Hybrid Positron Emission Tomography and Magnetic Resonance Imaging Guided Microsurgical Management of Glial Tumors: Case Series and Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
3. Results
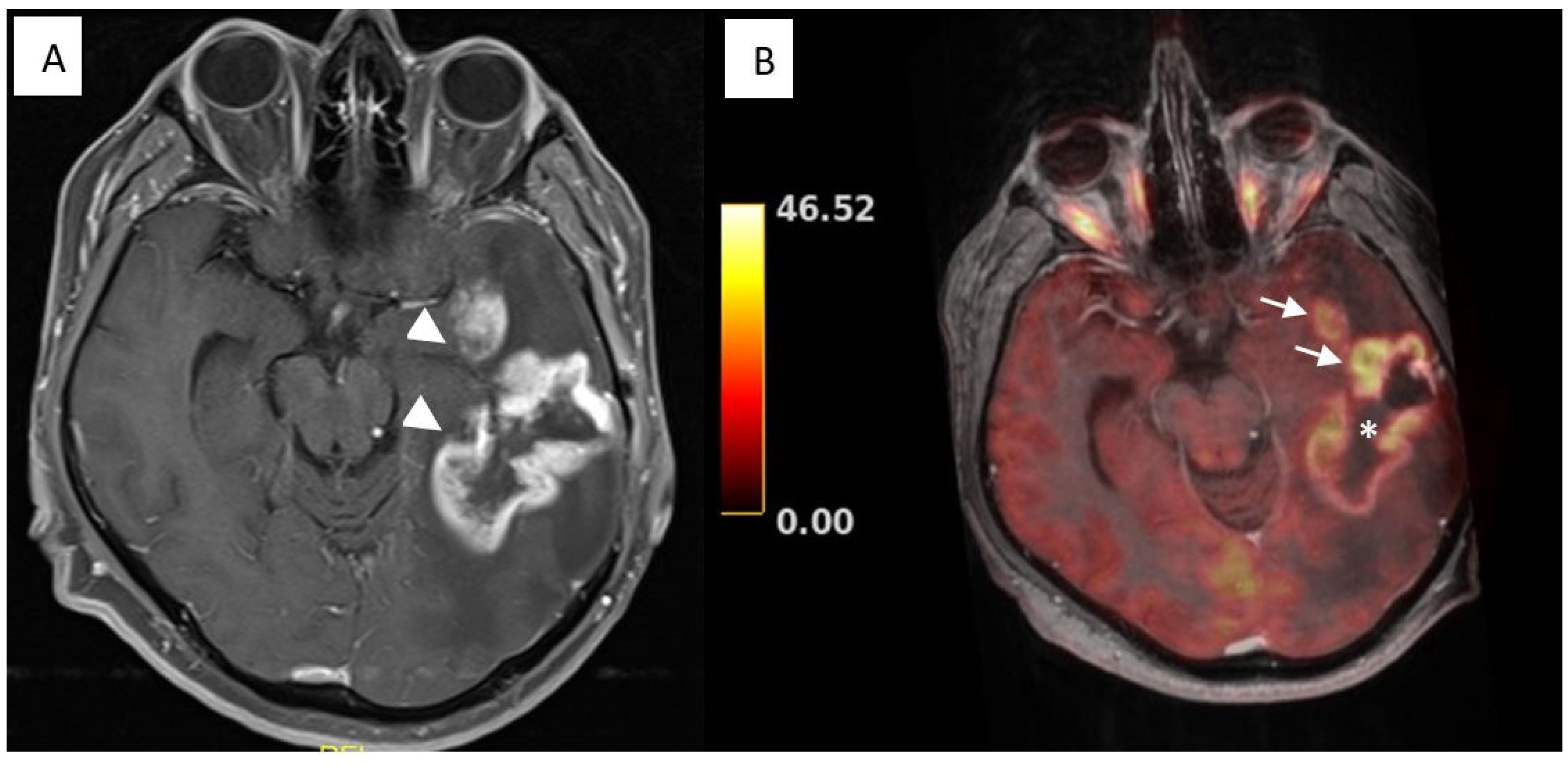
3.1. Illustrative Cases: Case 3
3.2. Illustrative Cases: Case 5
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.; Barnholtz-Sloan, J.S. Epidemiology of Intracranial Gliomas. Prog. Neurol. Surg. 2018, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.M. Epidemiology of Cancer. Clin. Chem. 2024, 70, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- McKinney, P.A. Brain tumours: Incidence, survival, and aetiology. J. Neurol. Neurosurg. Psychiatry 2004, 75, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.M.; Nabavi, A.; Mehdorn, H.M.; Blömer, U. Glioblastoma multiforme: Report of 267 cases treated at a single institution. Surg. Neurol. 2005, 63, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Mineo, J.F.; Bordron, A.; Baroncini, M.; Ramirez, C.; Maurage, C.A.; Blond, S.; Dam-Hieu, P. Prognosis factors of survival time in patients with glioblastoma multiforme: A multivariate analysis of 340 patients. Acta Neurochir. 2007, 149, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Song, K.; Wu, S.; Hameed, N.U.F.; Kudulaiti, N.; Xu, H.; Qin, Z.Y.; Wu, J.S. The prognosis of glioblastoma: A large, multifactorial study. Br. J. Neurosurg. 2021, 35, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Tempany, C.M.C.; Jayender, J.; Kapur, T.; Bueno, R.; Golby, A.; Agar, N.; Jolesz, F.A. Multimodal imaging for improved diagnosis and treatment of cancers. Cancer 2015, 121, 817–827. [Google Scholar] [CrossRef]
- Boellaard, R.; Quick, H.H. Current Image Acquisition Options in PET/MR. Semin. Nucl. Med. 2015, 45, 192–200. [Google Scholar] [CrossRef]
- Smits, M. MRI biomarkers in neuro-oncology. Nat. Rev. Neurol. 2021, 17, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Rao, P. Role of MRI in paediatric neurooncology. Eur. J. Radiol. 2008, 68, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.S.; Hawkins-Daarud, A.; Wang, L.; Li, J.; Swanson, K.R. Imaging of intratumoral heterogeneity in high-grade glioma. Cancer Lett. 2020, 477, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Peca, C.; Pacelli, R.; Elefante, A.; Del Basso De Caro, M.L.; Vergara, P.; Mariniello, G.; Giamundo, A.; Maiuri, F. Early clinical and neuroradiological worsening after radiotherapy and concomitant temozolomide in patients with glioblastoma: Tumour progression or radionecrosis? Clin. Neurol. Neurosurg. 2009, 111, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; van den Bent, M.J.; Smits, M. Pseudoprogression of brain tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.W.; Westerlaan, H.E.; Holtman, G.A.; Aden, K.M.; van Laar, P.J.; van der Hoorn, A. Incidence of Tumour Progression and Pseudoprogression in High-Grade Gliomas: A Systematic Review and Meta-Analysis. Clin. Neuroradiol. 2018, 28, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.L.; O’Neal, C.M.; Andrews, B.J.; Westrup, A.M.; Battiste, J.D.; Glenn, C.A. A systematic review of the utility of amino acid PET in assessing treatment response to bevacizumab in recurrent high-grade glioma. Neuro-Oncology Adv. 2021, 3, vdab003. [Google Scholar] [CrossRef] [PubMed]
- van de Weijer, T.; Broen, M.P.G.; Moonen, R.P.M.; Hoeben, A.; Anten, M.; Hovinga, K.; Compter, I.; van der Pol, J.A.J.; Mitea, C.; Lodewick, T.M. The Use of 18F-FET-PET-MRI in Neuro-Oncology: The Best of Both Worlds—A Narrative Review. Diagnostics 2022, 12, 1202. [Google Scholar] [CrossRef]
- Shah, R.; Vattoth, S.; Jacob, R.; Manzil, F.F.P.; O’Malley, J.P.; Borghei, P.; Patel, B.N.; Curé, J.K. Radiation Necrosis in the Brain: Imaging Features and Differentiation from Tumor Recurrence. RadioGraphics. 2012, 32, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Puttick, S.; Bell, C.; Dowson, N.; Rose, S.; Fay, M. PET, MRI, and simultaneous PET/MRI in the development of diagnostic and therapeutic strategies for glioma. Drug. Discov. Today 2015, 20, 306–316. [Google Scholar] [CrossRef]
- Aiello, M.; Cavaliere, C.; Marchitelli, R.; D’Albore, A.; De Vita, E.; Salvatore, M. Hybrid PET/MRI Methodology. Int. Rev. Neurobiol. 2018, 141, 97–128. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, U.; Herzog, H. Does PET/MR in human brain imaging provide optimal co-registration? A critical reflection. Magn. Reason. Mater. Phys. Biol. Med. 2013, 26, 137–147. [Google Scholar] [CrossRef]
- Preuss, M.; Werner, P.; Barthel, H.; Nestler, U.; Christiansen, H.; Hirsch, F.W.; Fritzsch, D.; Hoffmann, K.T.; Bernhard, M.K.; Sabri, O. Integrated PET/MRI for planning navigated biopsies in pediatric brain tumors. Child. Nerv. Syst. 2014, 30, 1399–1403. [Google Scholar] [CrossRef]
- Jena, A.; Taneja, S.; Gambhir, A.; Mishra, A.K.; D’souza, M.M.; Verma, S.M.; Hazari, P.P.; Negi, P.; Jhadav, G.K.; Sogani, S.K. Glioma Recurrence Versus Radiation Necrosis. Clin. Nucl. Med. 2016, 41, e228–e236. [Google Scholar] [CrossRef]
- Sogani, S.; Jena, A.; Taneja, S.; Gambhir, A.; Mishra, A.; D’Souza, M.; Verma, S.M.; Hazari, P.P.; Negi, P.; Jadhav, G.K. Potential for differentiation of glioma recurrence from radionecrosis using integrated 18F-fluoroethyl-L-tyrosine (FET) positron emission tomography/magnetic resonance imaging: A prospective evaluation. Neurol. India 2017, 65, 293. [Google Scholar] [CrossRef]
- Pyka, T.; Hiob, D.; Preibisch, C.; Gempt, J.; Wiestler, B.; Schlegel, J.; Straube, C.; Zimmer, C. Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur. J. Radiol. 2018, 103, 32–37. [Google Scholar] [CrossRef]
- Almansory, K.O.; Fraioli, F. Combined PET/MRI in brain glioma imaging. Br. J. Hosp. Med. 2019, 80, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Li, L.; Mu, W.; Wang, Y.; Liu, Z.; Liu, Z.; Wang, Y.; Ma, W.; Kong, Z.; Wang, S.; Zhou, X.; et al. A non-invasive radiomic method using 18F-FDG PET predicts isocitrate dehydrogenase genotype and prognosis in patients with Glioma. Front. Oncol. 2019, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response assessment in neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Meißner, A.K.; Kocher, M.; Bauer, E.K.; Werner, J.M.; Fink, G.R.; Shah, N.J.; Langen, K.J.; Galldiks, N. Feature-based PET/MRI radiomics in patients with brain tumors. Neurooncol. Adv. 2021, 2 (Suppl. S4), iv15–iv21. [Google Scholar] [CrossRef] [PubMed]
- Haubold, J.; Demircioglu, A.; Gratz, M.; Glas, M.; Wrede, K.; Sure, U.; Antoch, G.; Keyvani, K.; Nittka, M.; Kannengiesser, S.; et al. Non-invasive tumor decoding and phenotyping of cerebral gliomas utilizing multiparametric 18F-FET PET-MRI and MR Fingerprinting. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Ning, J.; Xu, B.; Liu, J.; Dang, H.; Lin, M.; Feng, X.; Grimm, R.; Tian, J. Histogram analysis of 11C-methionine integrated PET/MRI may facilitate to determine the O6-methylguanylmethyltransferase methylation status in gliomas. Nucl. Med. Commun. 2019, 40, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Lin, Y.; Jiang, C.; Li, L.; Liu, Z.; Wang, Y.; Dai, C.; Liu, D.; Qin, X.; Wang, Y.; et al. 18F-FDG-PET-based Radiomics signature predicts MGMT promoter methylation status in primary diffuse glioma. Cancer Imaging 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Stoffels, G.; Filss, C.P.; Piroth, M.D.; Sabel, M.; Ruge, M.I.; Herzog, H.; Shah, N.J.; Fink, G.R.; Coenen, H.H.; et al. Role of O-(2-18F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J. Nucl. Med. 2012, 53, 1367–1374. [Google Scholar] [CrossRef]
- Lohmann, P.; Kocher, M.; Ceccon, G.; Bauer, E.K.; Stoffels, G.; Viswanathan, S.; Ruge, M.I.; Neumaier, B.; Shah, N.J.; Fink, G.R.; et al. Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. Neuroimage Clin. 2018, 20, 537–542. [Google Scholar] [CrossRef]
- Wang, K.; Qiao, Z.; Zhao, X.; Li, X.; Wang, X.; Wu, T.; Chen, Z.; Fan, D.; Chen, Q.; Ai, L. Individualized discrimination of tumor recurrence from radiation necrosis in glioma patients using an integrated radiomics-based model. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Minamimoto, R.; Miwa, K. 11C-methionine-PET for differentiating recurrent brain tumor from radiation necrosis: Radiomics approach with random forest classifier. Sci. Rep. 2019, 9, 15666. [Google Scholar] [CrossRef]
- Castello, A.; Albano, D.; Muoio, B.; Castellani, M.; Panareo, S.; Rizzo, A.; Treglia, G.; Urso, L. Diagnostic Accuracy of PET with 18F-Fluciclovine ([18F]FACBC) in Detecting High-Grade Gliomas: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 3610. [Google Scholar] [CrossRef]
- Karlberg, A.; Pedersen, L.K.; Vindstad, B.E.; Skjulsvik, A.J.; Johansen, H.; Solheim, O.; Skogen, K.; Kvistad, K.A.; Bogsrud, T.V.; Myrmel, K.S.; et al. Diagnostic accuracy of anti-3-[18F]-FACBC PET/MRI in gliomas. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Beattie, B.J.; Akhurst, T.; Dunphy, M.; Zanzonico, P.; Finn, R.; Mauguen, A.; Schöder, H.; Weber, W.A.; Lassman, A.B.; et al. 18F-Fluciclovine (18F-FACBC) PET imaging of recurrent brain tumors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-M.; Nie, Q.; Wang, R.-M.; Chang, S.M.; Zhao, W.-R.; Zhu, Q.; Liang, Y.K.; Yang, P.; Zhang, J.; Jia, H.W.; et al. 11C-CHO PET in optimization of target volume delineation and treatment regimens in postoperative radiotherapy for brain gliomas. Nucl. Med. Biol. 2012, 39, 437–442. [Google Scholar] [CrossRef]
- Schwenck, J.; Tabatabai, G.; Skardelly, M.; Reischl, G.; Beschorner, R.; Pichler, B.; la Fougère, C. In vivo visualization of prostate-specific membrane antigen in glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Akgun, E.; Akgun, M.Y.; Selçuk, H.H.; Uzan, M.; Sayman, H.B. PSMA PET/MR in the differentiation of low and high grade gliomas: Is 68Ga PSMA PET/MRI useful to detect brain gliomas? Eur. J. Radiol. 2020, 130, 109199. [Google Scholar] [CrossRef] [PubMed]
- Muoio, B.; Albano, D.; Dondi, F.; Bertagna, F.; Garibotto, V.; Kunikowska, J.; Piccardo, A.; Annunziata, S.; Espeli, V.; Migliorini, D.; et al. Diagnostic Accuracy of PET/CT or PET/MRI Using PSMA-Targeting Radiopharmaceuticals in High-Grade Gliomas: A Systematic Review and a Bivariate Meta-Analysis. Diagnostics 2022, 12, 1665. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, G.; Ma, X.; Wang, S.; Zhao, X.; Zhang, W.; Yang, W.; Wang, J. PET/CT using 68Ga-PSMA-617 versus 18F-fluorodeoxyglucose to differentiate low- and high-grade gliomas. J. Neuroimaging 2021, 31, 733–742. [Google Scholar] [CrossRef]
- Brighi, C.; Puttick, S.; Woods, A.; Keall, P.; Tooney, P.A.; Waddington, D.E.J.; Sproule, V.; Rose, S.; Fay, M. Comparison between [68Ga]Ga-PSMA-617 and [18F]FET PET as Imaging Biomarkers in Adult Recurrent Glioblastoma. Int. J. Mol. Sci. 2023, 24, 16208. [Google Scholar] [CrossRef] [PubMed]
- Overcast, W.B.; Davis, K.M.; Ho, C.Y.; Hutchins, G.D.; Green, M.A.; Graner, B.D.; Veronesi, M.C. Advanced imaging techniques for neuro-oncologic tumor diagnosis, with an emphasis on PET-MRI imaging of malignant brain tumors. Curr. Oncol. Rep. 2021, 23, 34. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma, a randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Vincenzo Barbagallo, G.M.; Certo, F.; Di Gregorio, S.; Maione, M.; Garozzo, M.; Peschillo, S.; Altieri, R. Recurrent high-grade glioma surgery, a multimodal intraoperative protocol to safely increase extent of tumor resection and analysis of its impact on patient outcome. Neurosurg. Focus. 2021, 50, E20. [Google Scholar] [CrossRef] [PubMed]

| Case | Age (Years) | Sex | Histological Diagnosis of Primary Tumor | Localization | Size (cm) | Tracer | Surgery |
|---|---|---|---|---|---|---|---|
| 1 | 59 | M | Glioblastoma | L Frontal | 2.3 × 3.5 × 2.8 | FDG | None |
| 2 | 48 | M | Diffuse Astrocytoma | L Frontal | 6.9 × 4.8 × 4.6 | FDG | Gross total resection |
| 3 | 58 | F | Glioblastoma | L Frontoparietal | 3.0 × 3.2 × 3.4 | FDG | Gross total resection |
| 4 | 45 | M | Gemistocytic Astrocytoma | L Frontoparietal | 2.5 × 2.0 × 1.9 | FDG | Gross total resection |
| 5 | 53 | M | Glioblastoma | L Temporal | 2.0 × 2.0 × 2.3 | FDG | Gross total resection |
| 6 | 47 | F | Oligodendroglioma | R Temporal | 3.3 × 3.4 × 2.6 | FDG | Gross total resection |
| 7 | 28 | M | Glioblastoma | R Hippocampus | 6.0 × 4.5 × 4.5 | FDG | Gross total resection |
| 8 | 63 | F | Glioblastoma | L Frontoparietal | 5.4 × 4.7 × 3.7 | FDG | Gross total resection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caglar, Y.S.; Buyuktepe, M.; Sayaci, E.Y.; Dogan, I.; Bozkurt, M.; Peker, E.; Soydal, C.; Ozkan, E.; Kucuk, N.O. Hybrid Positron Emission Tomography and Magnetic Resonance Imaging Guided Microsurgical Management of Glial Tumors: Case Series and Review of the Literature. Diagnostics 2024, 14, 1551. https://doi.org/10.3390/diagnostics14141551
Caglar YS, Buyuktepe M, Sayaci EY, Dogan I, Bozkurt M, Peker E, Soydal C, Ozkan E, Kucuk NO. Hybrid Positron Emission Tomography and Magnetic Resonance Imaging Guided Microsurgical Management of Glial Tumors: Case Series and Review of the Literature. Diagnostics. 2024; 14(14):1551. https://doi.org/10.3390/diagnostics14141551
Chicago/Turabian StyleCaglar, Yusuf Sukru, Murat Buyuktepe, Emre Yagiz Sayaci, Ihsan Dogan, Melih Bozkurt, Elif Peker, Cigdem Soydal, Elgin Ozkan, and Nuriye Ozlem Kucuk. 2024. "Hybrid Positron Emission Tomography and Magnetic Resonance Imaging Guided Microsurgical Management of Glial Tumors: Case Series and Review of the Literature" Diagnostics 14, no. 14: 1551. https://doi.org/10.3390/diagnostics14141551
APA StyleCaglar, Y. S., Buyuktepe, M., Sayaci, E. Y., Dogan, I., Bozkurt, M., Peker, E., Soydal, C., Ozkan, E., & Kucuk, N. O. (2024). Hybrid Positron Emission Tomography and Magnetic Resonance Imaging Guided Microsurgical Management of Glial Tumors: Case Series and Review of the Literature. Diagnostics, 14(14), 1551. https://doi.org/10.3390/diagnostics14141551






