Impact of Obesity on Target Organ Damage in Patients with Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
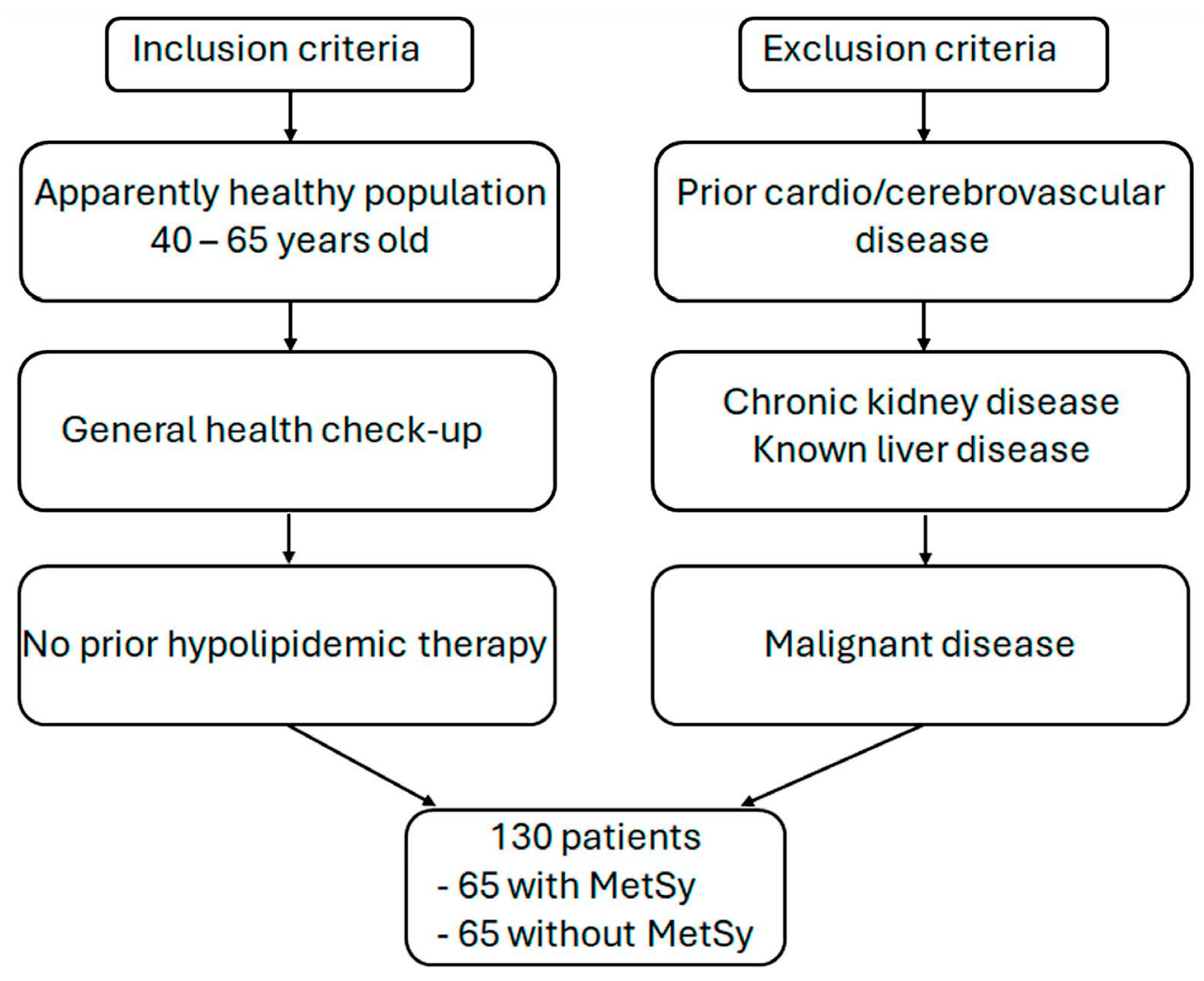
2.1. Subjects
2.2. Risk Factor Assessment
2.3. Anthropometric Measurements
2.4. Laboratory Analyses
2.5. Non-Invasive Assessment of Liver Disease
2.6. Imaging Modalities
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Dobrowolski, P.; Prejbisz, A.; Kuryłowicz, A.; Baska, A.; Burchardt, P.; Chlebus, K.; Dzida, G.; Jankowski, P.; Jaroszewicz, J.; Jaworski, P.; et al. Metabolic syndrome—A new definition and management guidelines. Arch. Med. Sci. 2022, 18, 1133–1156. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Heymsfield, S.B.; Roche, A.F. Human body composition and the epidemiology of chronic disease. Obes. Rev. 1995, 3, 73–95. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Chen, J.; Gallagher, D.; Albu, J.; Pi-Sunyer, X.; Lewis, C.E.; Grunfeld, C.; Heshka, S.; Heymsfield, S.B. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity 2006, 14, 727–736. [Google Scholar] [CrossRef]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63 Pt B, 2985–3023. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Morawiecka-Pietrzak, M.; Starzak, M.; Stanek, A.; Cieślar, G. Retrospective Cross-Sectional Study of the Relationship of Thyroid Volume and Function with Anthropometric Measurements, Body Composition Analysis Parameters, and the Diagnosis of Metabolic Syndrome in Euthyroid People Aged 18–65. Medicina 2024, 60, 1080. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diab. Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The Role of Oxidized Low-Density Lipoproteins in Atherosclerosis: The Myths and the Facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef]
- Hurtado-Roca, Y.; Bueno, H.; Fernandez-Oritz, A.; Ordovas, J.M.; Ibañez, B.; Fuster, V.; Rodriguez-Artalejo, F.; Laclaustra, M. Oxidized LDL Is Associated with Metabolic Syndrome Traits Independently of Central Obesity and Insulin Resistance. Diabetes 2017, 66, 474–482. [Google Scholar] [CrossRef]
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric Oxide in the Vasculature: Where Does It Come From and Where Does It Go? A Quantitative Perspective. Antioxid. Redox Signal. 2008, 10, 1185–1198. [Google Scholar] [CrossRef]
- Zahedi Asl, S.; Ghasemi, A.; Azizi, F. Serum nitric oxide metabolites in subjects with metabolic syndrome. Clin. Biochem. 2008, 41, 1342–1347. [Google Scholar] [CrossRef]
- Alessi, M.-C.; Juhan-Vague, I. PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2200–2207. [Google Scholar] [CrossRef]
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult. Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies. Eur. Heart J. 2021, 42, 3227–3337. [CrossRef]
- Calculate Your Body Mass Index. Available online: https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi-m.htm (accessed on 9 June 2024).
- Shuter, B.; Aslani, A. Body surface area: Du Bois and Du Bois revisited. Eur. J. Appl. Physiol. 2000, 82, 250–254. [Google Scholar] [CrossRef]
- Radulović, N.S.; Ranđelović, P.J.; Stojanović, N.M.; Cakić, N.D.; Bogdanović, G.A.; Živanović, A.V. Aboriginal bush foods: A major phloroglucinol from Crimson Bottlebrush flowers (Callistemon citrinus, Myrtaceae) displays strong antinociceptive and antiinflammatory activity. Food. Res. Int. 2015, 77, 280–289. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Fatar, M.; et al. Mannheim Carotid Intima-Media Thickness Consensus (2004–2006). An Update on Behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc. Dis. 2007, 23, 75–80. [Google Scholar] [CrossRef]
- April-Sanders, A.K.; Rodriguez, C.J. Metabolically Healthy Obesity Redefined. JAMA Netw. Open. 2021, 4, e218860. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist: Hip ratio as predictors of cardiovascular risk—A review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef]
- de Koning, L.; Merchant, A.T.; Pogue, J.; Anand, S.S. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur. Heart J. 2007, 28, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, C.F.; Lin, F.J.; Lee, T.L.; Yang, K.C.; Tseng, W.K.; Wu, Y.W.; Yin, W.-H.; Yeh, H.-I.; Chen, J.-W.; Wu, C.-C.; et al. The waist-to-body mass index ratio as an anthropometric predictor for cardiovascular outcome in subjects with established atherosclerotic cardiovascular disease. Sci. Rep. 2022, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Ntougou Assoumou, H.G.; Pichot, V.; Barthelemy, J.C.; Celle, S.; Garcin, A.; Thomas, T.; Roche, F. Obesity related to metabolic syndrome: Comparison of obesity indicators in an older french population. Diabetol. Metab. Syndr. 2023, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, N.A.; Arifani, R.; Irawan, R. Cut-off value of Waist-to-Hip Ratio as a predictor of metabolic syndrome in adolescents with obesity. Acta Biomed. 2023, 94, e2023076. [Google Scholar] [CrossRef]
- Kawamoto, R.; Kikuchi, A.; Akase, T.; Ninomiya, D.; Kumagi, T. Usefulness of waist-to-height ratio in screening incident metabolic syndrome among Japanese community-dwelling elderly individuals. PLoS ONE 2019, 14, e0216069. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817. [Google Scholar] [CrossRef] [PubMed]
- NAFLD (Non-Alcoholic Fatty Liver Disease) Fibrosis Score. Available online: https://www.mdcalc.com/calc/3081/nafld-non-alcoholic-fatty-liver-disease-fibrosis-score (accessed on 9 June 2024).
- Health Survey for England 2013, Trend Tables. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2013-trend-tables (accessed on 9 June 2024).
- Blak, B.T.; Thompson, M.; Dattani, H.; Bourke, A. Generalisability of The Health Improvement Network (THIN) database: Demographics, chronic disease prevalence and mortality rates. Inform. Prim. Care 2011, 19, 251–255. [Google Scholar] [CrossRef]
- Loomis, A.K.; Kabadi, S.; Preiss, D.; Hyde, C.; Bonato, V.; Louis, M.S.; Desai, J.; Gill, J.M.R.; Welsh, P.; Waterworth, D.; et al. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J. Clin. Endocrinol. Metab. 2016, 101, 945–952. [Google Scholar] [CrossRef]
- Yari, Z.; Fotros, D.; Hekmatdoost, A. Comparison of cardiometabolic risk factors between obese and non-obese patients with nonalcoholic fatty liver disease. Sci. Rep. 2023, 13, 14531. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Arrese, M.; Barrera, F.; Triantafilo, N.; Arab, J.P. Concurrent nonalcoholic fatty liver disease and type 2 diabetes: Diagnostic and therapeutic considerations. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 849–866. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.S.; Hooper, A.J.; Sullivan, D.R.; Burnett, J.R. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology 2019, 51, 148–154. [Google Scholar] [CrossRef]
- Wadström, B.N.; Wulff, A.B.; Pedersen, K.M.; Jensen, G.B.; Nordestgaard, B.G. Elevated remnant cholesterol increases the risk of peripheral artery Disease, Myocardial Infarction, and Ischaemic Stroke: A cohort-based study. Eur. Heart J. 2022, 43, 3258–3269. [Google Scholar] [CrossRef]
- Huang, H.; Guo, Y.; Liu, Z.; Zeng, Y.; Chen, Y.; Xu, C. Remnant cholesterol predicts long-term mortality of patients with metabolic dysfunction-associated fatty Liver Disease. J. Clin. Endocrinol. Metab. 2022, 107, e3295–e3303. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Roh, E.; Lee, S.J.; Ihm, S.H.; Han, K.D.; Kang, J.G. Remnant cholesterol is an Independent predictor of type 2 Diabetes: A Nationwide Population-based Cohort Study. Diabetes Care 2022, 46, 305–312. [Google Scholar] [CrossRef]
- Zou, Y.; Kuang, M.; Zhong, Y.; Jiang, C. Remnant cholesterol can identify individuals at higher risk of metabolic syndrome in the general population. Sci. Rep. 2023, 13, 5957. [Google Scholar] [CrossRef]
- Huang, H.; Xie, J.; Zeng, Y.; Liu, Z.; Miao, M.; Xu, L.; Xu, C. Remnant Cholesterol Independently Predicts the Development of Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2023, 108, 2907–2915. [Google Scholar] [CrossRef]
- Alsharoh, H.; Ismaiel, A.; Leucuta, D.C.; Popa, S.L.; Dumitrascu, D.L. Plasminogen Activator Inhibitor-1 Levels in Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. J. Gastrointestin. Liver Dis. 2022, 31, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Klisic, A.; Kocic, G.; Kavaric, N.; Pavlovic, R.; Soldatovic, I.; Ninic, A. Nitric Oxide Products are not Associated with Metabolic Syndrome. J. Med. Biochem. 2019, 38, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, L.; Atochin, D.N.; Fattakhov, N.; Vasilenko, M.; Zatolokin, P.; Kirienkova, E. Nitric oxide and mitochondria in metabolic syndrome. Front. Physiol. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total | MetSy Group (n = 65) | Control Group (n = 65) | p-Value |
|---|---|---|---|---|
| Age (years) | 52.2 ± 8.2 | 53.9 ± 8.4 | 50.4 ± 7.7 | 0.015 |
| Male gender (n, %) | 71 (54.6%) | 39 (60.0%) | 33 (49.2%) | 0.218 |
| MetSy components, n (%) | ||||
| 33 (25.4%) | 0 | 33 (25.4%) | |
| 10 (7.7%) | 0 | 10 (7.7%) | |
| 22 (16.9%) | 0 | 22 (16.9%) | |
| 21 (16.2%) | 21 (16.2%) | 0 | |
| 24 (18.5%) | 24 (18.5%) | 0 | |
| 20 (15.4%) | 20 (15.4%) | 0 | |
| Hypertension, n (%) | 55 (42.3%) | 49 (76.0%) | 6 (9.2%) | <0.001 |
| Hyperlipidemia, n (%) | 96 (74.0%) | 65 (98.0%) | 32 (49.0%) | <0.001 |
| Diabetes mellitus, n (%) | 20 (15.4%) | 19 (29.0%) | 1 (1.5%) | <0.001 |
| Smoking, n (%) | 33 (25.4%) | 19 (29.2%) | 14 (21.5%) | 0.314 |
| Risk factors, n (%) | 2.1 ± 1.3 | 3.0 ± 0.9 | 1.1 ± 1.0 | <0.001 |
| Obesity, n (%) | 44 (34.0%) | 34 (52.0%) | 11 (16.9%) | <0.001 |
| Body weight (kg), n (%) | 86.2 ± 20.5 | 94.4 ± 16.5 | 78.0 ± 20.8 | <0.001 |
| WC (cm), n (%) | 98.9 ± 18.6 | 108.7 ± 13.5 | 89.1 ± 17.9 | <0.001 |
| HC (cm), n (%) | 107.5 ± 12.1 | 112.7 ± 10.9 | 102.2 ± 10.9 | <0.001 |
| Waist-to-hip ratio, (mean ± SD) | 0.91 ± 0.11 | 0.96 ± 0.09 | 0.87 ± 0.11 | <0.001 |
| BMI (kg/m2), (mean ± SD) | 28.41 ± 5.56 | 31.06 ± 4.52 | 25.76 ± 10.94 | <0.001 |
| BSA, (mean ± SD) | 1.99 ± 0.29 | 2.10 ± 0.22 | 1.89 ± 0.32 | <0.001 |
| SCORE, (mean ± SD) | 3.88 ± 3.11 | 3.88 ± 3.11 | 1.45 ± 1.28 | <0.001 |
| SCORE 2, (mean ± SD) | 9.98 ± 5.34 | 12.53 ± 4.94 | 7.44 ± 4.47 | <0.001 |
| Characteristics | I Group MetSy BMI ≥ 30 kg/m2 (N = 32) | II Group MetSy BMI < 30 kg/m2 (N = 33) | III Group, Control (N = 65) | p-Value |
|---|---|---|---|---|
| Age (years), (mean ± SD) | 54.5 ± 8.7 | 53.4 ± 8.0 | 50.4 ± 7.7 | I:II n.s. I:III p < 0.01 II:III p < 0.01 |
| Male gender, n (%) | 19 (59%) | 20 (69%) | 33 (49.2%) | I:II n.s. I:III n.s. II:III n.s |
| BMI (kg/m2), (mean ± SD) | 34.44 ± 3.98 | 27.78 ± 1.72 | 25.76 ± 10.94 | I:II p <0.001 I:III p < 0.001 II:III p < 0.001 |
| BSA (m2), (mean ± SD) | 2.19 ± 0.18 | 1.99 ± 0.22 | 1.89 ± 0.32 | I:II p <0.001 I:III p < 0.001 II:III p < 0.01 |
| Waist-to-hip ratio, (mean ± SD) | 0.99 ± 0.10 | 0.94 ± 0.08 | 0.87 ± 0.11 | I:II p < 0.05 I:III p < 0.001 II:III p < 0.001 |
| Parameter | I Group MetSy BMI ≥ 30 kg/m2 (N = 32) | II Group MetSy BMI < 30 kg/m2 (N = 33) | III Group, Control (N = 65) | p-Value |
|---|---|---|---|---|
| Glycemia (mmol/L) | 7.24 ± 1.80 | 6.04 ± 0.90 | 5.56 ± 0.55 | I:II p < 0.01 I:III p < 0.001 II:III p < 0.01 |
| HbA1c (%) | 6.53 ± 1.35 | 5.84 ± 1.38 | 4.72 ± 1.04 | I:II p < 0.05 I:III p < 0.001 II:III p < 0.001 |
| Total cholesterol (mmol/L) | 6.87 ± 1.59 | 6.83 ± 1.18 | 6.16 ± 1.03 | I:II n.s. I:III p < 0.05 II:III p < 0.05 |
| LDL cholesterol (mmol/L) | 4.55 ± 1.07 | 4.53 ± 1.14 | 4.04 ± 0.98 | I:II n.s. I:III p < 0.05 II:III p < 0.05 |
| HDL cholesterol (mmnol/L) | 1.13 ± 0.29 | 1.17 ± 0.24 | 1.57 ± 0.37 | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| Non-HDL cholesterol (mmnol/L) | 5.74 ± 1.50 | 5.66 ± 1.12 | 4.51 ± 1.08 | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| Remnant cholesterol (mmnol/L) | 1.29 ± 0.99 | 1.14 ± 0.38 | 0.55 ± 0.31 | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| Triglycerides (mmnol/L) | 3.79 ± 2.84 | 2.75 ± 1.20 | 1.24 ± 0.66 | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| Uric acid (mmol/L) | 385.07 ± 95.42 | 356.43 ± 74.76 | 269.19 ± 93.90 | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| Creatinine (µmol/L) | 89.30 ± 15.13 | 93.08 ± 16.15 | 82.15 ± 12.35 | I:II n.s. I:III p < 0.01 II:III p < 0.001 |
| Creatinine clearance (mL/min) | 109.53 ± 26.25 | 91.82 ± 22.06 | 95.87 ± 28.76 | I:II p < 0.05 I:III p < 0.001 II:III n.s. |
| NO metabolites (µmol/L) | 16.34 ± 11.73 | 14.12 ± 10.88 | 33.85 ± 30.09 | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| iNOS (pg/mL) | 115.93 ± 33.19 | 130.10 ± 35.84 | 77.78 ± 50.10 | I:II p < 0.01 I:III p < 0.001 II:III p < 0.05 |
| Ox-LDL-Chol | 1263.08 ± 326.57 | 1272.62 ± 430.60 | 954.37 ± 336.50 | I:II n.s. I:III p < 0.001 II:III p < 0.01 |
| PAI-1 (ng/mL) | 171.21 ± 48.85 | 149.21 ± 74.67 | 88.25 ± 58.90 | I:II n.s. I:III p < 0.001 II:III p < 0.01 |
| CRP (mg/L) | 3.39 ± 6.72 | 2.02 ± 4.94 | 1.63 ± 2.63 | I:II n.s. I:III n.s. II:III n.s. |
| Parameter | I Group MetSy BMI ≥ 30 kg/m2 (N = 32) | II Group MetSy BMI < 30 kg/m2 (N = 33) | III Group, Control (N = 65) | p-Value |
|---|---|---|---|---|
| NAFLD, n (%) | 32 (100%) | 23 (69.7%) | 12 (18.5%) | I:II p < 0.001 I:III p < 0.001 II:III p < 0.001 |
| FLI, Me (IQR) | 95.0 (87.0–98.0) | 76.5 (49.7–84.0) | 23.0 (6.0–54.0) | I:II p < 0.001 I:III p < 0.001 II:III p < 0.001 |
| IMC thickness (mm), (mean ± SD) | 0.82 ± 0.22 | 0.77 ± 0.19 | 0.64 ± 0.12 | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| Presence of carotid plaques, n (%) | 17 (53%) | 22 (66.7%) | 13 (20%) | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| Number of carotid plaques, Me (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.0 (0.0–0.0) | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| Carotid stenosis, %, Me (IQR) | 30.0 (0.0–35.0) | 29.0 (0.0–40.0) | 0.0 (0.0–0.0) | I:II n.s. I:III p < 0.001 II:III p < 0.001 |
| Bilateral carotid plaques, n (%) | 13 (40%) | 10 (30%) | 5 (7.7%) | I:II n.s. I:III p < 0.001 II:III p < 0.01 |
| LVMI (g/m2), Me (IQR) | 91.3 (79.6–108.0) | 77.5 (71.2–100.0) | 84.7 (68.9–97.2) | I:II n.s. I:III p < 0.01 II:III n.s. |
| Diastolic dysfunction, n (%) | 15 (46.8%) | 14 (42%) | 10 (15%) | I:II n.s. I:III p < 0.001 II:III p < 0.01 |
| E/a < 0.8, n (%) | 11 (34.4%) | 10 (30.3%) | 6 (9.2%) | I:II n.s. I:III p < 0.01 II:III p < 0.01 |
| E/a 0.8–2.0, n (%) | 21 (65.6%) | 23 (69.7%) | 59 (90.8%) | |
| DP (mmHg/min), Me (IQR) | 10,920.0 (9750.0–11,960.0) | 10,070.0 (8927.5–12,340.0) | 10,300.0 (9367.5–12,180.0) | I:II n.s. I:III n.s. II:III n.s. |
| Characteristics | OR (95% CI) | p-Value |
|---|---|---|
| Age (years) | 1.111 (0.984–1.254) | 0.090 |
| Male gender | 0.587 (0.052–6.675) | 0.668 |
| Hypertension | 7.943 (1.032–61.130) | 0.047 |
| Waist-to hip ratio 0.88–0.97 * | 24.997 (1.963–318.172) | 0.013 |
| Waist-to-hip ratio 0.98–1.16 * | 73.117 (4.250–1259.936) | 0.003 |
| NO | 0.954 (0.897–1.015) | 0.136 |
| iNOS | 0.992 (0.974–1.011) | 0.399 |
| PAI-1 | 1.025 (1.006–1.043) | 0.008 |
| Remnant cholesterol | 102.436 (6.565–1598.297) | 0.001 |
| Characteristics | OR (95% CI) | p Value |
|---|---|---|
| Age (years) | 1.123 (1.053–1.197) | <0.001 |
| Male gender | 1.460 (0.422–5.048) | 0.550 |
| Hypertension | 7.127 (1.865–27.238) | 0.004 |
| Waist-to-hip ratio 0.88–0.97 * | 5.134 (1.126–23.420) | 0.035 |
| Waist-to-hip ratio 0.98–1.16 * | 4.238 (1.702–10.554) | 0.002 |
| NO | 0.611 (0.167–2.243) | 0.458 |
| iNOS | 0.561 (0.193–1.634) | 0.290 |
| PAI-1 | 1.123 (1.053–1.197) | <0.001 |
| Remnant cholesterol | 1.460 (0.422–5.048) | 0.550 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, S.; Tasić, I.; Stojanović, N.; Rakočević, J.; Deljanin Ilić, M.; Đorđević, D.; Stoičkov, V.; Tasić, I. Impact of Obesity on Target Organ Damage in Patients with Metabolic Syndrome. Diagnostics 2024, 14, 1569. https://doi.org/10.3390/diagnostics14141569
Kostić S, Tasić I, Stojanović N, Rakočević J, Deljanin Ilić M, Đorđević D, Stoičkov V, Tasić I. Impact of Obesity on Target Organ Damage in Patients with Metabolic Syndrome. Diagnostics. 2024; 14(14):1569. https://doi.org/10.3390/diagnostics14141569
Chicago/Turabian StyleKostić, Svetlana, Ivan Tasić, Nikola Stojanović, Jelena Rakočević, Marina Deljanin Ilić, Dragan Đorđević, Viktor Stoičkov, and Isidora Tasić. 2024. "Impact of Obesity on Target Organ Damage in Patients with Metabolic Syndrome" Diagnostics 14, no. 14: 1569. https://doi.org/10.3390/diagnostics14141569
APA StyleKostić, S., Tasić, I., Stojanović, N., Rakočević, J., Deljanin Ilić, M., Đorđević, D., Stoičkov, V., & Tasić, I. (2024). Impact of Obesity on Target Organ Damage in Patients with Metabolic Syndrome. Diagnostics, 14(14), 1569. https://doi.org/10.3390/diagnostics14141569







