Liquid Biopsies in Follicular Thyroid Carcinomas—A Brief Report
Abstract
:1. Introduction
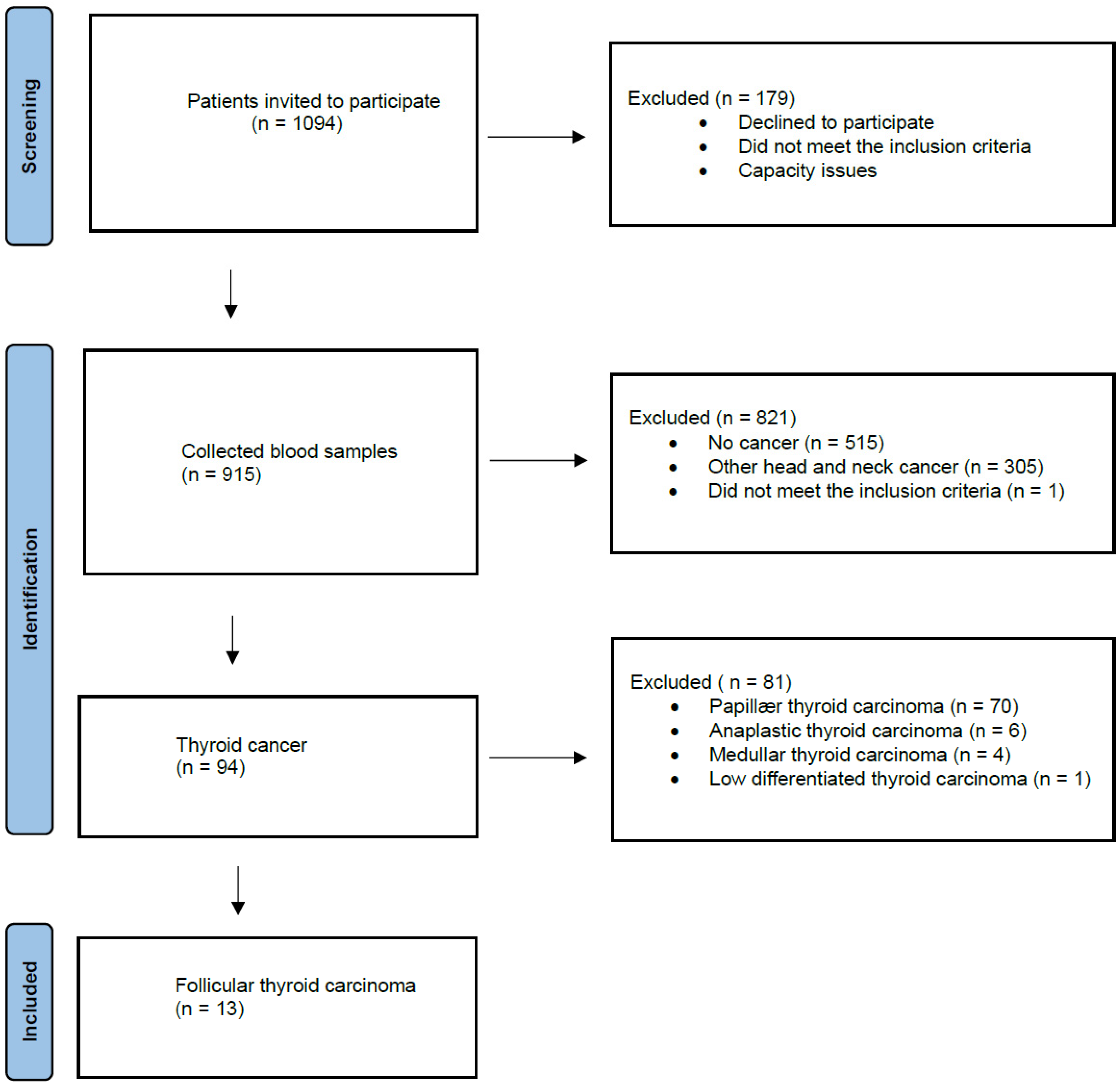
2. Materials and Methods
2.1. Blood Samples and DNA Extraction
2.2. Variant Filtering
3. Results
3.1. The Participants
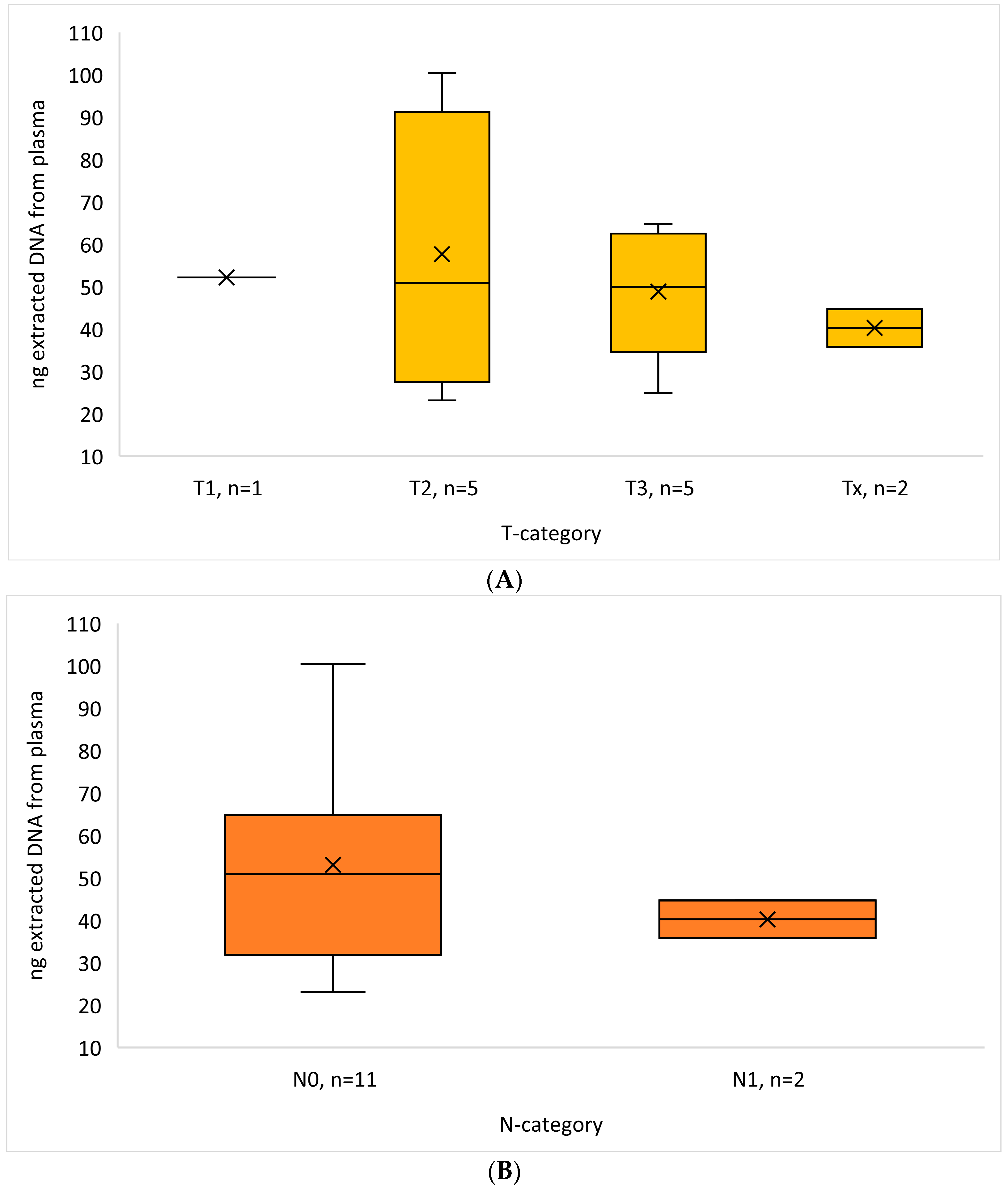
3.2. DNA Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirian, C.; Grønhøj, C.; Jensen, D.H.; Jakobsen, K.K.; Karnov, K.; Jensen, J.S.; Hahn, C.H.; Klitmøller, T.A.; Bentzen, J.; von Buchwald, C. Trends in thyroid cancer: Retrospective analysis of incidence and survival in Denmark 1980–2014. Cancer Epidemiol. 2018, 55, 81–87. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, A.; Treseler, P.; Ituarte, P.H.G.; Wong, M.; Streja, L.; Greenspan, F.S.; Siperstein, A.E.; Duh, Q.; Clark, O.H. Follicular thyroid carcinoma: Histology and prognosis. Cancer 2004, 100, 1123–1129. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Grogan, R.H.; Ward, M.H.; Kaplan, E.; Devesa, S.S. Follicular Thyroid Cancer Incidence Patterns in the United States, 1980–2009. Thyroid 2013, 23, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Lasithiotakis, K.; Grisbolaki, E.; Koutsomanolis, D.; Venianaki, M.; Petrakis, I.; Vrachassotakis, N.; Chrysos, E.; Zoras, O.; Chalkiadakis, G. Indications for surgery and significance of unrecognized cancer in endemic multinodular goiter. World J. Surg. 2012, 36, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, P.; Minuto, M.N.; Galleri, D.; D’Agostino, J.; Basolo, F.; Antonangeli, L.; Aghini-Lombardi, F.; Berti, P. Incidental thyroid carcinoma in a large series of consecutive patients operated on for benign thyroid disease. ANZ J. Surg. 2006, 76, 123–126. [Google Scholar] [CrossRef]
- Miller, J.M.; Kini, S.R.; Hamburger, J.I. The diagnosis of malignant follicular neoplasms of the thyroid by needle biopsy. Cancer 1985, 55, 2812–2817. [Google Scholar] [CrossRef]
- McHenry, C.R.; Thomas, S.R.; Slusarczyk, S.J.; Khiyami, A. Follicular or Hürthle cell neoplasm of the thyroid: Can clinical factors be used to predict carcinoma and determine extent of thyroidectomy? Surgery 1999, 126, 794–798. [Google Scholar] [CrossRef]
- Kim, E.S.; Nam-Goong, I.S.; Gong, G.; Hong, S.J.; Kim, W.B.; Shong, Y.K. Postoperative findings and risk for malignancy in thyroid nodules with cytological diagnosis of the so-called “follicular neoplasm”. Korean J. Intern. Med. 2003, 18, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Görges, R.; Maniecki, M.; Jentzen, W.; Sheu, S.N.-Y.; Mann, K.; Bockisch, A.; E Janssen, O. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur. J. Endocrinol. 2005, 153, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.; Fatemi, S. Thyroglobulin antibody (TgAb) methods—Strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 701–712. [Google Scholar] [CrossRef] [PubMed]
- van Veelen, W.; de Groot, J.W.B.; Acton, D.S.; Hofstra, R.M.W.; Höppener, J.W.M.; Links, T.P.; Lips, C.J. Medullary thyroid carcinoma and biomarkers: Past, present and future. J. Intern. Med. 2009, 266, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Offi, C.; Patrone, R.; Clarizia, G.; Mauriello, C.; Tartaglia, E.; Di Capua, F.; Di Martino, S.; Romano, R.M.; Fiore, L.; et al. Calcitonin negative Medullary Thyroid Carcinoma: A challenging diagnosis or a medical dilemma? BMC Endocr. Disord. 2019, 19 (Suppl. S1), 45. [Google Scholar] [CrossRef] [PubMed]
- Khatami, F.; Tavangar, S.M. Liquid Biopsy in Thyroid Cancer: New Insight. Int. J. Hematol. Stem Cell Res. 2018, 12, 235–248. [Google Scholar]
- Allin, D.M.; Shaikh, R.; Carter, P.; Thway, K.; Sharabiani, M.T.A.; Gonzales-de-Castro, D.; O'Leary, B.; Garcia-Murillas, I.; Bhide, S.; Hubank, M.; et al. Circulating tumour DNA is a potential biomarker for disease progression and response to targeted therapy in advanced thyroid cancer. Eur. J. Cancer. 2018, 103, 165–175. [Google Scholar] [CrossRef]
- Lupo, M.; Guttler, R.; Geck, Z.; Tonozzi, T.R.; Kammesheidt, A.; Braunstein, G.D. Is measurement of circulating tumor DNA of diagnostic use in patients with thyroid nodules? Endocr. Pract. 2018, 24, 453–459. [Google Scholar] [CrossRef]
- Zeyghami, W.; Hansen, M.-L.U.; Jakobsen, K.K.; Groenhøj, C.; Feldt-Rasmussen, U.; von Buchwald, C.; Hahn, C.H. Liquid biopsies in thyroid cancers: A systematic review and meta-analysis. Endocr. Relat. Cancer 2023, 30, e230002. [Google Scholar] [CrossRef]
- Zane, M.; Agostini, M.; Enzo, M.V.; Casal Ide, E.; Del Bianco, P.; Torresan, F.; Boschin, I.M.; Pennelli, G.; Saccani, A.; Rubello, D.; et al. Circulating cell-free DNA, SLC5A8 and SLC26A4 hypermethylation, BRAFV600E: A non-invasive tool panel for early detection of thyroid cancer. Biomed. Pharmacother. 2013, 67, 723–730. [Google Scholar] [CrossRef]
- Illumina. Available online: https://emea.illumina.com/products/by-type/clinical-research-products/trusight-oncology-500.html (accessed on 20 May 2024).
- Robinson, J.T.; Thorvaldsdóttir, H.; Wenger, A.M.; Zehir, A.; Mesirov, J.P. Variant Review with the Integrative Genomics Viewer. Cancer Res. 2017, 77, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Vu-Phan, D.; Koenig, R.J. Genetics and epigenetics of sporadic thyroid cancer. Mol. Cell Endocrinol. 2014, 386, 55–66. [Google Scholar] [CrossRef]
- Giordano, T.J. Follicular cell thyroid neoplasia: Insights from genomics and The Cancer Genome Atlas research network. Curr. Opin. Oncol. 2016, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Catalogue of Somatic Mutations in Cancer. Available online: https://cancer.sanger.ac.uk/cosmic/browse/tissue?wgs=off&sn=thyroid&ss=all&hn=carcinoma&sh=follicular_carcinoma&in=t&src=tissue&all_data=n (accessed on 20 May 2024).
- Chuang, T.C.Y.; Chuang, A.Y.C.; Poeta, L.; Koch, W.M.; Califano, J.A.; Tufano, R.P. Detectable BRAF mutation in serum DNA samples from patients with papillary thyroid carcinomas. Head Neck 2010, 32, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Pupilli, C.; Pinzani, P.; Salvianti, F.; Fibbi, B.; Rossi, M.; Petrone, L.; Perigli, G.; De Feo, M.L.; Vezzosi, V.; Pazzagli, M.; et al. Circulating BRAFV600E in the diagnosis and follow-up of differentiated papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 3359–3365. [Google Scholar] [CrossRef]
- Paulsson, J.O.; Rafati, N.; DiLorenzo, S.; Chen, Y.; Haglund, F.; Zedenius, J.; Juhlin, C.C. Whole-genome Sequencing of Follicular Thyroid Carcinomas Reveal Recurrent Mutations in MicroRNA Processing Subunit DGCR8. J. Clin. Endocrinol. Metab. 2021, 106, 3265–3282. [Google Scholar] [CrossRef]
- Leiden Open Variation Database. Available online: https://databases.lovd.nl/shared/genes/SDHA (accessed on 10 June 2024).
| FTC + n (%) | |
|---|---|
| Sex | |
| Female | 8 (61.5) |
| Male | 5 (38.5) |
| Age, years | |
| <45 | 0 (0) |
| 45–50 | 2 (15.4) |
| 50–60 | 4 (30.8) |
| 60–70 | 2 (15.4) |
| 70–80 | 4 (30.8) |
| 80–90 | 1 (7.7) |
| CCI at diagnosis (not including the TC) * | |
| 0 | 2 (15.4) |
| 1 | 3 (23.1) |
| 2 | 2 (15.4) |
| 3 | 3 (23.1) |
| 4 | 3 (23.1) |
| 5 | 0 (0) |
| Ultrasonography EU-TIRADS score | |
| 1 | 4 (30.8) ** |
| 2 | 0 (0) |
| 3 | 4 (30.8) |
| 4 | 4 (30.8) |
| 5 | 1 (7.7) |
| Fine-needle aspiration diagnosis | |
| Follicular neoplasia | 6 (46.2) |
| Malignant cells | 2 (15.4) |
| Follicular carcinoma | 1 (7.7) |
| To sparse material | 1 (7.7) |
| No malignant cells | 1 (7.7) |
| No fine-needle aspiration | 1 (7.7) *** |
| Papillary thyroid carcinoma | 1 (7.7) |
| T-category | |
| x | 2 (15.4) |
| 0 | 0 (0) |
| 1a | 0 (0) |
| 1b | 1 (7.7) |
| 2 | 5 (38.5) |
| 3a | 4 (30.8) |
| 3b | 1 (7.7) |
| 4a + 4b | 0 (0) |
| N-category | |
| 0 | 11 (84.6) |
| 1a | 0 (0) |
| 1b | 2 (15.4) |
| M-category | |
| x | 1 (7.7) |
| 0 | 10 (76.9) |
| 1 | 2 (15.4) |
| Case ID | Gene | Mutation Classification | Chromosome | Function | Gene Region |
|---|---|---|---|---|---|
| 1 | RET | Missense | 10 | Normal | Exonic |
| 2 | SLX4 | Missense | 16 | Loss | Exonic |
| 3 | SDHA | Missense | 5 | Normal | Exonic |
| 4 | BCORL1 | Missense | X | Normal | Exonic |
| 5 | ESR1 | Missense | 6 | Loss | Promoter; Exonic |
| Case ID | Transcript ID | Transcript Variant | Protein Variant | Allele Frequency (%) | Variation | Mean Cov. TSO500 |
|---|---|---|---|---|---|---|
| 1 | NM_020975.6; NM_001355216.1; ENST00000340058.6; NM_020630.6 | c.1241G>A; c.479G>A | p.R414K; p.R160K | 0.86 | SNV | 576 |
| 2 | NM_032444.4 | c.947A>C | p.N316T | 4.73 | SNV | 624 |
| 3 | NM_004168.4; ENST00000510361.5; NM_001294332.2; ENST00000264932.11; NM_001330758.2 | c.1216G>A; c.1360G>A | p.A406T; p.A454T | 0.95 | SNV | 642 |
| 4 | NM_001184772.3; NM_001379451.1; NM_021946.5; NM_001379450.1; ENST00000540052.6 | c.2869C>T | p.L957F | 1.09 | SNV | 611 |
| 5 | ENST00000206249.8; NM_001385568.1; NM_001291241.2; NM_001291230.2; NM_001385570.1; NM_001122741.2; NM_000125.4; NM_001385569.1; NM_001385571.1; NM_001122740.2; NST00000427531.6; NM_001328100.2; NM_001385572.1; ENST00000406599.5; M_001122742.2; ENST00000456483.3 | c.166G>A; c.-1261G>A | p.E56K | 1.03 | SNV | 743 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, M.-L.U.; Bendtsen, S.K.; Jakobsen, K.K.; Schmidt, A.Y.; Hahn, C.H.; von Buchwald, C.; Grønhøj, C. Liquid Biopsies in Follicular Thyroid Carcinomas—A Brief Report. Diagnostics 2024, 14, 1577. https://doi.org/10.3390/diagnostics14141577
Hansen M-LU, Bendtsen SK, Jakobsen KK, Schmidt AY, Hahn CH, von Buchwald C, Grønhøj C. Liquid Biopsies in Follicular Thyroid Carcinomas—A Brief Report. Diagnostics. 2024; 14(14):1577. https://doi.org/10.3390/diagnostics14141577
Chicago/Turabian StyleHansen, Marie-Louise Uhre, Simone Kloch Bendtsen, Kathrine Kronberg Jakobsen, Ane Yde Schmidt, Christoffer Holst Hahn, Christian von Buchwald, and Christian Grønhøj. 2024. "Liquid Biopsies in Follicular Thyroid Carcinomas—A Brief Report" Diagnostics 14, no. 14: 1577. https://doi.org/10.3390/diagnostics14141577
APA StyleHansen, M.-L. U., Bendtsen, S. K., Jakobsen, K. K., Schmidt, A. Y., Hahn, C. H., von Buchwald, C., & Grønhøj, C. (2024). Liquid Biopsies in Follicular Thyroid Carcinomas—A Brief Report. Diagnostics, 14(14), 1577. https://doi.org/10.3390/diagnostics14141577







