification
Abstract
1. Introduction
2. Materials and Methods
2.1. DL-Powered Algorithm for AD Detection: Architecture and Training
2.2. Data Selection
2.3. Ethical Considerations for Data
2.4. The Ground Truth
2.5. Data Processing
2.6. Statistical Analysis
3. Results
3.1. Data Distribution
3.2. Performance Statistical Results
3.3. Stanford AD Type Classification
3.4. Stratified Statistical Analysis Results
3.5. Time to Notification Evaluation Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coady, M.A.; Rizzo, J.A.; Goldstein, L.J.; Elefteriades, J.A. Natural History, Pathogenesis, and Etiology of Thoracic Aortic Aneurysms and Dissections. Cardiol. Clin. 1999, 17, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Awal, S.S.; Prasad, N.; Biswas, S. CT Evaluation of Aortic Dissection and Other Acute Aortic Syndromes: An Update. Int. J. Radiol. Radiat. Ther. 2022, 9, 159–165. [Google Scholar] [CrossRef]
- Bossone, E.; LaBounty, T.M.; Eagle, K.A. Acute Aortic Syndromes: Diagnosis and Management, an Update. Eur. Heart J. 2018, 39, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Criado, F.J. Aortic Dissection: A 250-Year Perspective. Tex. Heart Inst. J. 2011, 38, 694–700. [Google Scholar]
- Gawinecka, J.; Schönrath, F.; von Eckardstein, A. Acute Aortic Dissection: Pathogenesis, Risk Factors and Diagnosis. Swiss Med. Wkly. 2017, 147, w14489. [Google Scholar] [CrossRef]
- Harris, R.J.; Kim, S.; Lohr, J.; Towey, S.; Velichkovich, Z.; Kabachenko, T.; Driscoll, I.; Baker, B. Classification of Aortic Dissection and Rupture on Post-Contrast CT Images Using a Convolutional Neural Network. J. Digit. Imaging 2019, 32, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Pourafkari, L.; Tajlil, A.; Ghaffari, S.; Parvizi, R.; Chavoshi, M.; Kolahdouzan, K.; Khaki, N.; Parizad, R.; Hobika, G.G.; Nader, N.D. The Frequency of Initial Misdiagnosis of Acute Aortic Dissection in the Emergency Department and Its Impact on Outcome. Intern. Emerg. Med. 2017, 12, 1185–1195. [Google Scholar] [CrossRef]
- Zaschke, L.; Habazettl, H.; Thurau, J.; Matschilles, C.; Göhlich, A.; Montagner, M.; Falk, V.; Kurz, S.D. Acute Type A Aortic Dissection: Aortic Dissection Detection Risk Score in Emergency Care—Surgical Delay Because of Initial Misdiagnosis. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, S40–S47. [Google Scholar] [CrossRef]
- Lovatt, S.; Wong, C.W.; Schwarz, K.; Borovac, J.A.; Lo, T.; Gunning, M.; Phan, T.; Patwala, A.; Barker, D.; Mallen, C.D.; et al. Misdiagnosis of Aortic Dissection: A Systematic Review of the Literature. Am. J. Emerg. Med. 2022, 53, 16–22. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Chang, P.D.; Kuoy, E.; Grinband, J.; Weinberg, B.D.; Thompson, M.; Homo, R.; Chen, J.; Abcede, H.; Shafie, M.; Sugrue, L.; et al. Hybrid 3D/2D Convolutional Neural Network for Hemorrhage Evaluation on Head CT. AJNR Am. J. Neuroradiol. 2018, 39, 1609–1616. [Google Scholar] [CrossRef]
- 45 CFR § 164.514 (e); Code of Federal Regulation. Government Publishing Office: Washington, DC, USA, 2023.
- 45 CFR § 46.101; Code of Federal Regulation. Government Publishing Office: Washington, DC, USA, 2023.
- GDPR 2016/679; Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation). Official Journal of the European Union: Brussels, Belgium, 2016.
- Hata, A.; Yanagawa, M.; Yamagata, K.; Suzuki, Y.; Kido, S.; Kawata, A.; Doi, S.; Yoshida, Y.; Miyata, T.; Tsubamoto, M.; et al. Deep Learning Algorithm for Detection of Aortic Dissection on Non-Contrast-Enhanced CT. Eur. Radiol. 2021, 31, 1151–1159. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Q.; Lu, X.; Liu, S.; Cao, C.; Wang, Z.; Zhang, X.; Xia, S. Impact of Encephalomalacia and White Matter Hyperintensities on ASPECTS in Patients with Acute Ischemic Stroke: Comparison of Automated and Radiologist-Derived Scores. Am. J. Roentgenol. 2022, 218, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Mao, L.; Wang, C.; Guo, Y.; Luo, X.; Jia, D.; Lei, Y.; Pan, J.; Li, J.; Li, S.; et al. Advanced Warning of Aortic Dissection on Non-Contrast CT: The Combination of Deep Learning and Morphological Characteristics. Front. Cardiovasc. Med. 2022, 8, 762958. [Google Scholar] [CrossRef] [PubMed]
- FDA 510(k) Summary Aidoc Medical, Ltd.’s BriefCase 2022. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf22/K222329.pdf (accessed on 10 June 2024).
- Matthews, C.R.; Madison, M.; Timsina, L.R.; Namburi, N.; Faiza, Z.; Lee, L.S. Impact of Time between Diagnosis to Treatment in Acute Type A Aortic Dissection. Sci. Rep. 2021, 11, 3519. [Google Scholar] [CrossRef]
- Rapezzi, C.; Longhi, S.; Graziosi, M.; Biagini, E.; Terzi, F.; Cooke, R.M.T.; Quarta, C.; Sangiorgi, D.; Ciliberti, P.; Di Pasquale, G.; et al. Risk Factors for Diagnostic Delay in Acute Aortic Dissection. Am. J. Cardiol. 2008, 102, 1399–1406. [Google Scholar] [CrossRef]
- Harris, K.M.; Strauss, C.E.; Eagle, K.A.; Hirsch, A.T.; Isselbacher, E.M.; Tsai, T.T.; Shiran, H.; Fattori, R.; Evangelista, A.; Cooper, J.V.; et al. Correlates of Delayed Recognition and Treatment of Acute Type A Aortic Dissection: The International Registry of Acute Aortic Dissection (IRAD). Circulation 2011, 124, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, T.; Tomotsuka, S.; Takehara, M.; Tsumaru, S. Three Cases of Diagnostic Delay of Type A Acute Aortic Dissection. Egypt. Heart J. 2024, 76, 10. [Google Scholar] [CrossRef]
- Sullivan, P.R.; Wolfson, A.B.; Leckey, R.D.; Burke, J.L. Diagnosis of Acute Thoracic Aortic Dissection in the Emergency Department. Am. J. Emerg. Med. 2000, 18, 46–50. [Google Scholar] [CrossRef]
- Vos, M.D.; Ranschaert, W.; Vergauwen, W.; Graulus, E.; Verrelst, P.; Schepens, M. Acute Type A Aortic Dissection: Timeline between Onset and Treatment. J. Vis. Surg. 2021, 7, 24. [Google Scholar] [CrossRef]
- Froehlich, W.; Tolenaar, J.L.; Harris, K.M.; Strauss, C.; Sundt, T.M.; Tsai, T.T.; Peterson, M.D.; Evangelista, A.; Montgomery, D.G.; Kline-Rogers, E.; et al. Delay from Diagnosis to Surgery in Transferred Type A Aortic Dissection. Am. J. Med. 2018, 131, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Graber, M.; Gordon, R.; Franklin, N. Reducing Diagnostic Errors in Medicine: What’s the Goal? Acad. Med. J. Assoc. Am. Med. Coll. 2002, 77, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.; Nicola, L.; Breau-Brunel, M.; Sensini, F.; Tanova-Yotova, N.; Atanasov, P.; Lobig, F.; Blankenburg, M. Unlocking the Value: Quantifying the Return on Investment of Hospital Artificial Intelligence. JACR J. Am. Coll. Radiol. 2024, 16. [Google Scholar] [CrossRef] [PubMed]
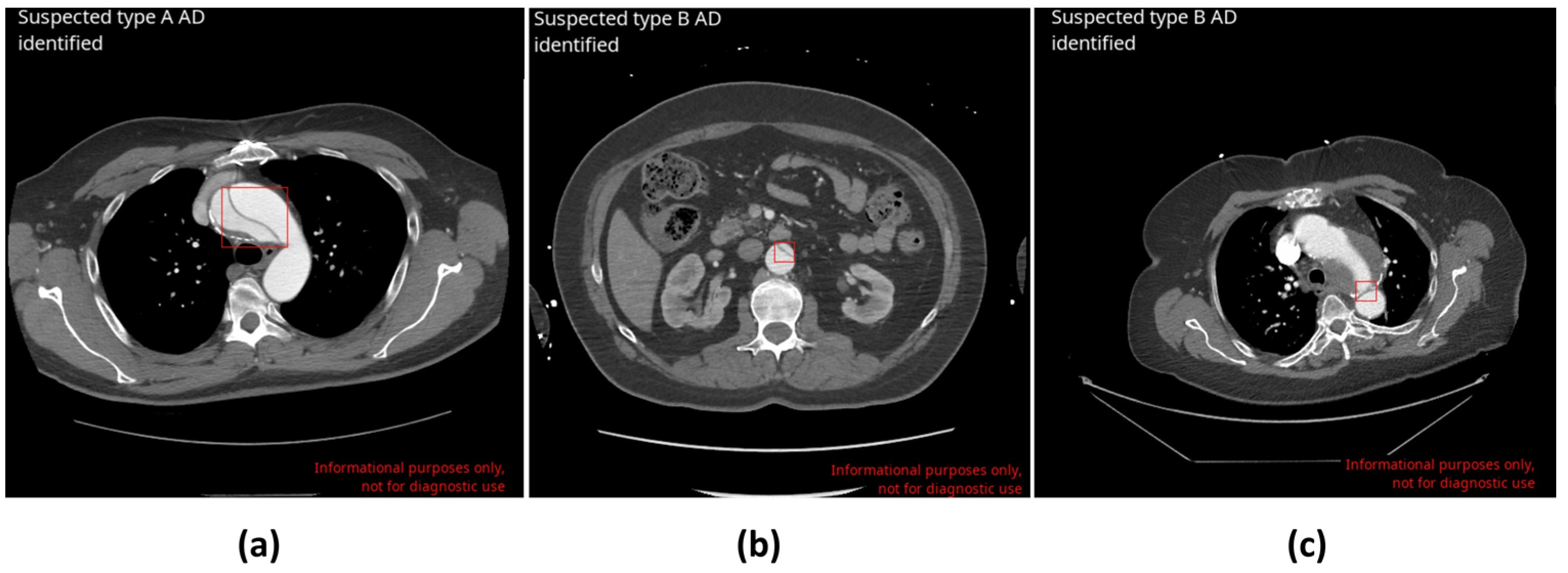
| The Inclusion Criteria for CINA-CHEST (AD) |
| Chest or thoraco-abdominal CTA scans Age ≥ 18 y/o Matrix size ≥ 512 × 512 (rectangular matrix accepted) Axial acquisition only Slice thickness ≤ 3 mm with no gap between successive slices Radiation dose parameters: 60 kVp to 160 kVp Reconstruction diameter above 200 mm Density threshold in the aorta ≥ 140 HU Soft tissue reconstruction kernel Field of view including the aortic arch and thoracic aorta |
| The Exclusion Criteria for CINA-CHEST (AD) |
| Parameters not compatible with acquisition protocol Thoracic aorta out of the field of view Significant motion artefacts (uninterpretable images) Significant streak artefacts (uninterpretable images) Significant noise (uninterpretable images) Bad bolus timing (uninterpretable images) |
| Characteristic | Parameters | AD Dataset (1303 Cases) | AD Positive Cases (137 Cases) |
|---|---|---|---|
| Age | Mean ± SD | 58.8 ± 16.4 y/o | 59.0 ± 13.3 y/o |
| Sex | Male | 609 (46.7%) | 84 (61.3%) |
| Female | 692 (53.3%) | 53 (38.7%) | |
| Scanner makers | GE | 259 (19.9%) | 77 (56.2%) |
| Philips | 489 (37.5%) | 14 (10.2%) | |
| Siemens | 474 (36.4%) | 33 (24.1%) | |
| Canon | 76 (5.8%) | 13 (9.5%) | |
| Hitachi | 4 (0.3%) | 0 (0.0%) | |
| PNMS | 1 (0.1%) | 0 (0.0%) | |
| Slice thickness | <1.5 mm | 456 (35%) | 53 (38.7%) |
| 1.5 mm < ST < 3 mm | 629 (48%) | 56 (40.9%) | |
| =3 mm | 218 (17%) | 28 (20.4%) |
| Confusion Matrix | Ground Truth | |||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| CINA-CHEST (AD) * | Positive | 129 (TP) | 8 (FN) | 137 |
| Negative | 32 (FP) | 1134 (TN) | 1166 | |
| Total | 161 | 1142 | 1303 | |
| Main Reasons for False Negatives (n = 8) | Main Reasons for False Positives (n = 32) |
|---|---|
| Intramural hematoma (IMH) (4) | Inadequate contrast opacification (13) |
| Penetrating atherosclerotic ulcer (PAU) (2) | Motion artefacts (10) |
| Acquisition artefacts (2) | Instances of pathology mimicking dissection (7) |
| Interference from stent grafts (2) |
| AD Type | Sensitivity [95% CI], % | Specificity [95% CI], % | Accuracy [95% CI], % |
|---|---|---|---|
| Type A | 100 [92.8–100] (TP = 63; FN = 0) | 99.4 [98.8–99.8] (TN = 1233; FP = 7) | 99.5 [98.9–99.8] |
| Type B | 89.2 [79.3–94.9] (TP = 66; FN = 8) | 97.9 [97.0–98.7] (TN = 1204; FP = 25) | 97.5 [96.4–98.3] |
| Parameter | Condition | Sensitivity [95% CI], % | Specificity [95% CI], % | Accuracy [95% CI], % |
|---|---|---|---|---|
| Age | 18 ≤ Age < 40 | 100 [47.8–100] | 97.7 [94.3–99.4] | 97.9 [94.5–99.4] |
| 40 ≤ Age ≤ 60 | 97.1 [89.9–99.6] | 98.2 [96.3–99.3] | 98.0 [96.3–99.1] | |
| Age > 60 | 90.5 [80.4–96.4] | 96.5 [94.7–97.8] | 95.9 [94.1–97.3] | |
| Sex | Male | 96.4 [89.9–99.2] | 96.9 [95.1–98.3] | 96.9 [95.1–98.1] |
| Female | 90.6 [79.3–96.9] | 97.5 [95.9–98.6] | 96.9 [95.4–98.1] | |
| Scanner makers * | GE | 94.8 [87.2–98.6] | 96.2 [92.2–98.4] | 95.8 [92.5–97.9] |
| Philips | 92.2 [66.1–99.8] | 96.8 [94.8–98.2] | 96.7 [94.6–98.1] | |
| Siemens | 93.9 [79.8–99.3] | 97.7 [95.8–98.9] | 97.5 [95.6–98.7] | |
| Canon | 93.3 [62.1–99.6] | 100 [92.8–100] | 98.7 [92.9–99.9] | |
| Slice thickness | <1.5 mm | 90.5 [79.3–96.9] | 97.7 [95.8–98.9] | 96.9 [94.9–98.3] |
| 1.5 mm < ST < 3 mm | 98.2 [90.5–99.9] | 96.7 [94.9–98.0] | 96.8 [95.1–98.0] | |
| =3 mm | 92.9 [76.5–99.1] | 97.8 [94.7–99.4] | 97.2 [94.1–99.0] |
| Parameter | Harris et al., 2019 [6] | Hata et al., 2020 [15] | Huang et al., 2022 [16] | Yi et al., 2022 [17] | Current Study |
|---|---|---|---|---|---|
| Image type | CTA | Non-enhanced CT | CTA | Non-enhanced CT | CTA |
| Architecture | 5-layer CNN | CNN Xception | 2-step network: attention U-net and ResNeXt | Deep integrated model: 2.5D U-net, ResNet34 | 2-step 2.5D U-Net: aorta isolation and dissection detection |
| Model | 2D | 2D | 3D | 3D | 3D |
| Population | 34,577 cases (112 AD pos) | 170 cases (85 AD pos) | 298 cases (51 pos: 22 type A; 29 type B) | 452 cases (internal cohort (341): 139 AD pos. external cohort (111): 46 AD pos.) | 1303 cases (137 AD pos) |
| Enrolment | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective |
| Samples | Multicenter and multiscanner | One center | One center | Internal center and external center | Multicenter, multiscanner, and multinational |
| Sensitivity | 87.8% | 91.8% | Type A: 95.5% Type B: 79.3% | Internal: 86.2% External: 97.8% | All: 94.2% Type A: 100% Type B: 89.2% |
| Specificity | 96.0% | 88.2% | Type A: 98.5% Type B: 94.0% | Internal: 92.3% External: 55.4% | All: 97.3% Type A: 99.4% Type B: 97.9% |
| Features | Triage Mean time to notification: 23.5 ± 21.0 [SD] seconds | Triage Comparison with experts (5 readers): Sensitivity: 90.6% Specificity: 94.1% | Type A/B classification | Triage Comparison with experts (3 readers): Internal experts: Mean sensitivity: 72.7% Mean specificity: 98.3% External experts: Mean sensitivity: 40.6% Mean specificity: 94.0% | Triage Type A/B classification Mean time to notification: 27.9 ± 8.2 [SD] seconds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laletin, V.; Ayobi, A.; Chang, P.D.; Chow, D.S.; Soun, J.E.; Junn, J.C.; Scudeler, M.; Quenet, S.; Tassy, M.; Avare, C.; et al. Diagnostic Performance of a Deep Learning-Powered Application for Aortic Dissection Triage Prioritization and Classification. Diagnostics 2024, 14, 1877. https://doi.org/10.3390/diagnostics14171877
Laletin V, Ayobi A, Chang PD, Chow DS, Soun JE, Junn JC, Scudeler M, Quenet S, Tassy M, Avare C, et al. Diagnostic Performance of a Deep Learning-Powered Application for Aortic Dissection Triage Prioritization and Classification. Diagnostics. 2024; 14(17):1877. https://doi.org/10.3390/diagnostics14171877
Chicago/Turabian StyleLaletin, Vladimir, Angela Ayobi, Peter D. Chang, Daniel S. Chow, Jennifer E. Soun, Jacqueline C. Junn, Marlene Scudeler, Sarah Quenet, Maxime Tassy, Christophe Avare, and et al. 2024. "Diagnostic Performance of a Deep Learning-Powered Application for Aortic Dissection Triage Prioritization and Classification" Diagnostics 14, no. 17: 1877. https://doi.org/10.3390/diagnostics14171877
APA StyleLaletin, V., Ayobi, A., Chang, P. D., Chow, D. S., Soun, J. E., Junn, J. C., Scudeler, M., Quenet, S., Tassy, M., Avare, C., Roca-Sogorb, M., & Chaibi, Y. (2024). Diagnostic Performance of a Deep Learning-Powered Application for Aortic Dissection Triage Prioritization and Classification. Diagnostics, 14(17), 1877. https://doi.org/10.3390/diagnostics14171877







