Right Ventricular and Right Atrial Strain Are Associated with Kidney Dysfunction in Acute Heart Failure
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Echocardiographic Analysis
2.3. Statistical Analysis
3. Results
3.1. Clinical and Echocardiographic Characteristics
3.2. Independent Associates of Low eGFR
4. Discussion
4.1. Cardiac Output and Renal Impairment
4.2. Venous Congestion and Renal Impairment
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| eGFR | estimated glomerular filtration rate |
| HF | heart failure |
| LV | left ventricular/ventricle |
| NT-pro-BNP | N-terminal pro B-type natriuretic peptide |
| RA | right atrial/atrium |
| RV | right ventricular/ventricle |
References
- Gheorghiade, M.; Pang, P.S. Acute heart failure syndromes. J. Am. Coll. Cardiol. 2009, 53, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Velavan, P.; Khan, N.K.; Goode, K.; Rigby, A.S.; Loh, P.H.; Komajda, M.; Follath, F.; Swedberg, K.; Madeira, H.; Cleland, J.G. Predictors of short term mortality in heart failure—Insights from the Euro Heart Failure survey. Int. J. Cardiol. 2010, 138, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.D.; Hernandez, A.F.; Liang, L.; Peterson, E.D.; LaBresh, K.A.; Yancy, C.W.; Albert, N.M.; Ellrodt, G.; Fonarow, G.C. Quality of care and outcomes among patients with heart failure and chronic kidney disease: A Get With the Guidelines—Heart Failure Program study. Am. Heart J. 2008, 156, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, S.; Laragh, J.H.; Cody, R.J. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 1990, 39 (Suppl. S4), 10–21, discussion 22–24. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef]
- McLean, A.S. Echocardiography in shock management. Crit. Care 2016, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Valentova, M.; von Haehling, S.; Bauditz, J.; Doehner, W.; Ebner, N.; Bekfani, T.; Elsner, S.; Sliziuk, V.; Scherbakov, N.; Murín, J.; et al. Intestinal congestion and right ventricular dysfunction: A link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur. Heart J. 2016, 37, 1684–1691. [Google Scholar] [CrossRef]
- Dini, F.L.; Demmer, R.T.; Simioniuc, A.; Morrone, D.; Donati, F.; Guarini, G.; Orsini, E.; Caravelli, P.; Marzilli, M.; Colombo, P.C. Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur. J. Heart Fail. 2012, 14, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.B.; Ram, P.; Kanjanahattakij, N.; Gupta, S.; Pressman, G.S.; Rangaswami, J. Right Ventricular Free Wall Strain Is Associated With Long-Term Renal Function in Heart Failure With Preserved Ejection Fraction. J. Card. Fail. 2018, 24, 719–720. [Google Scholar] [CrossRef]
- Tafciu, E.; Niro, L.; Iseppi, M.; Fanti, D.; Maffeis, C.; Bergamini, C.; Benfari, G.; Rossi, A.; Ribichini, F.L. Right Atrial Function Role in Tricuspid Regurgitation-Related Systemic Venous Congestion. Am. J. Cardiol. 2023, 204, 320–324. [Google Scholar] [CrossRef]
- Anastasiou, V.; Peteinidou, E.; Moysidis, D.V.; Daios, S.; Gogos, C.; Liatsos, A.C.; Didagelos, M.; Gossios, T.; Efthimiadis, G.K.; Karamitsos, T.; et al. Multiorgan Congestion Assessment by Venous Excess Ultrasound Score in Acute Heart Failure. J. Am. Soc. Echocardiogr. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, V.; Daios, S.; Moysidis, D.V.; Bazmpani, M.A.; Zegkos, T.; Karamitsos, T.; Makedou, K.; Savopoulos, C.; Efthimiadis, G.; Ziakas, A.; et al. Clinical Value of Novel Echocardiographic Biomarkers Assessing Myocardial Work in Acute Heart Failure-Rationale and Design of the “Beyond Myo-HF Study”. Diagnostics 2023, 13, 1191. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate eGFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for eGFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am. J. Kidney Dis. 2022, 79, 268–288.e1. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [PubMed]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef]
- Gorter, T.M.; van Veldhuisen, D.J.; Bauersachs, J.; Borlaug, B.A.; Celutkiene, J.; Coats, A.J.S.; Crespo-Leiro, M.G.; Guazzi, M.; Harjola, V.P.; Heymans, S.; et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: Mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Vonk Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J. Am. Coll. Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.D.; Raine, A.E.; Ledingham, J.G. Raised venous pressure: A direct cause of renal sodium retention in oedema? Lancet 1988, 1, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, V.; Papazoglou, A.S.; Moysidis, D.V.; Daios, S.; Barmpagiannos, K.; Gossios, T.; Efthimiadis, G.K.; Karamitsos, T.; Ziakas, A.; Kamperidis, V. The prognostic impact of right ventricular-pulmonary arterial coupling in heart failure: A systematic review and meta-analysis. Heart Fail. Rev. 2023, 29, 13–26. [Google Scholar] [CrossRef]
- Goldsmith, S.R.; Bart, B.A.; Burnett, J. Decongestive therapy and renal function in acute heart failure: Time for a new approach? Circ. Heart Fail. 2014, 7, 531. [Google Scholar] [CrossRef]

| Variable | All (n = 377) | eGFR ≥ 45 mL/min/1.73 m2 (n = 252) | eGFR < 45 mL/min/1.73 m2 (n = 125) | p-Value |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Age, years | 73.6 ± 11.9 | 71.4 ± 12.5 | 77.8 ± 9.4 | <0.001 |
| Male, n (%) | 228 (60.5) | 159 (63.1) | 69 (55.2) | 0.15 |
| BSA, m2 | 1.93 ± 0.23 | 1.94 ± 0.23 | 1.90 ± 0.23 | 0.048 |
| Systolic blood pressure, mmHg | 124.1 ± 19.0 | 124.4 ± 18.1 | 123.0 ± 19.8 | 0.51 |
| Diastolic blood pressure, mmHg | 74.0 ± 15.5 | 75.6 ± 16.0 | 70.6 ± 14.1 | 0.004 |
| Heart rate, bpm | 82.6 ± 18.0 | 83.9 ±18.0 | 80.3 ± 17.9 | 0.07 |
| NYHA III-IV, % | 129 (34.2) | 70 (27.8) | 59 (47.2) | <0.001 |
| Past Medical History | ||||
| Ischemic heart disease, n (%) | 145 (38.4) | 97 (38.5) | 48 (38.4) | 1.00 |
| Dilated cardiomyopathy, n (%) | 41 (10.9) | 27 (10.7) | 14 (11.2) | 0.86 |
| New-onset heart failure, n (%) | 164 (43.5) | 133 (52.8) | 31 (24.8) | <0.001 |
| Chronic heart failure, n (%) | 211 (56.0) | 118 (46.8) | 93 (76.8) | <0.001 |
| Severe valvular heart disease, n (%) | 77 (20.4) | 52 (20.6) | 25 (20.0) | 1.00 |
| Chronic atrial fibrillation, n (%) | 98 (26.0) | 54 (21.4) | 44 (35.2) | 0.006 |
| Hypertension, n (%) | 196 (52.0) | 124 (49.2) | 72 (57.6) | 0.13 |
| Type 2 diabetes mellitus, n (%) | 136 (36.1) | 73 (29.0) | 63 (50.4) | <0.001 |
| Chronic obstructive pulmonary disease, n (%) | 58 (15.4) | 40 (15.9) | 18 (14.4) | 0.76 |
| Medication on Admission | ||||
| Furosemide, n (%) | 168 (44.6) | 100 (39.7) | 68 (54.4) | 0.019 |
| Furosemide dose, mg/day (range) | 38 (0–40) | 32 (0–40) | 53 (0–80) | 0.002 |
| ACEi/ARBs, n (%) | 126 (33.4) | 83 (32.9) | 43 (34.4) | 0.82 |
| ARNI, n (%) | 32 (8.5) | 17 (6.7) | 15 (12.0) | 0.12 |
| MRAs, n (%) | 102 (27.1) | 57 (22.6) | 45 (36.0) | 0.007 |
| B-blockers, n (%) | 164 (43.5) | 103 (40.9) | 61 (48.8) | 0.15 |
| SGLT2i, n (%) | 76 (20.2) | 43 (17.1) | 33 (26.4) | 0.08 |
| Laboratory Indices | ||||
| NT-pro-BNP, pg/mL (range) | 4376 (2113–10,697) | 3199 (1701–7064) | 9387 (3985–25,246) | <0.001 |
| Admission troponin I, ng/L (range) | 40 (25–81) | 36 (22–69) | 55 (36–97) | <0.001 |
| AST, U/L (range) | 27 (20–39) | 28 (20–38) | 26 (20–40) | 0.63 |
| ALT, U/L (range) | 20 (13–33) | 21 (14–35) | 17 (11–30) | 0.06 |
| CPK, U/L (range) | 76 (46–149) | 83 (48–150) | 71 (45–142) | 0.19 |
| Creatitine, mg/dL | 1.4 ± 0.65 | 1.10 ± 0.24 | 2.01 ± 0.79 | <0.001 |
| Blood urea nitrogen, mg/dL (range) | 56 (40–79) | 47 (37–61) | 94 (62–122) | <0.001 |
| Sodium, mEq/L | 136.8 ± 12.6 | 137.3 ± 12.5 | 135.7 ± 12.9 | 0.25 |
| Potassium, mEq/L | 4.3 ± 0.6 | 4.2 ± 0.6 | 4.4 ± 0.7 | 0.011 |
| Hemoglobin, g/dL | 12.3 ± 2.2 | 12.6 ± 2.1 | 11.6 ± 2.1 | <0.001 |
| Platelets, K/dL (range) | 222 (174–281) | 229 (182–285) | 209 (166–266) | 0.06 |
| Variable | All (n = 377) | eGFR ≥ 45 mL/min/1.73 m2 (n = 252) | eGFR < 45 mL/min/1.73 m2 (n = 125) | p-Value |
|---|---|---|---|---|
| LV end-diastolic diameter, mm | 55.7 ± 11.5 | 55.6 ± 11.5 | 55.9 ± 11.7 | 0.80 |
| LV end-systolic diameter, mm | 45.1 ±12.6 | 45.2 ± 12.3 | 45.2 ± 13.3 | 1.00 |
| LV end-diastolic volume indexed, mL/m2 | 87.9 ± 37.8 | 86.8 ± 37.4 | 90.6 ± 39.2 | 0.36 |
| LV end-systolic volume indexed, mL/m2 | 57.3 ± 35.3 | 56.4 ± 34.7 | 59.5 ± 37.0 | 0.42 |
| LV ejection fraction, % | 39.4 ± 14.7 | 39.6 ± 14.1 | 39.1 ± 15.7 | 0.75 |
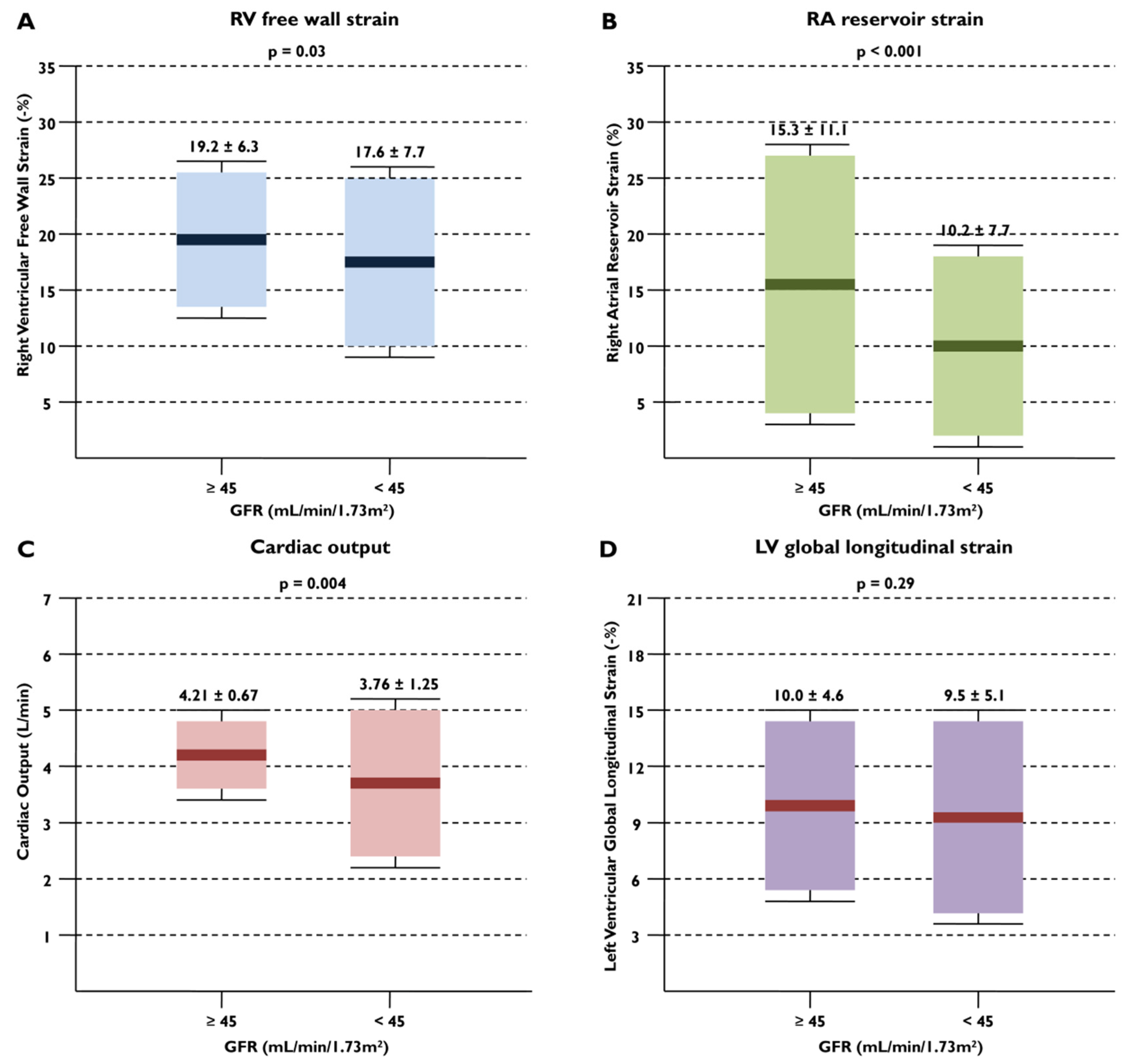
| LV GLS, % | 9.9 ± 4.8 | 10.0 ± 4.6 | 9.5 ± 5.1 | 0.29 |
| Stroke volume, mL | 50.37 ± 18.44 | 51.46 ± 18.64 | 48.26 ± 18.19 | 0.12 |
| Cardiac output, L/min | 4.06 ±1.40 | 4.21 ± 0.67 | 3.76 ± 1.25 | 0.004 |
| E, cm/s | 102.6 ± 27.1 | 101.7 ± 27.6 | 104.6 ± 26.3 | 0.34 |
| A, cm/s | 58.7 ± 28.0 | 57.2 ± 26.1 | 62.3 ± 31.7 | 0.18 |
| E/A | 2.06 ±1.15 | 2.08 ± 1.19 | 1.99 ± 1.06 | 0.60 |
| Mean E/e’ | 18.9 ± 6.5 | 18.2 ± 6.0 | 20.5 ± 7.3 | 0.002 |
| RV end-diastolic area, cm2 | 22.8 ±7.1 | 22.2 ± 6.9 | 24.0 ± 7.4 | 0.021 |
| RV end-systolic area, cm2 | 15.4 ± 6.1 | 14.8 ± 5.9 | 16.5 ± 6.5 | 0.016 |
| Tricuspid annular diameter, mm | 35.5 ± 7.5 | 34.9 ±7.5 | 36.7 ±7.4 | 0.042 |
| Basal RV end-diastolic diameter, mm | 46.3 ± 8.1 | 45.5 ± 8.2 | 47.8 ± 7.8 | 0.01 |
| Mid RV end-diastolic diameter, mm | 34.7 ± 8.7 | 34.1 ± 8.7 | 36.0 ± 8.8 | 0.05 |
| Apex-to-base RV end-diastolic diameter, mm | 76.4 ± 13.5 | 76.7 ± 13.5 | 75.9 ±13.8 | 0.62 |
| Fractional area change, % | 33.7 ± 9.6 | 34.3 ± 9.3 | 32.7 ± 10.0 | 0.14 |
| S’TDI tricuspid, cm/s | 9.5 ± 3.0 | 9.8 ± 2.8 | 8.8 ± 3.1 | 0.002 |
| TAPSE, mm | 16.5 ± 4.1 | 16.9 ± 3.9 | 15.8 ± 4.5 | 0.015 |
| RV E, cm/s | 57.9 ± 18.9 | 56.6 ± 17.7 | 60.4 ± 20.4 | 0.10 |
| PV acceleration time, ms | 83.9 ± 24.1 | 86.7 ± 24.8 | 78.3 ± 22.2 | 0.002 |
| RV free wall LS, % | 18.6 ± 6.8 | 19.2 ± 6.3 | 17.6 ± 7.7 | 0.03 |
| RV GLS, % | 14.8 ± 5.4 | 15.1 ± 5.1 | 14.1 ± 5.9 | 0.08 |
| LA end-systolic volume indexed, mL/m2 | 58.2 ± 20.7 | 56.8 ± 20.1 | 61.2 ± 22.1 | 0.06 |
| LASr, % | 8.5 ± 4.5 | 8.9 ± 4.5 | 8.0 ± 4.5 | 0.07 |
| RA volume, mL | 86.4 ± 49.6 | 84.2 ± 50.8 | 90.4 ± 47.4 | 0.26 |
| RASr, % | 13.6 ± 10.4 | 15.3 ± 11.1 | 10.2 ± 7.7 | <0.001 |
| Severe left-sided heart disease, % | 77 (20.4) | 44 (17.4) | 30 (24.0) | 0.08 |
| Severe tricuspid regurgitation, % | 146 (38.7) | 86 (34.0) | 60 (48.0) | 0.005 |
| RV systolic pressure, mmHg | 49.3 ± 14.0 | 48.4 ± 14.0 | 51.1 ± 13.9 | 0.08 |
| Inferior vena cava diameter, mm | 23.1 ± 5.8 | 22.7 ± 5.6 | 23.9 ± 6.2 | 0.06 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Systolic blood pressure, mmHg | 1.00 | 0.99–1.02 | 0.51 | |||
| Diastolic blood pressure, mmHg | 1.02 | 1.01–1.04 | 0.005 | 1.02 | 0.99–1.04 | 0.14 |
| NHYA III, IV | 2.32 | 1.49–3.63 | <0.001 | 1.43 | 0.76–2.70 | 0.27 |
| Chronic heart failure | 3.30 | 2.06–5.29 | <0.001 | 2.36 | 1.15–4.89 | 0.019 |
| Chronic atrial fibrillation | 1.99 | 1.24–3.20 | 0.004 | 1.03 | 0.51–2.10 | 0.93 |
| Type 2 diabetes mellitus | 2.49 | 1.60–3.88 | <0.001 | 2.52 | 1.38–4.62 | 0.003 |
| NT-pro-BNP, pg/mL * | 5.65 | 3.27–9.78 | <0.001 | 5.78 | 2.84–11.63 | <0.001 |
| LV end-diastolic volume indexed, mL/m2 | 1.00 | 0.99–1.01 | 0.36 | |||
| LV ejection fraction, % | 0.99 | 0.98–1.01 | 0.75 | |||
| LV GLS, % | 0.98 | 0.93–1.02 | 0.29 | |||
| Stroke volume, mL | 1.01 | 0.99–1.02 | 0.12 | |||
| Cardiac output, L/min | 0.79 | 0.67–0.93 | 0.005 | 0.95 | 0.75–1.20 | 0.65 |
| Mean E/e’ ratio | 1.06 | 1.02–1.09 | 0.003 | 1.01 | 0.96–1.06 | 0.78 |
| Basal RV end-diastolic diameter, mm | 1.04 | 1.01–1.06 | 0.011 | 1.04 | 0.99–1.07 | 0.12 |
| Fractional area change, % ** | 0.98 | 0.96–1.01 | 0.14 | |||
| S’TDI tricuspid, cm/s ** | 0.88 | 0.82–0.96 | 0.002 | |||
| TAPSE, mm ** | 0.94 | 0.89–0.99 | 0.016 | |||
| RV free wall LS, %, % ** | 0.97 | 0.93–0.99 | 0.031 | 0.94 | 0.89–0.99 | 0.027 |
| RV GLS, % ** | 0.96 | 0.93–1.00 | 0.08 | |||
| LA volume indexed, mL/m2 | 1.01 | 1.00–1.02 | 0.06 | |||
| LASr, % | 0.95 | 0.91–1.00 | 0.07 | |||
| RA volume, mL | 1.00 | 0.99–1.01 | 0.26 | |||
| RASr, % | 0.95 | 0.92–0.97 | <0.001 | 0.95 | 0.91–0.99 | 0.029 |
| Severe left heart valvular disease | 1.51 | 0.89–2.55 | 0.12 | |||
| Severe tricuspid regurgitation | 1.82 | 1.17–2.82 | 0.008 | 2.10 | 0.96–4.57 | 0.06 |
| RV systolic pressure, mmHg | 1.01 | 0.99–1.03 | 0.08 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiou, V.; Peteinidou, E.; Tountas, C.; Daios, S.; Moysidis, D.V.; Fardoulis, E.; Gogos, C.; Theodorakopoulou, M.; Iatridi, F.; Sarafidis, P.; et al. Right Ventricular and Right Atrial Strain Are Associated with Kidney Dysfunction in Acute Heart Failure. Diagnostics 2024, 14, 1576. https://doi.org/10.3390/diagnostics14141576
Anastasiou V, Peteinidou E, Tountas C, Daios S, Moysidis DV, Fardoulis E, Gogos C, Theodorakopoulou M, Iatridi F, Sarafidis P, et al. Right Ventricular and Right Atrial Strain Are Associated with Kidney Dysfunction in Acute Heart Failure. Diagnostics. 2024; 14(14):1576. https://doi.org/10.3390/diagnostics14141576
Chicago/Turabian StyleAnastasiou, Vasileios, Emmanouela Peteinidou, Christos Tountas, Stylianos Daios, Dimitrios V. Moysidis, Emmanouil Fardoulis, Christos Gogos, Marieta Theodorakopoulou, Fotini Iatridi, Pantelis Sarafidis, and et al. 2024. "Right Ventricular and Right Atrial Strain Are Associated with Kidney Dysfunction in Acute Heart Failure" Diagnostics 14, no. 14: 1576. https://doi.org/10.3390/diagnostics14141576
APA StyleAnastasiou, V., Peteinidou, E., Tountas, C., Daios, S., Moysidis, D. V., Fardoulis, E., Gogos, C., Theodorakopoulou, M., Iatridi, F., Sarafidis, P., Giannakoulas, G., Karamitsos, T., Delgado, V., Ziakas, A., & Kamperidis, V. (2024). Right Ventricular and Right Atrial Strain Are Associated with Kidney Dysfunction in Acute Heart Failure. Diagnostics, 14(14), 1576. https://doi.org/10.3390/diagnostics14141576










