A Comprehensive Multidisciplinary Approach to Diagnosing Chronic Inflammatory Bowel Diseases: Integration of Clinical, Endoscopic, and Imaging Modalities
Abstract
1. Introduction
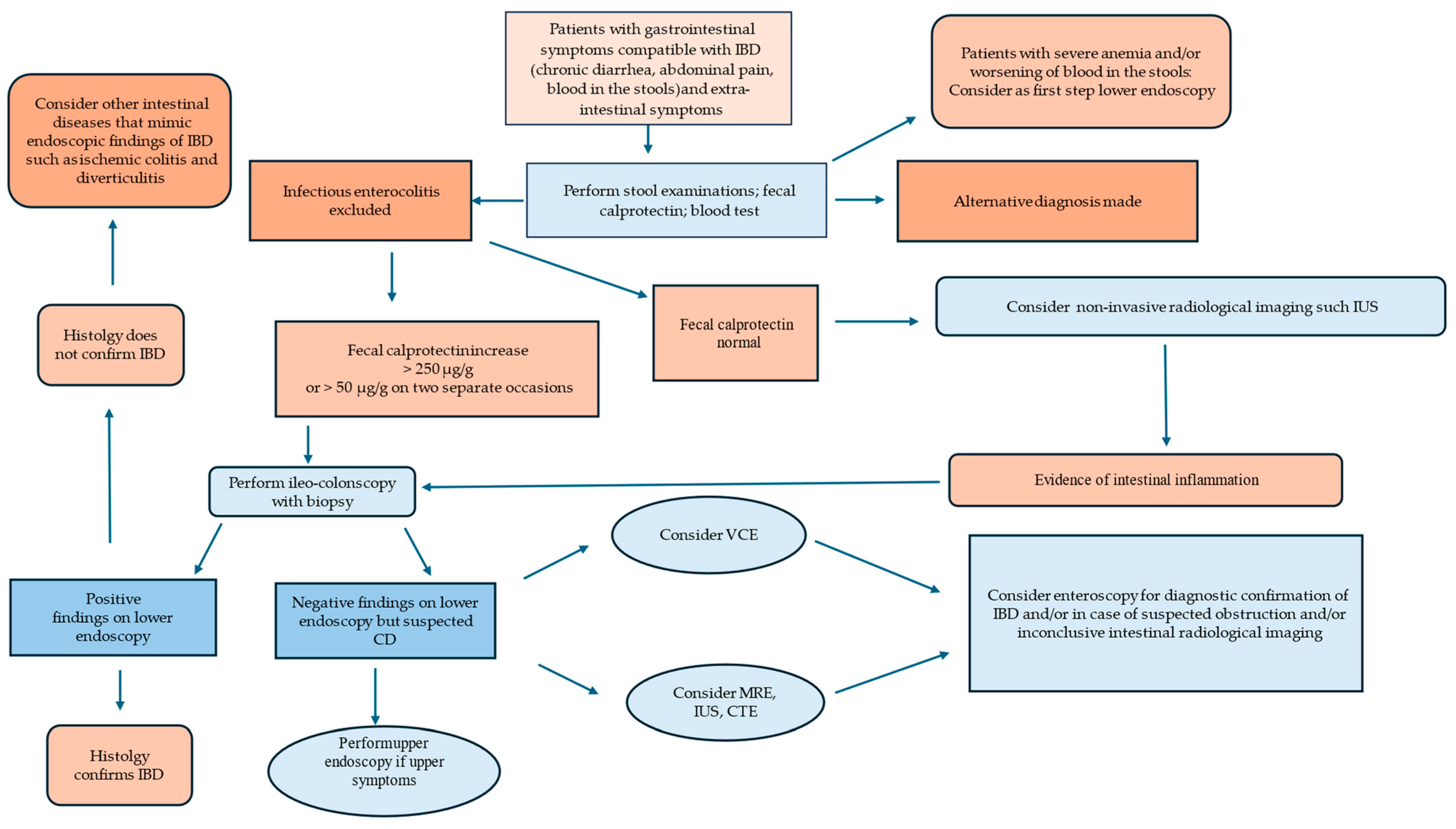
2. Clinical Approach
Biomarkers
3. Endoscopy
4. Histological Diagnosis
4.1. Crypt Architectural
4.2. Lamina Propria Cellularity: Chronic and Acute Inflammation and Epithelioid Cell Granuloma
4.3. Epithelial Abnormalities
5. New Advance Endoscopic Techniques
6. Wireless Video Capsule Endoscopy and Enteroscopy
7. Advanced Imaging Techniques
8. Future Directions—Pre-Clinical Diagnosis of Inflammatory Bowel Disease
9. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Nunes, P.B.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007, 117, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Garrone, A.; Bertolino, C.; Vanni, R.; Bretto, E.; Poshnjari, A.; Tribocco, E.; Frara, S.; Armandi, A.; Astegiano, M.; et al. Epidemiology of Inflammatory Bowel Diseases: A Population Study in a Healthcare District of North-West Italy. J. Clin. Med. 2023, 13, 641. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Wu, J. Meta-analysis of the association between appendiceal orifice inflammation and appendectomy and ulcerative colitis. Rev. Esp. Enferm. Dig. 2016, 108, 401–410. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Jackson, T.; Sands, B.E.; Frisch, M.; Andersson, R.E.; Korzenik, J. The risk of developing Crohn’s disease after an appendectomy: A meta-analysis. Am. J. Gastroenterol. 2008, 103, 2925–2931. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.Y.; Blanchard, J.F.; Bernstein, C.N. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 2687–2692. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental risk factors for inflammatory bowel diseases: An umbrella review of meta-analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef]
- Dignass, A.; Eliakim, R.; Magro, F.; Maaser, C.; Chowers, Y.; Geboes, K.; Mantzaris, G.; Reinisch, W.; Colombel, J.F.; Vermeire, S. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: Definitions and diagnosis. J. Crohn’s Colitis 2012, 6, 965–990. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; Dignass, A.; Panes, J.; Beaugerie, L.; Karagiannis, J.; Allez, M.; Ochsenkühn, T.; Orchard, T.; Rogler, G.; Louis, E.; et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J. Crohn’s Colitis 2010, 4, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Holubar, S.; Rieder, F. Fibrostenotic strictures in Crohn’s disease. Intest. Res. 2020, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350.e6. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.J.; Ford, A.C. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: Systematic review and meta-analysis. Am. J. Gastroenterol. 2012, 107, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Bruce, E.S. From Symptom to Diagnosis: Clinical Distinctions Among Various Forms of Intestinal Inflammation. Gastroenterology 2004, 126, 1518–1532. [Google Scholar]
- Zeitz, J.; Ak, M.; Müller-Mottet, S.; Scharl, S.; Biedermann, L.; Fournier, N.; Frei, P.; Pittet, V.; Scharl, M.; Fried, M.; et al. Pain in IBD Patients: Very Frequent and Frequently Insufficiently Taken into Account. PLoS ONE 2016, 11, e0156666. [Google Scholar] [CrossRef] [PubMed]
- Agha, F.P.; Ghahremani, G.G.; Panella, J.S.; Kaufman, M.W. Appendicitis as the initial manifestation of Crohn’s disease: Radiologic features and prognosis. AJR Am. J. Roentgenol. 1987, 149, 515–518. [Google Scholar] [CrossRef]
- Han, H.; Kim, H.; Rehman, A.; Jang, S.M.; Paik, S.S. Appendiceal Crohn’s disease clinically presenting as acute appendicitis. World J. Clin. Cases 2014, 2, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Baumgart, D.C.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohn’s Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Acosta, M.B.-D.; Borberg, K.; Burisch, J.; De Vos, M.; De Vries, A.-M.; Dick, A.D.; et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J. Crohn’s Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Prenzel, F.; Uhlig, H.H. Frequency of indeterminate colitis in children and adults with IBD—A metaanalysis. J. Crohn’s Colitis. 2009, 3, 277–281. [Google Scholar] [CrossRef]
- Burakoff, R.J. Indeterminate colitis: Clinical spectrum of disease. Clin. Gastroenterol. 2004, 38 (Suppl. S1), S41–S43. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Gomes, C.; Jensen, C.B.; Agrawal, M.; Ribeiro-Mourão, F.; Jess, T.; Colombel, J.F.; Allin, K.H.; Burisch, J. Risk Factors for Developing Inflammatory Bowel Disease within and across Families with a Family History of IBD. J. Crohn’s Colitis 2023, 27, 30–36. [Google Scholar] [CrossRef]
- Eder, P.; Verstock, B.; Culver, E.; Dragoni, G.; Kredel, L.I.; Wypych, J.; de Paredes, A.G.G.; Kaniewska, M.; Leibovitzh, H.; Lobaton, T.; et al. Autoimmune Pancreatitis in Patients with Inflammatory Bowel Disease: A Real-World Multicentre Collaborative ECCO CONFER Study. J. Crohn’s Colitis 2023, 24, 1791–1799. [Google Scholar] [CrossRef]
- Menees, S.B.; Powell, C.; Kurlander, J.; Goel, A.; Chey, W.D. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am. J. Gastroenterol. 2015, 110, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Dajti, E.; Frazzoni, L.; Iascone, V.; Secco, M.; Vestito, A.; Fuccio, L.; Eusebi, L.H.; Fusaroli, P.; Rizzello, F.; Calabrese, C.; et al. Systematic review with meta-analysis: Diagnostic performance of faecal calprotectin in distinguishing inflammatory bowel disease from irritable bowel syndrome in adults. Aliment. Pharmacol. Ther. 2023, 58, 1120–1131. [Google Scholar] [CrossRef]
- Colugo, J.; Colwill, M.; Pollok, A.P.R.; Patel, K.; Honap, S. Biomarkers in inflammatory bowel disease: A practical guide. Herap Adv. Gastroenterol. 2024, 9, 17562848241251600. [Google Scholar]
- McFarlane, M.; Chambers, S.; Malik, A.; Lee, B.; Sung, E.; Nwokolo, C.; Waugh, N.; Arasaradnam, R. Clinical outcomes at 12 months and risk of inflammatory bowel disease in patients with an intermediate raised fecal calprotectin: A ‘real-world’ view. BMJ Open 2016, 6, e011041. [Google Scholar] [CrossRef]
- Dróżdż, M.; Biesiada, G.; Pituch, H.; Wultańska, D.; Obuch-Woszczatyński, P.; Piotrowski, M.; Kędzierska, J.; Michalak, M.; Garlicki, A.; Czepiel, J. The level of fecal calprotectin significantly correlates with Clostridium difficile infection severity. Folica Med. Cracov 2019, 59, 53–65. [Google Scholar]
- Eckard, A.R.; Hughes, H.Y.; Hagood, N.L.B.; O’Riordan, M.A.; Labbato, D.; Kosco, J.C.; Scott, S.E.; McComsey, G.A. Fecal calprotectin is elevated in HIV and related to systemic inflammation. J. Acquis. Immune Defic. Syndr. 2021, 86, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Fang, C.B.; Rolim, E.G.; Klug, W.A.; Steinwurz, F.; Rossini, L.G.; Candelária, P.A. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: Correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res. Notes 2009, 2, 221. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Cobo, P.; Salvador-Martín, S.; Velasco, M.; Palomino, L.M.; Clemente, S.; Segarra, O.; Moreno-Álvarez, A.; Fernández-Lorenzo, A.; Pérez-Moneo, B.; Montraveta, M. Polymorphisms indicating risk of inflammatory bowel disease or antigenicity to anti-TNF drugs as biomarkers of response in children. Pharmacol. Res. 2023, 194, 106859. [Google Scholar] [CrossRef] [PubMed]
- Venema, W.T.U.; Voskuil, M.D.; Dijkstra, G.; Weersma, R.K.; Festen, E.A. The genetic background of inflammatory bowel disease: From correlation to causality. J. Pathol. 2017, 241, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Shergill, A.K.; Lightdale, J.R.; Bruining, D.H.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; et al. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. The role of endoscopy in inflammatory bowel disease. Gastrointest. Endosc. 2015, 81, 1101–1121.e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Mowat, C.; Cole, A.; Windsor, A.; Ahmad, T.; Arnott, I.; Driscoll, R.; Mitton, S.; Orchard, T.; Rutter, M.; Younge, L.; et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011, 60, 571–607. [Google Scholar] [CrossRef] [PubMed]
- McHugh, J.B.; Appelman, H.D.; McKenna, B.J. The diagnostic value of endoscopic terminal ileum biopsies. Am. J. Gastroenterol. 2007, 102, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Pera, A.; Bellando, P.; Caldera, D.; Ponti, V.; Astegiano, M.; Barletti, C.; David, E.; Arrigoni, A.; Rocca, G.; Verme, G. Colonoscopy in inflammatory bowel disease. Diagnostic accuracy and proposal of an endoscopic score. Gastroenterology 1987, 92, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Gastroenterology, H.N.A.S.F.P.; America, C.F.O.; Bousvaros, A.; A Antonioli, D.; Colletti, R.B.; Dubinsky, M.C.; Glickman, J.N.; Gold, B.D.; Griffiths, A.M.; Jevon, G.P.; et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: Report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 653–674. [Google Scholar]
- Park, S.H.; Loftus, E.V.; Jr Yang, S.K. Appendiceal skip inflammation and ulcerative colitis. Dig. Dis. Sci. 2014, 59, 2050–2057. [Google Scholar] [CrossRef]
- Anzai, H.; Hata, K.; Kishikawa, J.; Ishii, H.; Yasuda, K.; Otani, K.; Nishikawa, T.; Tanaka, T.; Kiyomatsu, T.; Kawai, K.; et al. Appendiceal orifice inflammation is associated with proximal extension of disease in patients with ulcerative colitis. Colorectal Dis. 2016, 18, O278–O282. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.; Odze, R.D. Rectal sparing and skip lesions in ulcerative colitis: A comparative study of endoscopic and histologic findings in patients who underwent proctocolectomy. Am. J. Surg. Pathol. 2010, 34, 689–696. [Google Scholar] [CrossRef]
- Abdelrazeq, A.S.; Wilson, T.R.; Leitch, D.L.; Lund, J.N.; Leveson, S.H. Ileitis in ulcerative colitis: Is it a backwash? Dis. Colon. Rectum. 2005, 48, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Simpson, P.; Papadakis, K.A. Endoscopic evaluation of patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2008, 14, 1287–1297. [Google Scholar] [CrossRef]
- Goldstein, N.; Dulai, M. Contemporary morphologic definition of backwash ileitis in ulcerative colitis and features that distinguish it from Crohn disease. Am. J. Clin. Pathol. 2006, 126, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.L.; Caviglia, R.; Papparella, L.G.; Cicala, M. Upper gastrointestinal involvement of Crohn’s disease: A prospective study on the role of upper endoscopy in the diagnostic work-up. Dig. Dis. Sci. 2012, 57, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- RFeakins, P.; Nunes, B.; Driessen, A.; Gordon, I.O.; Zidar, N.; Baldin, P.; Christensen, B.; Danese, S.; Herlihy, N.; Iacucci, M.; et al. Definitions of Histological Abnormalities in Inflammatory Bowel Disease: An ECCO Position Paper. J. Crohn’s Colitis 2024, 18, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, C.; Oesterreicher, C.; Angelberger, S. Diagnosing colitis: A prospective study on essential parameters for reaching a diagnosis. Endoscopy 2003, 35, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Feakins, R.M. Inflammatory bowel disease biopsies: Updated British Society of Gastroenterology reporting guidelines British Society of Gastroenterology. J. Clin. Pathol. 2013, 66, 1005–1026. [Google Scholar] [CrossRef]
- Jenkins, D.; Balsitis, M.; Gallivan, S.; Dixon, M.F.; Gilmour, H.M.; Shepherd, N.A.; Theodossi, A.; Williams, G.T. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. Br. Soc. Gastroenterol. Initiat. J. Clin. Pathol. 1997, 50, 93–105. [Google Scholar]
- Levine, D.S.; Haggitt, R.C. Normal histology of the colon. Am. J. Surg. Pathol. 1989, 13, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Hunyady, B.; Mezey, E.; Palkovits, M. Gastrointestinal immunology: Cell types in the lamina propria—A morphological review. Acta Physiol. Hung. 2000, 87, 305–328. [Google Scholar] [PubMed]
- Polydorides, A.D.; Banner, B.F.; Hannaway, P.J.; Yantiss, R.K. Evaluation of site-specific and seasonal variation in colonic mucosal eosinophils. Hum. Pathol. 2008, 39, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.; Belic, L. Rectal biopsy helps to distinguish acute self-limited colitis from idiopathic inflammatory bowel disease. Gastroenterology 1984, 86, 104–113. [Google Scholar] [CrossRef]
- Stange, E.F.; Travis, S.P.L.; Vermeire, S.; Beglinger, C.; Kupcinkas, L.; Geboes, K.; Barakauskiene, A.; Villanacci, V.; Von Herbay, A.; Warren, B.F.; et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. Gut 2006, 55 (Suppl. S1), i1–i15. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.; Haggitt, R.C.; Husseman, M.; McFarland, L.V. Mucosal biopsy diagnosis of colitis: Acute self-limited colitis and idiopathic inflammatory bowel disease. Gastroenterology 1994, 107, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, G.; Kollberg, B.; Sandstedt, B. A prospective study of first attacks of inflammatory bowel disease and infectious colitis. Histologic course during the first year after presentation. Scand. J. Gastroenterol. 1994, 29, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Theodossi, A.; Spiegelhalter, D.J.; Jass, J.; Firth, J.; Dixon, M.; Leader, M.; A Levison, D.; Lindley, R.; Filipe, I.; Price, A. Observer variation and discriminatory value of biopsy features in inflammatory bowel disease. Gut 1994, 35, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.S.; Harrison, R.F. Discriminant histological features in the diagnosis of chronic idiopathic inflammatory bowel disease: Analysis of a large dataset by a novel data visualisation technique. J. Clin. Pathol. 2002, 55, 51–57. [Google Scholar] [CrossRef]
- Nostrant, T.T.; Kumar, N.B.; Appelman, H.D. Histopathology differentiates acute self-limited colitis from ulcerative colitis. Gastroenterology 1987, 92, 318–328. [Google Scholar] [CrossRef]
- Kiesslich, R.; Neurath, M.F. Chromoendoscopy and other novel imaging techniques. Gastroenterol. Clin. N. Am. 2006, 35, 605–619. [Google Scholar] [CrossRef]
- Hoang, T.T.; Leung, Y.; Rosenfeld, G.; Bressler, B. High-definition chromoendoscopy results in more significant dysplasia detection than white light endoscopy with random biopsies in ulcerative colitis patients: A single-center retrospective study. Medicine 2024, 103, e36836. [Google Scholar] [CrossRef]
- Neumann, H.; Neurath, M.F.; Mudte, J. New endoscopic approaches in IBD. World J. Gastroenterol. 2011, 17, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Kodashima, S.; Fujishiro, M. Novel image-enhanced endoscopy with i-scan technology. World J. Gastroenterol. 2010, 16, 1043. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kudo, T.; Jo, Y.; Esaki, M.; Yao, T.; Iida, M. Magnifying colonoscopy with narrow band imaging system for the diagnosis of dysplasia in ulcerative colitis: A pilot study. Gastrointest. Endosc. 2007, 66, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Iacucci, M.; Furfaro, F.; Matsumoto, T.; Uraoka, T.; Smith, S.; Ghosh, S.; Kiesslich, R. Advanced endoscopic techniques in the assessment of inflammatory bowel disease: New technology, new era. Gut 2019, 68, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Varadarajulu, S.; Banerjee, S.; Barth, B.A.; Desilets, D.J.; Kaul, V.; Kethu, S.R.; Pedrosa, M.C.; Pfau, P.R.; Tokar, J.L.; Wang, A.; et al. GI endoscopes. Gastrointest. Endosc. 2011, 74, 1–6.e6. [Google Scholar] [CrossRef]
- Manfredi, M.A.; Abu Dayyeh, B.K.; Bhat, Y.M.; Chauhan, S.S.; Gottlieb, K.T.; Hwang, J.H.; Komanduri, S.; Konda, V.; Lo, S.K.; Maple, J.T.; et al. Electronic chromoendoscopy. Gastrointest. Endosc. 2015, 81, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Vieth, M.; Günther, C.; Neufurt, C.; Kiesslich, R.; Grauer, M.; Atreya, R.; Neurath, M.F. Virtual chromoendoscopy for prediction of severity and disease extent in patients with inflammatory bowel disease: A randomized controlled study. Inflamm. Bowel Dis. 2013, 19, 1935–1942. [Google Scholar] [CrossRef]
- Kiesslich, R.; Burg, J.; Vieth, M.; Gnaendiger, J.; Enders, M.; Delaney, P.; Polglase, A.; McLaren, W.; Janell, D.; Thomas, S.; et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasia and colorectal cancer in vivo. Gastroenterology 2004, 127, 706–713. [Google Scholar] [CrossRef]
- Watanabe, O.; Ando, T.; Maeda, O.; Hasegawa, M.; Ishikawa, D.; Ishiguro, K.; Ohmiya, N.; Niwa, Y.; Goto, H. Confocal endomicroscopy in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. S2), S286–S290. [Google Scholar] [CrossRef]
- Bessho, R.; Kanai, T.; Hosoe, N.; Kobayashi, T.; Takayama, T.; Inoue, N.; Mukai, M.; Ogata, H.; Hibi, T. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J. Gastroenterol. 2011, 46, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Vieth, M.; Atreya, R.; Grauer, M.; Siebler, J.; Bernatik, T.; Neurath, M.F.; Mudter, J. Assessment of Crohn’s disease activity by confocal laser endomicroscopy. Inflamm. Bowel Dis. 2012, 18, 2261–2269. [Google Scholar] [CrossRef]
- Li, C.Q.; Xie, X.J.; Yu, T.; Gu, X.M.; Zuo, X.L.; Zhou, C.J.; Huang, W.Q.; Chen, H.; Li, Y.Q. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am. J. Gastroenterol. 2010, 105, 1391–1396. [Google Scholar] [CrossRef]
- Hundorfean, G.; Chiriac, M.T.; Mudter, J.; Neurath, M.F. Confocal laser endomicroscopy provides potential differentiation criteria between Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, E61–E64. [Google Scholar] [CrossRef] [PubMed]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Pennazio, M.; Rondonotti, E.; Despott, E.J.; Dray, X.; Keuchel, M.; Moreels, T.; Sanders, D.S.; Spada, C.; Carretero, C.; CortegosoValdivia, P.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2022. Endoscopy 2023, 55, 58–95. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, R.; Fischer, D.; Suissa, A.; Yassin, K.; Katz, D.; Guttman, N.; Migdal, M. Wireless capsule video endoscopy is a superior diagnostic tool in comparison to barium follow-through and computerized tomography in patients with suspected Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2003, 15, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.D. Video capsule endoscopy in inflammatory bowel disease. World J. Gastrointest. Endosc. 2016, 8, 477–488. [Google Scholar] [CrossRef]
- Petruzziello, C.; Calabrese, E.; Onali, S.; Zuzzi, S.; Condino, G.; Ascolani, M.; Zorzi, F.; Pallone, F.; Biancone, L. Small bowel capsule endoscopy vs conventional techniques in patients with symptoms highly compatible with Crohn’s disease. J. Crohn’s Colitis 2011, 5, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, C.; Onali, S.; Calabrese, E.; Zorzi, F.; Ascolani, M.; Condino, G.; Lolli, E.; Naccarato, P.; Pallone, F.; Biancone, L. Wireless capsule endoscopy and proximal small bowel lesions in Crohn’s disease. World J. Gastroenterol. 2010, 16, 3299–3304. [Google Scholar] [CrossRef] [PubMed]
- Cheifetz, A.S.; Kornbluth, A.A.; Legnani, P.; Schmelkin, I.; Brown, A.; Lichtiger, S.; Lewis, B.S. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am. J. Gastroenterol. 2006, 101, 2218–2222. [Google Scholar] [CrossRef] [PubMed]
- Bourreille, A.; Ignjatovic, A.; Aabakken, L.; Loftus EVJr Eliakim, R.; Pennazio, M.; Bouhnik, Y.; Seidman, E.; Keuchel, M.; Albert, J.G.; Ardizzone, S.; et al. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: An international OMED-ECCO consensus. World Organisation of Digestive Endoscopy (OMED) and the European Crohn’s and Colitis Organisation (ECCO). Endoscopy 2009, 41, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Manfredi, M.A.; Abu Dayyeh, B.K.; Brintha, K.E.; Fujii-Lau, L.L.; Sri, K.; Vani, K.; John, T.M.; Faris, M.M.; Rahul, P.; et al. Enteroscopy. Gastrointest. Endosc. 2015, 82, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.D.; Hadithi, M.; Groenen, M.J.; Kuipers, E.J.; Jacobs, M.A.; Mulder, C.J. Double-balloon enteroscopy: Indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy 2006, 38, 42–48. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Yang, F.; Wang, G.; Ge, N.; Wang, S.; Guo, J.; Sun, S. Diagnostic efficacy of double-balloon enteroscopy inpatients with suspected isolated small bowel Crohn’s disease. BMC Gastroenterol. 2020, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Tielbeek, J.; Carter, D.; Taylor, S.A.; Tolan, D.; Wilkens, R.; Bryant, R.V.; Hoeffel, C.; De Kock, I.; Maaser, C.; et al. ECCO-ESGAR Topical Review on Optimizing Reporting for Cross-Sectional Imaging in Inflammatory Bowel Disease. J. Crohn’s Colitis 2022, 26, 523–543. [Google Scholar] [CrossRef] [PubMed]
- JPanes, Y.; Bouhnik, W.; Reinisch, J.; Stoker, S.A.; Taylor, D.C.; Baumgart, S.; Danese, S.; Halligan, B.; Marincek, C.; Matos, L.; et al. Imaging techniques for assessment of inflammatory bowel disease: Joint ECCO and ESGAR evidence-based consensus guidelines. J. Crohn’s Colitis 2013, 7, 556–585. [Google Scholar]
- Bruining, D.H.; Zimmermann, E.M.; Loftus, E.V., Jr.; Sandborn, W.J.; Sauer, C.G.; Strong, S.A. Society of Abdominal Radiology Crohn’s Disease-Focused Panel. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Gastroenterology 2018, 154, 1172–1194. [Google Scholar] [CrossRef]
- Fletcher, J.G.; Fidler, J.L.; Bruining, D.H.; Hu-prich, J.E. New concepts in intestinal imaging for inflammatory bowel diseases. Gastroenterology 2011, 140, 1795–1806. [Google Scholar] [CrossRef]
- Kunihiro, K.; Hata, J.; Haruma, K.; Manabe, N.; Tanaka, S.; Chayama, K. Sonographic detection of longitudinal ulcers in Crohn disease. Scand. J. Gastroenterol. 2004, 39, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Buisson, A.; Joubert, A.; Montoriol, P.; Ines, D.D.; Hordonneau, C.; Pereira, B.; Garcier, J.; Bommelaer, G.; Petitcolin, V. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment. Pharmacol. Ther. 2013, 37, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kunisaki, R.; Kinoshita, H.; Kimura, H.; Kodera, T.; Nozawa, A.; Hanzawa, A.; Shibata, N.; Yonezawa, H.; Miyajima, E.; et al. Doppler ultrasound findings correlate with tissue vascularity and inflammation in surgical pathology specimens from patients with small intestinal Crohn’s disease. BMC Res. Notes 2014, 7, 363. [Google Scholar] [CrossRef]
- Taylor, S.A.; Mallett, S.; Bhatnagar, G.; Baldwin-Cleland, R.; Bloom, S.; Gupta, A.; Hamlin, P.J.; Hart, A.L.; Higginson, A.; Jacobs, I.; et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): A multicentre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 548–558. [Google Scholar] [CrossRef]
- Na, J.E.; Kim, H.S.; Hong, S.N.; Song, K.D.; Kim, J.E.; Kim, E.R.; Kim, Y.H.; Chang, D.K. Comparison of an Endoscopic Scoring System and the Simplified Magnetic Resonance Index of Activity in Patients with Small Bowel Crohn’s Disease. Gut Liver 2024, 18, 97–105. [Google Scholar] [CrossRef]
- Maira, H.; Stuart, A.T. Small bowel imaging in inflammatory bowel disease: Updates for 2023. Expert. Rev. Gastroenterol. Hepatol. 2023, 17, 1117–1134. [Google Scholar]
- Barchi, A.; D’Amico, F.; Zilli, A.; Furfaro, F.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; Dal Buono, A.; Allocca, M. Recent advances in the use of ultrasound in Crohn’s disease. Expert. Rev. Med. Devices 2023, 20, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Fiorino, G.; Bonifacio, C.; Furfaro, F.; Gilardi, D.; Argollo, M.; Peyrin-Biroulet, L.; Danese, S. Comparative accuracy of bowel ultrasound versus Magnetic resonance enterography in combination with colonoscopy in assessing Crohn’s disease and guiding clinical decision-making. J. Crohn’s Colitis 2018, 12, 1280–1287. [Google Scholar] [CrossRef]
- Ordás, I.; Rimola, J.; Alfaro, I.; Rodríguez, S.; Castro-Poceiro, J.; Ramírez-Morros, A.; Gallego, M.; Giner, À.; Barastegui, R.; Fernández-Clotet, A.; et al. Development and validation of a simplified magnetic resonance index of activity for Crohn’s disease. Gastroenterology 2019, 157, 432–439.e1. [Google Scholar] [CrossRef]
- Alyami, A.S.; Madkhali, Y.; Majrashi, N.A.; Alwadani, B.; Elbashir, M.; Ali, S.; Ageeli, W.; El-Bahkiry, H.S.; Althobity, A.A.; Refaee, T. The role of molecular imaging in detecting fibrosis in Crohn’s disease. Ann. Med. 2024, 56, 2313676. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.; Ribeiro, H.; Maconi, G. Bowel Thickening in Crohn’s Disease: Fibrosis or Inflammation? Diagnostic Ultrasound Imaging Tools. Inflamm. Bowel Dis. 2017, 23, 23–34. [Google Scholar] [CrossRef]
- Catalano, O.A.; Gee, M.S.; Nicolai, E.; Selvaggi, F.; Pellino, G.; Cuocolo, A.; Luongo, A.; Catalano, M.; Rosen, B.R.; Gervais, D. Evaluation of quantitative PET/MR enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease. Radiology 2015, 278, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Coronella, M.; Spatola, C.; Puzzo, L.; Garro, R.; Inserra, G.; Riguccio, G.; et al. Can conventional and diffusion-weighted mr enterography biomarkers differentiate inflammatory from fibrotic strictures in crohn’s disease? Medicina 2021, 5, 265. [Google Scholar] [CrossRef] [PubMed]
- Fraquelli, M.; Colli, A.; Casazza, G.; Paggi, S.; Colucci, A.; Massironi, S.; Duca, P.; Conte, D. Role of US in detection of Crohn disease: Meta-analysis. Radiology 2005, 236, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.; Saxena, S.; Jayasooriya, N.; Bottle, A.; Petersen, I.; Hotopf, M.; Alexakis, C.; Pollok, R.C.J. Prevalence and Duration of Gastrointestinal Symptoms before Diagnosis of Inflammatory Bowel Disease and Predictors of Timely Specialist Review: A Population-Based Study. POP-IBD study group. Crohn’s Colitis. 2020, 15, jjaa146. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Petralia, F.; Sato, T.; Wang, P.; Telesco, S.E.; Choung, R.S.; Strauss, R.; Li, X.J.; Laird, R.M.; Gutierrez, R.L.; et al. Serum Biomarkers Identify Patients Who Will Develop Inflammatory Bowel Diseases up to 5 Years Before Diagnosis. Gastroenterology 2020, 159, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.V.; Allin, K.H.; Poulsen, G.J.; Lee, J.C.; Jess, T. Characterizing the pre-clinical phase of inflammatory bowel disease. Cell Rep. Med. 2023, 4, 101263. [Google Scholar] [CrossRef] [PubMed]
- Umar, N.; Wambua, S.; Harvey, P.; Haroon, S.; Adderley, N.J.; Trudgill, N. P488 Development and validation of a risk prediction tool for inflammatory bowel disease (IBD) diagnosis in patients presenting in primary care with abdominal symptoms, a UK based study. J. Crohn’s Colitis 2024, 18 (Suppl. S1), i976. [Google Scholar] [CrossRef]
- Danese, S.; Fiorino, G.; Mary, J.-Y.; Lakatos, P.L.; D’haens, G.; Moja, L.; D’hoore, A.; Panes, J.; Reinisch, W.; Sandborn, W.J.; et al. Development of red flags index for early referral of adults with symptoms and signs suggestive of Crohn’s disease: An IOIBD initiative. J. Crohn’s Colitis 2015, 9, 601–606. [Google Scholar] [CrossRef]
- Fiorino, G.; Bonovas, S.; Gilardi, D.; Di Sabatino, A.; Allocca, M.; Furfaro, F.; Roda, G.; Lenti, M.V.; Aronico, N.; Mengoli, C.; et al. Validation of the red flags index for early diagnosis of Crohn’s disease: A prospective observational IG-IBD study among general practitioners. J. Crohn’s Colitis 2020, 14, 1777–1779. [Google Scholar] [CrossRef] [PubMed]
- Novacek, G.; Austrian IBD Study Group (ATISG); Gröchenig, H.P.; Haas, T.; Wenzl, H.; Steiner, P.; Koch, R.; Feichtenschlager, T.; Eckhardt, G.; Mayer, A.; et al. Diagnostic delay in patients with inflammatory bowel disease in Austria. Wien. Klin. Wochenschr. 2019, 131, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Dehlavi, M.A.; Fournier, N.; Safroneeva, E.; Straumann, A.; Pittet, V.; Peyrin-Biroulet, L.; Michetti, P.; Rogler, G.; Vavricka, S.R.; et al. Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am. J. Gastroenterol. 2013, 108, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Dilillo, D.; Zuccotti, G.V.; Galli, E.; Meneghin, F.; Dell’Era, A.; Penagini, F.; Colella, G.; Lewindon, P.; Carmagnola, S.; Farina, E.; et al. Noninvasive testing in the management of children with suspected inflammatory bowel disease. Scand. J. Gastroenterol. 2019, 54, 586–591. [Google Scholar] [CrossRef]
- Maconi, G.; Nylund, K.; Ripolles, T.; Calabrese, E.; Dirks, K.; Dietrich, C.F.; Hollerweger, A.; Sporea, I.; Saftoiu, A.; Maaser, C.; et al. EFSUMB Recommendations and Clinical Guidelines for Intestinal Ultrasound (GIUS) in Inflammatory Bowel Diseases. Ultraschall Med. Eur. J. Ultrasound 2018, 39, 304–317. [Google Scholar] [CrossRef]
| Tools for Diagnosis of IBD | |
|---|---|
| Clinical evaluation | Evaluate presence of gastrointestinal symptomsa and Extra intestinal symptoms; family history of IBD; autoimmune disease |
| Laboratory tests | Stool examinations for enteric infections; fecal calprotectin and lactoferrin test; laboratory tests such as C-reactive protein, blood cell count, iron, and vitamins |
| Endoscopy | Ileocolonscopy with biopsies: in all patients with suspected IBD; Esophagogastroduodenoscopy with biopsies: if upper symptoms; VCE: if suspected CD and negative findings on ileocolonoscopy; DAE: to take biopsies or when stenosis is expected; CLE: for inflammation assessment |
| Imaging techniques | IUS, MRE, or CTE to evaluate the extent of small bowel involvement in CD, disease activity, and complications; IUS and CTE to evaluate the extent of disease and complications in ulcerative colitis |
| Partial Mayo Score [Index] | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Stool frequency | Normal | 1–2/day > normal | 3–4/day > normal | 5/day > normal |
| Rectal bleeding | None | Streaks | Obvious | Mostly blood |
| Physician’s global assessment | Normal | Mild | Moderate | Severe |
| Variable | Variable Description |
|---|---|
| General well-being | 0 = very well; 1 = slightly below average; 2 = poor; 3 = very poor; 4 = terrible |
| Abdominal pain | 0 = none; 1 = mild; 2 = moderate; 3 = severe |
| Number of liquid stools | 0 = 0–1 stools; 1 = 2–3 stools; 2 = 4–5 stools; 3 = 6–7 stools; 4 = 8–9 stools; 5 = 10+ stools |
| Abdominal mass | 0 = none; 1 = dubious; 2 = definite; 3 = tender |
| Complications | None, uveitis, arthalgia, erythema nodosum, aphthous gangrenosum, anal fissure, new fistula, abscess; one point each |
| Variable | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Size of ulcers | None | Afthous ulcers (Diameter 0.1 to 0.5 cm) | Large ulcers (Diameter 0.5 to 2 cm) | Very large ulcers (Diameter > 2 cm) |
| Ulcerated surface | None | <10% | 10–30% | >30% |
| Affected surface | Unaffected segment | <50% | 50–75% | >75% |
| Presence of narrowings | None | Single, can be passed | Multiple, can be passed | Cannot be passed |
| Mayo Endoscopic Score | Endoscopic Features |
|---|---|
| 0 | None |
| 1 | Erythema, decreased vascular pattern, mild friability |
| 2 | Marked erythema, absent vascular pattern, friability, erosions |
| 3 | Spontaneous bleeding, ulcerations |
| Typical Histological Features | Ulceratis Colitis | Crohn’s Disease | Infectious Colitis |
|---|---|---|---|
| Lymphoid aggregates | Frequent in mucosa | Common, transmural | Present in mucosa |
| Granulomas | Absent | Common, transmural | Possible in tubercolosis enteritis |
| Localization of inflammation | Limited to the mucosa | Transmural | Limited to the mucosa |
| Active inflammation | Diffuse | Focal with skip lesions | Diffuse |
| Cryptitis, crypt abscesses | Diffuse continuous | Focal discontinuous | Frequent, diffuse |
| Crypt architectural distortion | Diffuse | Focal, frequent | Usually absent |
| Atrophy | Present | Uncommon, mild | Rare |
| Pyloric metaplasia | Rare | Present | Rare except in tuberculosis enteritis |
| Basal plasmacytosis | Present | Present | Usually absent |
| Radiological Imaging Techniques in Inflammatory Bowel Disease | Advantages of Radiological Imaging Techniques in IBD | Disadvantages of Radiological Imaging Techniques in IBD |
|---|---|---|
| Magnetic resonance enterography | Non-invasive nature Assessment of disease extent and severity Evaluation of disease complications Sensitivity > 97% | Radiation exposure Contrast agent administration High cost Contraindicated in patients with certain medical devices or claustrophobia Diagnostic accuracy for stenosis is based on the use of luminal contrast |
| Intestinal ultrasound | Non-invasive nature Assessment of disease extent and severity Cost-effective No oral preparation Easily reproducible Evaluation of disease complications Sensitivity > 92% | Operator dependence Poor image quality in patients with excess abdominal weight |
| Computed tomography enterography | Non-invasive nature Assessment of disease extent and severity Evaluation of disease complications Sensitivity > 84% | Radiation exposure Contrast agent administration High cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicerone, C.; D’Amico, F.; Allocca, M.; Zilli, A.; Parigi, T.L.; Danese, S.; Furfaro, F. A Comprehensive Multidisciplinary Approach to Diagnosing Chronic Inflammatory Bowel Diseases: Integration of Clinical, Endoscopic, and Imaging Modalities. Diagnostics 2024, 14, 1530. https://doi.org/10.3390/diagnostics14141530
Cicerone C, D’Amico F, Allocca M, Zilli A, Parigi TL, Danese S, Furfaro F. A Comprehensive Multidisciplinary Approach to Diagnosing Chronic Inflammatory Bowel Diseases: Integration of Clinical, Endoscopic, and Imaging Modalities. Diagnostics. 2024; 14(14):1530. https://doi.org/10.3390/diagnostics14141530
Chicago/Turabian StyleCicerone, Clelia, Ferdinando D’Amico, Mariangela Allocca, Alessandra Zilli, Tommaso Lorenzo Parigi, Silvio Danese, and Federica Furfaro. 2024. "A Comprehensive Multidisciplinary Approach to Diagnosing Chronic Inflammatory Bowel Diseases: Integration of Clinical, Endoscopic, and Imaging Modalities" Diagnostics 14, no. 14: 1530. https://doi.org/10.3390/diagnostics14141530
APA StyleCicerone, C., D’Amico, F., Allocca, M., Zilli, A., Parigi, T. L., Danese, S., & Furfaro, F. (2024). A Comprehensive Multidisciplinary Approach to Diagnosing Chronic Inflammatory Bowel Diseases: Integration of Clinical, Endoscopic, and Imaging Modalities. Diagnostics, 14(14), 1530. https://doi.org/10.3390/diagnostics14141530







