Evaluation of the Accuracy of Estimated Endpoint Titer of NOVA View in Indirect Immunofluorescent Antinuclear Antibody Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Detection of ANA and Determination of Endpoint Titer by Serial Dilution and Estimation Function
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
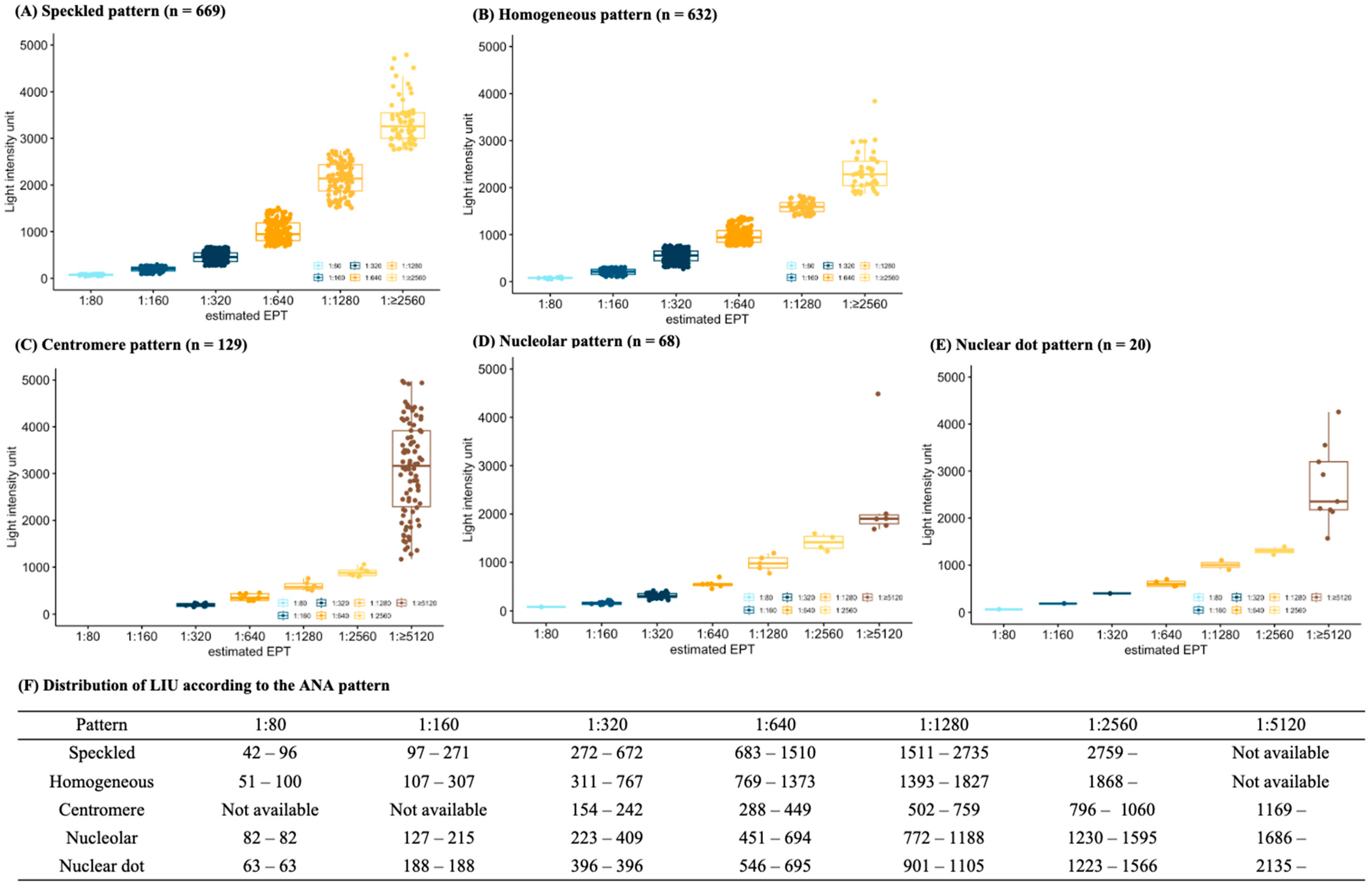
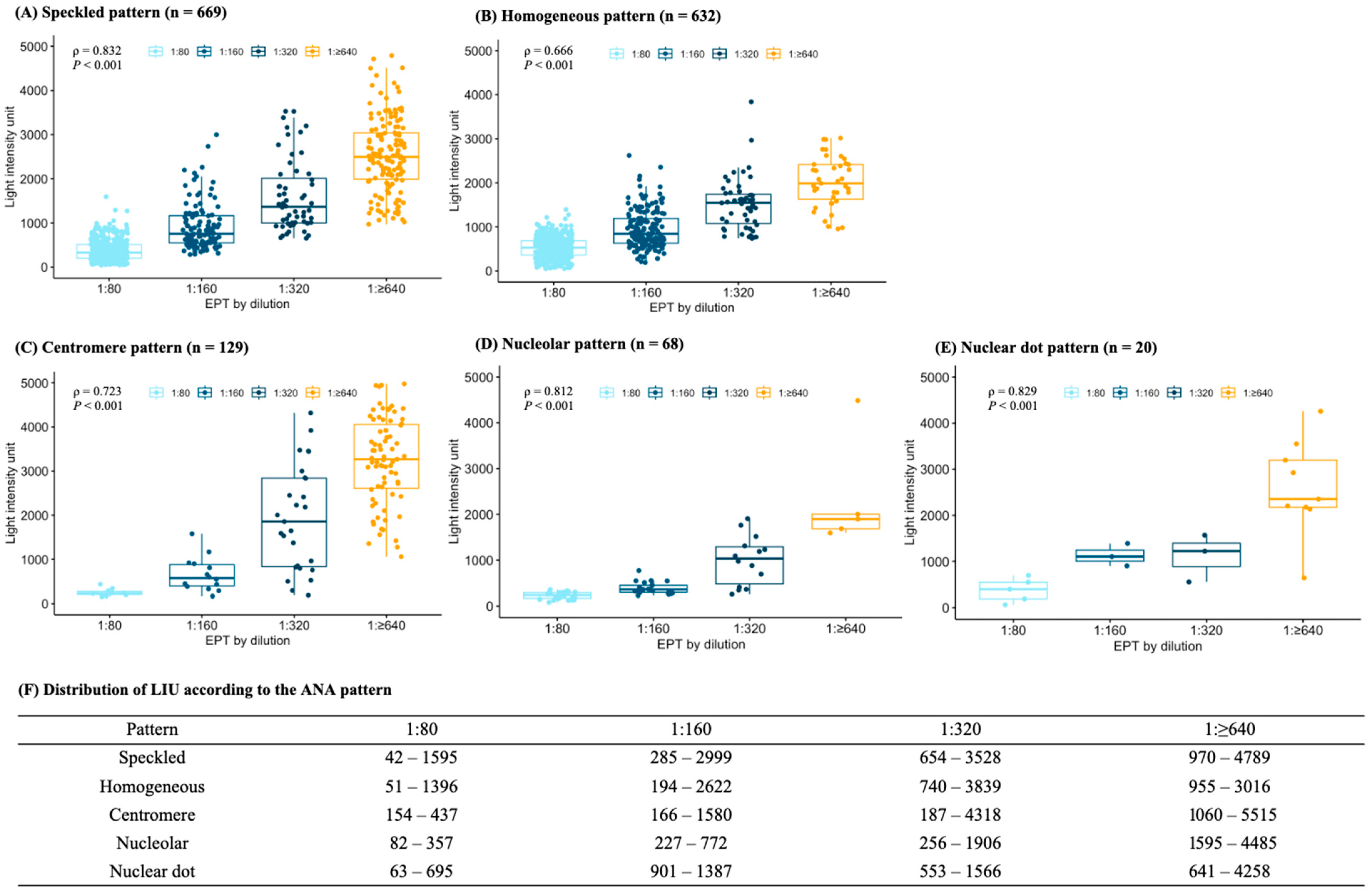
3.2. Relationship between Light Intensity Units and Endpoint Titers
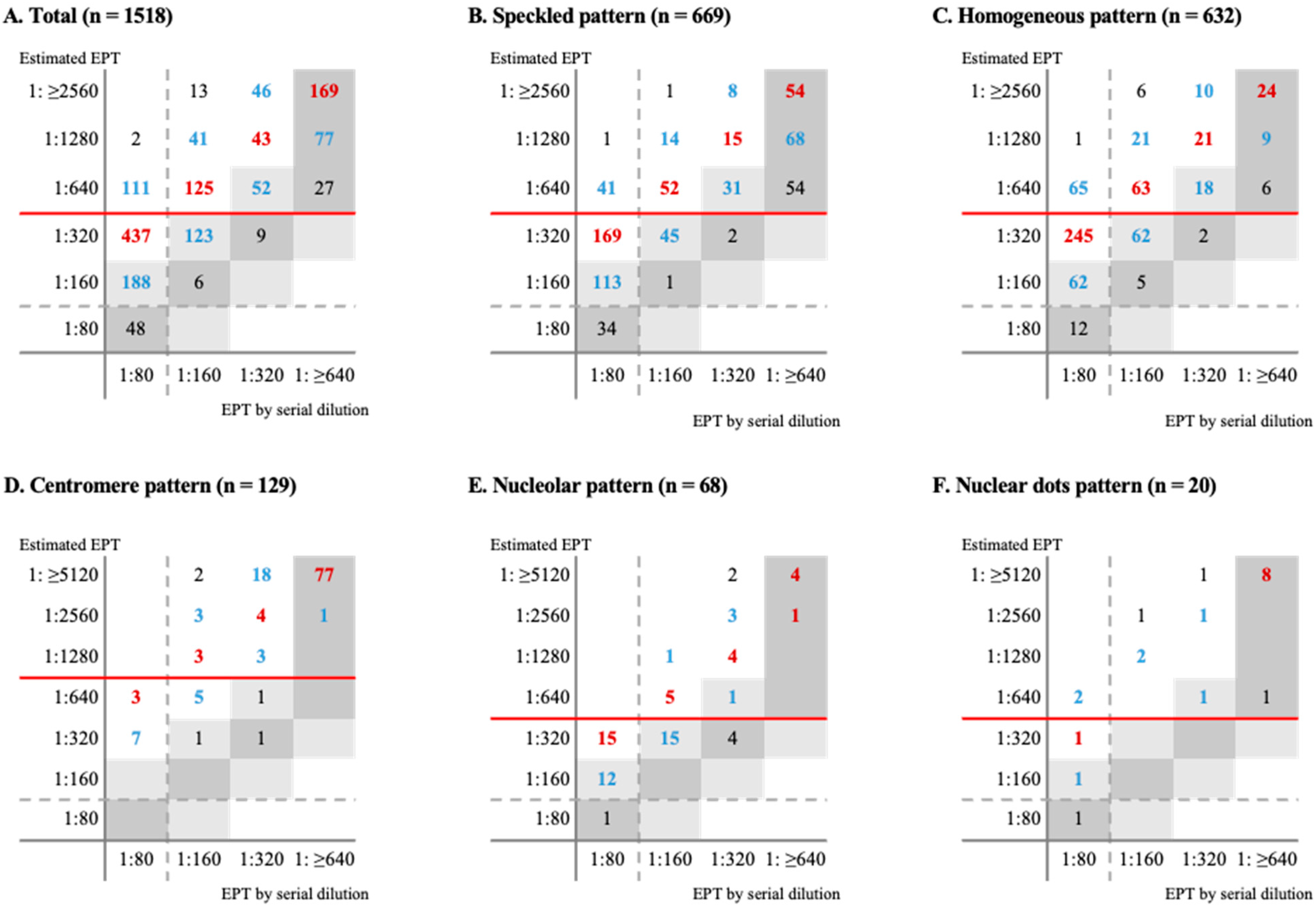
3.3. Concordance of dEPT and eEPT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Agmon-Levin, N.; Damoiseaux, J.; Kallenberg, C.; Sack, U.; Witte, T.; Herold, M.; Bossuyt, X.; Musset, L.; Cervera, R.; Plaza-Lopez, A.; et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheum. Dis. 2014, 73, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.; Andrade, L.E.C.; Carballo, O.G.; Conrad, K.; Francescantonio, P.L.C.; Fritzler, M.J.; Garcia de la Torre, I.; Herold, M.; Klotz, W.; de Melo Cruvinel, W. Clinical relevance of HEp-2 indirect immunofluorescent patterns: The International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum. Dis. 2019, 78, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Barbara, D.; John, L.S.; Robert, G.H. Manual of Molecular and Clinical Laboratory Immunology, 8th ed.; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
- Meroni, P.L.; Schur, P.H. ANA screening: An old test with new recommendations. Ann. Rheum. Dis. 2010, 69, 1420–1422. [Google Scholar] [CrossRef] [PubMed]
- Tozzoli, R.; Bonaguri, C.; Melegari, A.; Antico, A.; Bassetti, D.; Bizzaro, N. Current state of diagnostic technologies in the autoimmunology laboratory. Clin. Chem. Lab. Med. 2013, 51, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Tebo, A.E. Recent Approaches To Optimize Laboratory Assessment of Antinuclear Antibodies. Clin. Vaccine. Immunol. 2017, 24, e00270-17. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, X.; Hendrickx, A.; Frans, J. Antinuclear antibody titer and antibodies to extractable nuclear antigens. Ann. Rheum. Dis. 2005, 53, 987–988. [Google Scholar] [CrossRef]
- Pham, B.N.; Albarede, S.; Guyard, A.; Burg, E.; Maisonneuve, P. Impact of external quality assessment on antinuclear antibody detection performance. Lupus 2005, 14, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Copple, S.S.; Giles, S.R.; Jaskowski, T.D.; Gardiner, A.E.; Wilson, A.M.; Hill, H.R. Screening for IgG antinuclear autoantibodies by HEp-2 indirect fluorescent antibody assays and the need for standardization, Am. J. Clin. Pathol. 2012, 137, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Tozzoli, R.; Antico, A.; Porcelli, B.; Bassetti, D. Automation in indirect immunofluorescence testing: A new step in the evolution of the autoimmunology laboratory. Auto. Immun. Highlights 2012, 3, 59–65. [Google Scholar] [CrossRef]
- Mahler, M.; Meroni, P.L.; Bossuyt, X.; Fritzler, M.J. Current concepts and future directions for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. J. Immunol. Res. 2014, 14, 315179. [Google Scholar] [CrossRef]
- Meroni, P.L.; Bizzaro, N.; Cavazzana, I.; Borghi, M.O.; Tincani, A. Automated tests of ANA immunofluorescence as throughput autoantibody detection technology: Strengths and limitations. BMC Med. 2014, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Ricchiuti, V.; Adams, J.; Hardy, D.J.; Katayev, A.; Fleming, J.K. Automated Processing and Evaluation of Anti-Nuclear Antibody Indirect Immunofluorescence Testing. Front. Immunol. 2018, 9, 927. [Google Scholar] [CrossRef]
- Choi, H.W.; Kwon, Y.J.; Park, J.H.; Lee, S.Y.; Chun, S.; Won, E.J.; Lee, J.H.; Choi, H.J.; Kim, S.H.; Shin, M.G.; et al. Evaluation of a Fully Automated Antinuclear Antibody Indirect Immunofluorescence Assay in Routine Use. Front. Immunol. 2020, 11, 607541. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, N.; Antico, A.; Platzgummer, S.; Tonutti, E.; Bassetti, D.; Pesente, F.; Tozzoli, R.; Tampoia, M.; Villalta, D. Automated antinuclear immunofluorescence antibody screening: A comparative study of six computer-aided diagnostic systems. Autoimmun. Rev. 2014, 13, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Li, E.; Zhu, H.; Lu, J.; Shi, X.; Zhang, J.; Li, M. Automated antinuclear immunofluorescence antibody analysis is a reliable approach in routine clinical laboratories. Clin. Chem. Lab. Med. 2017, 55, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Van Hoovels, L.; Schouwers, S.; Van den Bremt, S.; Bogaert, L.; Vandeputte, N.; Vercammen, M.; Bossuyt, X. Analytical performance of the single well titer function of NOVA View®: Good enough to omit ANA IIF titer analysis? Clin. Chem. Lab. Med. 2018, 56, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Van den Bremt, S.; Schouwers, S.; Van Blerk, M.; Van Hoovels, L. ANA IIF Automation: Moving towards Harmonization? Results of a Multicenter Study. J. Immunol. Res. 2017, 12, 6038137. [Google Scholar] [CrossRef] [PubMed]
- Schouwers, S.; Bonnet, M.; Verschueren, P.; Westhovens, R.; Blockmans, D.; Mariën, G.; Bossuyt, X. Value-added reporting of antinuclear antibody testing by automated indirect immunofluorescence analysis. Clin. Chem. Lab. Med. 2014, 52, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Bonroy, C.; Verfaillie, C.; Smith, V.; Persijn, L.; De Witte, E.; De Keyser, F.; Devreese, K. Automated indirect immunofluorescence antinuclear antibody analysis is a standardized alternative for visual microscope interpretation. Clin. Chem. Lab. Med. 2013, 51, 1771–1779. [Google Scholar] [CrossRef]
- Roggenbuck, D.; Hiemann, R.; Schierack, P.; Reinhold, D.; Conrad, K. Digital immunofluorescence enables automated detection of antinuclear antibody endpoint titers avoiding serial dilution. Clin. Chem. Lab. Med. 2014, 52, e9–e11. [Google Scholar] [CrossRef]
- Yoo, I.Y.; Oh, J.W.; Cha, H.S.; Koh, E.M.; Kang, E.S. Performance of an Automated Fluorescence Antinuclear Antibody Image Analyzer. Ann. Lab. Med. 2017, 37, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Carbone, T.; Gilio, M.; Padula, M.C.; Tramontano, G.; D’Angelo, S.; Pafundi, V. A step towards standardization: A method for end-point titer determination by fluorescence index of an automated microscope. End-point titer determination by fluorescence index. J. Immunol. Methods 2018, 456, 67–71. [Google Scholar] [CrossRef] [PubMed]

| Total n = 1518 (%) | Autoimmune Disease n = 697 (%) | Non-Autoimmune Disease n = 821 (%) | p Value | |
|---|---|---|---|---|
| Age, year [1st–3rd interquartile range] | 57.0 [41.0–67.0] | 53.0 [39.0–64.0] | 59.0 [43.0–70.0] | <0.001 |
| Female | 1161 (76.5) | 589 (84.5) | 572 (69.7) | <0.001 |
| Underlying disease | ||||
| Hypertension | 92 (6.1) | 43 (6.2) | 59 (7.2) | 0.493 |
| Diabetes mellitus | 64 (4.2) | 28 (4.0) | 36 (4.4) | 0.820 |
| Chronic kidney disease | 81 (5.3) | 25 (3.6) | 56 (6.8) | 0.007 |
| Malignancy | 53 (3.5) | 8 (1.1) | 45 (5.5) | <0.001 |
| Autoantibody Pattern | 0.019 | |||
| Homogeneous | 632 (41.6) | 265 (38.0) | 367 (44.7) | |
| Speckled | 669 (44.1) | 319 (45.8) | 350 (42.6) | |
| Nucleolar | 68 (4.5) | 29 (4.2) | 39 (4.8) | |
| Centromere | 129 (8.5) | 73 (10.5) | 56 (6.8) | |
| Nuclear dots | 20 (1.3) | 11 (1.6) | 9 (1.1) | |
| Endpoint titer of autoantibody by dilution | <0.001 | |||
| 1:80 | 786 (51.8) | 248 (35.6) | 538 (65.5) | |
| 1:160 | 308 (20.3) | 145 (20.8) | 163 (19.9) | |
| 1:320 | 151 (9.9) | 87 (12.5) | 64 (7.8) | |
| ≥1:640 | 273 (18.0) | 217 (31.1) | 56 (6.8) |
| Total (n = 1518) | Speckled Pattern (n = 669) | Homogeneous Pattern (n = 632) | Centromere Pattern (n = 129) | Nucleolar Pattern (n = 68) | Nuclear Dot Pattern (n = 20) | |
|---|---|---|---|---|---|---|
| Before adjustment | ||||||
| Exact match, n (%) | 336 (22.1) | 213 (31.8) | 58 (9.2) | 79 (61.2) | 10 (14.7) | 10 (50.0) |
| ±One-titer match, n (%) | 699 (46.0) | 402 (60.1) | 200 (31.6) | 81 (62.8) | 38 (55.9) | 12 (60.0) |
| Categorical match, n (%) | 780 (51.4) | 345 (51.6) | 259 (41.0) | 119 (92.3) | 49 (61.2) | 16 (80.0) |
| After downgraded adjustment of eEPT * | ||||||
| Exact match, n (%) | 774 (51.0) | 290 (43.4) | 353 (66.4) | 87 (67.4) | 29 (42.7) | 9 (45.0) |
| ±One-titer match, n (%) | 1412 (93.0) | 576 (86.1) | 500 (94.0) | 124 (96.1) | 61 (89.7) | 16 (80.0) |
| Categorical match, n (%) | 1267 (83.5) | 579 (86.6) | 397 (74.6) | 121 (93.8) | 49 (72.1) | 18 (90.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.W.; Jeong, S.-H.; Hong, J.S.; Kim, D.; Park, Y.; Jeong, S.H. Evaluation of the Accuracy of Estimated Endpoint Titer of NOVA View in Indirect Immunofluorescent Antinuclear Antibody Testing. Diagnostics 2024, 14, 1580. https://doi.org/10.3390/diagnostics14151580
Cho HW, Jeong S-H, Hong JS, Kim D, Park Y, Jeong SH. Evaluation of the Accuracy of Estimated Endpoint Titer of NOVA View in Indirect Immunofluorescent Antinuclear Antibody Testing. Diagnostics. 2024; 14(15):1580. https://doi.org/10.3390/diagnostics14151580
Chicago/Turabian StyleCho, Hae Weon, Soon-Ho Jeong, Jun Sung Hong, Dokyun Kim, Yongjung Park, and Seok Hoon Jeong. 2024. "Evaluation of the Accuracy of Estimated Endpoint Titer of NOVA View in Indirect Immunofluorescent Antinuclear Antibody Testing" Diagnostics 14, no. 15: 1580. https://doi.org/10.3390/diagnostics14151580
APA StyleCho, H. W., Jeong, S.-H., Hong, J. S., Kim, D., Park, Y., & Jeong, S. H. (2024). Evaluation of the Accuracy of Estimated Endpoint Titer of NOVA View in Indirect Immunofluorescent Antinuclear Antibody Testing. Diagnostics, 14(15), 1580. https://doi.org/10.3390/diagnostics14151580






