Classification-Predictive Model Based on Artificial Neural Network Validated by Histopathology and Direct Immunofluorescence for the Diagnosis of Oral Lichen Planus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basic Statistics
2.2. Variants of Classification
2.3. Artificial Neural Networks
3. Results
3.1. Study Population
3.2. Direct Immunofluorescence vs. Histopathology
3.3. Artificial Neural Network
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slebioda, Z.; Szponar, E.; Kowalska, A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: Literature review. Arch. Immunol. Ther. Exp. 2014, 62, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Chavez, E.M.; Doerr, P.A.; Henson, B.S.; Sarmadi, M. Recurrent aphthous stomatitis. Quintessence Int. 2000, 31, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Chou, M.Y.; Ho, C.C.; Kao, C.T.; Tsai, C.H.; Wang, L.; Yang, C.C. Study of the viral infections and cytokines associated with recurrent aphthous ulceration. Microbes Infect. 2005, 7, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.C.; Gomez, R.S.; Zina, L.G.; Amaral, F.R. Recurrent aphthous stomatitis and Helicobacter pylori. Med. Oral Patol. Oral Cir. Bucal 2016, 21, E187–E191. [Google Scholar] [CrossRef]
- Bankvall, M.; Sjöberg, F.; Gale, G.; Wold, A.; Jontell, M.; Östman, S. The oral microbiota of patients with recurrent aphthous stomatitis. J. Oral Microbiol. 2014, 6, 25739. [Google Scholar] [CrossRef] [PubMed]
- Dudding, T.; Haworth, S.; Lind, P.A.; Sathirapongsasuti, J.F.; Tung, J.Y.; Mitchell, R.; Colodro-Conde, L.; Medland, S.E.; Gordon, S.; Elsworth, B.; et al. Genome wide analysis for mouth ulcers identifies associations at immune regulatory loci. Nat. Commun. 2019, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.H.; Torres, J.; Roda, G.; Brygo, A.; Delaporte, E.; Colombel, J.F. Review article: Non-malignant oral manifestations in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2015, 42, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Chiewchengchol, D.; Murphy, R.; Morgan, T.; Edwards, S.W.; Leone, V.; Friswell, M.; Pilkington, C.; Tullus, K.; Rangaraj, S.; McDonagh, J.E.; et al. Mucocutaneous manifestations in a UK national cohort of juvenile-onset systemic lupus erythematosus patients. Rheumatology 2014, 53, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, E. Behcet’s disease: A comprehensive review with a focus on epidemiology, etiology and clinical features, and management of mucocutaneous lesions. J. Dermatol. 2016, 43, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Gilvetti, C.; Porter, S.R.; Fedele, S. Traumatic chemical oral ulceration: A case report and review of the literature. Br. Dent. J. 2010, 208, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Saunders, D.P.; Peterson, D.E. Chemotherapy or radiationinduced oral mucositis. Dent. Clin. N. Am. 2014, 58, 341–349. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Ramos-García, P.; González-Moles, M.Á.; Warnakulasuriya, S. Oral cancer development in lichen planus and related conditions-3.0 evidence level: A systematic review of systematic reviews. Oral Dis. 2021, 27, 1919–1935. [Google Scholar] [CrossRef]
- Roopashree, M.R.; Gondhalekar, R.V.; Shashikanth, M.C.; George, J.; Thippeswamy, S.H.; Shukla, A. Pathogenesis of oral lichen planus—A review. J. Oral Pathol. Med. 2010, 39, 729–734. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Gould, A.; Kurago, Z.; Fantasia, J.; Muller, S. Diagnosis of oral lichen planus: A position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 332–354. [Google Scholar] [CrossRef]
- Gururaj, N.; Hasinidevi, P.; Janani, V.; Divynadaniel, T. Diagnosis and management of oral lichen planus—Review. J. Oral Maxillofac. Pathol. 2021, 25, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Yamashita, M.; Innocentini, L.; Macedo, L.D.; Chahud, F.; Ribeiro-Silva, A.; Roselino, A.M.; Rocha, M.J.A.; Motta, A.C. Direct Immunofluorescence as a Helpful Tool for the Differential Diagnosis of Oral Lichen Planus and Oral Lichenoid Lesions. Am. J. Dermatopathol. 2018, 40, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Buajeeb, W.; Okuma, N.; Thanakun, S.; Laothumthut, T. Direct immunofluorescence in oral lichen planus. J. Clin. Diagn. Res. 2015, 9, ZC34–ZC37. [Google Scholar] [CrossRef] [PubMed]
- van der Meij, E.H.; van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef]
- Bilodeau, E.A.; Lalla, R.V. Recurrent oral ulceration: Etiology, classification, management, and diagnostic algorithm. Periodontol. 2000 2019, 80, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Masaryk University Information System. Oral Mucosal Diseases—Repetitorium and Atlas. Available online: https://is.muni.cz/do/rect/el/estud/lf/ps21/onemocneni_ustni_dutiny/web/en/ch8.html (accessed on 12 June 2024).
- Rashid, H.; Lamberts, A.; Diercks, G.F.H.; Pas, H.H.; Meijer, J.M.; Bolling, M.C.; Horváth, B. Oral Lesions in Autoimmune Bullous Diseases: An Overview of Clinical Characteristics and Diagnostic Algorithm. Am. J. Clin. Dermatol. 2019, 20, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Carrozzo, M.; Porter, S.; Mercadante, V.; Fedele, S. Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontol. 2000 2019, 80, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Akaike, Y.; Morita, K.-I.; Sakamoto, K.; Tsushima, F.; Kayamori, K.; Maruta, N.; Yamazaki, K.; Anzai, E.; Tonouchi, E.; Harada, H.; et al. Automatic detection of spongiosis associated with oral lichenoid lesions using machine learning. J. Oral Maxil. Surg. Med. Pathol. 2023, 35, 368–374. [Google Scholar] [CrossRef]
- Keller, L.M.; Lombardi, T. Gingival lichen planus: A clinical and pathological study. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101354. [Google Scholar] [CrossRef] [PubMed]
- De Porras-Carrique, T.; González-Moles, M.Á.; Warnakulasuriya, S.; Ramos-García, P. Depression, anxiety, and stress in oral lichen planus: A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 1391–1408. [Google Scholar] [CrossRef] [PubMed]
- Huling, L.B.; Baccaglini, L.; Choquette, L.; Feinn, R.S.; Lalla, R.V. Effect of stressful life events on the onset and duration of recurrent aphthous stomatitis. J. Oral Pathol. Med. 2012, 41, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Albanidou-Farmaki, E.P.A.; Epivatianos, A.; Farmakis, K.; Karamouzis, M.; Antoniades, D. Increased anxiety level and high salivary and serum cortisol concentrations in patients with recurrent aphthous stomatitis. Tohoku J. Exp. Med. 2008, 214, 291–296. [Google Scholar] [CrossRef]
- Gallo Cde, B.; Mimura, M.A.; Sugaya, N.N. Psychological stress and recurrent aphthous stomatitis. Clinics 2009, 64, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Al-Omiri, M.K.; Karasneh, J.; Alhijawi, M.M.; Zwiri, A.M.; Scully, C.; Lynch, E. Recurrent aphthous stomatitis (RAS): A preliminary within-subject study of quality of life, oral health impacts and personality profiles. J. Oral Pathol. Med. 2015, 44, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ding, L.; Yang, C.; Hao, X.; Wang, C. Exploring the Relationship between Psychiatric Traits and the Risk of Mouth Ulcers Using Bi-Directional Mendelian Randomization. Front. Genet. 2020, 11, 608630. [Google Scholar] [CrossRef] [PubMed]
- Masquijo-Bisio, P.A.; Gandolfo, M.S.; Keszler, A.; Itoiz, M.E.; Paparella, M.L. Usefulness of a direct immunofluorescence in the diagnosis of plaque type oral lichen planus. Ann. Diagn. Pathol. 2017, 31, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Dong, Y.; Wang, Z.; Cai, L.; Pan, D.; Zhang, C.; Li, T.; Zhou, Y. Direct immunofluorescence and immune function in patients with oral lichen planus. J. Dent. Sci. 2022, 17, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, M.A.; Abutarbush, S.M. Using Artificial Intelligence to Predict Survivability Likelihood and Need for Surgery in Horses Presented with Acute Abdomen (Colic). J. Eq. Vet. Sci. 2020, 90, 102973. [Google Scholar] [CrossRef] [PubMed]
- Achararit, P.; Manaspon, C.; Jongwannasiri, C.; Phattarataratip, E.; Osathanon, T.; Sappayatosok, K. Artificial Intelligence-Based Diagnosis of Oral Lichen Planus Using Deep Convolutional Neural Networks. Eur. J. Dent. 2023, 17, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Keser, G.; Bayrakdar, İ.Ş.; Pekiner, F.N.; Çelik, Ö.; Orhan, K. A deep learning algorithm for classification of oral lichen planus lesions from photographic images: A retrospective study. J. Stomatol. Oral Max. Surg. 2023, 124, 101264. [Google Scholar] [CrossRef] [PubMed]
- Renaud, M.; Delpierre, A.; Becquet, H.; Mahalli, R.; Savard, G.; Micheneau, P.; Carayon, D.; Denis, F. Intraoral Ultrasonography for Periodontal Tissue Exploration: A Review. Diagnostics 2023, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Izzetti, R.; Vitali, S.; Oranges, T.; Dini, V.; Romanelli, M.; Caramella, D.; Gabriele, M. Intraoral Ultra-High Frequency Ultrasound study of oral lichen planus: A pictorial review. Skin Res. Technol. 2020, 26, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Izzetti, R.; Nisi, M.; Aringhieri, G.; Vitali, S.; Oranges, T.; Romanelli, M.; Caramella, D.; Graziani, F.; Gabriele, M. Ultra-high frequency ultrasound in the differential diagnosis of oral pemphigus and pemphigoid: An explorative study. Skin Res. Technol. 2021, 27, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745 (accessed on 17 April 2024).
- Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device#transforming (accessed on 17 April 2024).
| Lichen Confirmed in HP | LP Not Excluded in HP | |||||
|---|---|---|---|---|---|---|
| No (n = 57) | Yes (n = 4) | p-Value | No (n = 30) | Yes (n = 30) | p-Value | |
| Sex Female Male | 46 (93.9) 11 (91.7) | 3 (6.1) 1 (8.3) | 0.781 | 25 (51.0) 6 (50.0) | 24 (49.0) 6 (50.0) | 0.949 |
| Age at onset Median (IQR) | 60 (40–64) | 59 (52–64) | 0.751 | 62 (41–65) | 60 (43–64) | 1.0 |
| Stress at onset No Yes | 25 (96.1) 22 (88.0) | 1 (3.9) 3 (12.0) | 0.279 | 19 (73.1) 10 (40.0) | 7 (26.9) 15 (60.0) | 0.017 |
| Patient previously treated by a GP No Yes | 41 (91.1) 4 (100.0) | 4 (8.9) 0 (0.0) | 0.533 | 23 (51.1) 4 (100.0) | 22 (48.9) 0 (0.0) | 0.059 |
| White patches under the tongue No Yes | 43 (91.5) 5 (100.0) | 4 (8.5) 0 (0.0) | 0.497 | 23 (48.9) 5 (100.0) | 24 (51.1) 0 (0.0) | 0.029 |
| White patches on buccal mucosa No Yes | 12 (100.0) 36 (90.0) | 0 (0.0) 4 (10.0) | 0.254 | 9 (75.0) 19 (47.5) | 3 (25.0) 21 (52.5) | 0.094 |
| Erosions on mandibular gingiva No Yes | 31 (91.2) 19 (95.0) | 3 (8.8) 1 (5.0) | 0.604 | 14 (41.2) 14 (70.0) | 20 (58.8) 6 (30.0) | 0.041 |
| Erosions under the tongue No Yes | 45 (91.8) 4 (100.0) | 4 (8.2) 0 (0.0) | 0.552 | 24 (49.0) 4 (100.0) | 25 (51.0) 0 (0.0) | 0.049 |
| N = 63 | Lichen Confirmed on HP | p-Value | Lichen not Excluded on HP | p-Value | ||
|---|---|---|---|---|---|---|
| No (n = 57) | Yes (n = 4) | No (n = 33) | Yes (n = 30) | |||
| DIF IgG (−) | 53 (93.0) | 4 (7.0) | 0.635 | 29 (50.9) | 28 (49.1) | 0.594 |
| DIF IgG + | 3 (100.0) | 0 (0.0) | 2 (66.7) | 1 (33.3) | ||
| DIF IgA (−) | 55 (93.2) | 4 (6.8) | 0.787 | 31 (52.5) | 28 (47.5) | 0.297 |
| DIF IgA (+) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | ||
| DIF IgM (−) | 53 (93.0) | 4 (7.0) | 0.635 | 29 (50.9) | 28 (49.1) | 0.594 |
| DIF IgM (+) | 3 (100.0) | 0 (0.0) | 2 (66.7) | 1 (33.3) | ||
| DIF C3 (−) | 45 (91.8) | 4 (8.2) | 0.327 | 25 (51.0) | 24 (49.0) | 0.832 |
| DIF C3 (+) | 11 (100.0) | 0 (0.0) | 6 (54.6) | 5 (45.4) | ||
| DIF F1 (−) | 40 (90.9) | 4 (9.1) | 0.212 | 25 (56.8) | 19 (43.2) | 0.185 |
| DIF F1 (+) | 16 (100.0) | 0 (0.0) | 6 (37.5) | 10 (62.5) | ||
| DIF F2 (−) | 42 (91.3) | 4 (8.7) | 0.253 | 26 (56.5) | 20 (43.5) | 0.173 |
| DIF F2 (+) | 14 (100.0) | 0 (0.0) | 20 (35.7) | 9 (64.3) | ||
| IgG | IgA | IgM | C3 | F1 | F2 | N (% among OLP Confirmed) | N (% among OLP Not Confirmed) | N (% among OLP Not Excluded) | N (% among OLP Excluded) |
|---|---|---|---|---|---|---|---|---|---|
| − | − | − | − | − | − | 4 (100.0) | 33 (58.9) | 15 (51.7) | 22 (71.0) |
| − | − | − | + | + | + | 0 (0) | 6 (10.8) | 3 (10.3) | 3 (9.7) |
| − | − | − | − | + | − | 0 (0) | 2 (3.6) | 1 (3.4) | 1 (3.2) |
| − | − | − | − | + | + | 0 (0) | 7 (12.5) | 6 (20.7) | 1 (3.2) |
| + | − | + | + | − | − | 0 (0) | 1 (1.8) | 0 (0) | 1 (3.2) |
| − | − | + | − | + | + | 0 (0) | 1 (1.8) | 0 (0) | 1 (3.2) |
| + | + | − | − | − | − | 0 (0) | 1 (1.8) | 1 (3.5) | 0 (0) |
| − | − | − | + | − | − | 0 (0) | 3 (5.7) | 2 (6.9) | 1 (3.2) |
| + | − | − | + | − | − | 0 (0) | 1 (1.8) | 0 (0) | 1 (3.2) |
| − | − | + | − | − | − | 0 (0) | 1 (1.8) | 1 (3.5) | 0 (0) |
| DIF F1 (−) | DIF F1 (+) | p-Value | DIF F2 (−) | DIF F2 (+) | p-Value | |
|---|---|---|---|---|---|---|
| DIF IgG (+) | ||||||
| No | 52 (71.2) | 21 (28.8) | 0.208 | 54 (74.0) | 19 (26.0) | 0.240 |
| Yes | 4 (100.0) | 0 (0.0) | 4 (100.0) | 0 (0.0) | ||
| DIF IgA (+) | ||||||
| No | 54 (72.0) | 21 (28.0) | 0.840 | 56 (74.7) | 19 (25.3) | 0.756 |
| Yes | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | ||
| DIF IgM (+) | ||||||
| No | 54 (73.0) | 20 (27.0) | 0.320 | 56 (75.7) | 18 (24.3) | 0.252 |
| Yes | 2 (50.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | ||
| DIF C3 (+) | ||||||
| No | 51 (77.3) | 15 (22.7) | 0.028 | 53 (80.3) | 13 (19.7) | 0.013 |
| Yes | 5 (45.5) | 6 (54.5) | 5 (45.5) | 6 (54.5) |
| Variable | Chi Value | p-Value |
|---|---|---|
| Age at onset | 6.219780 | 0.622628 |
| Stress at onset | 5.684772 | 0.017113 |
| White patches under the tongue | 4.741641 | 0.029441 |
| Erosions on mandibular gingiva | 4.190498 | 0.040651 |
| Erosions under the tongue | 3.862974 | 0.049363 |
| Patient previously treated by a GP | 3.548971 | 0.059582 |
| White patches on buccal mucosa (left side) | 2.808929 | 0.093741 |
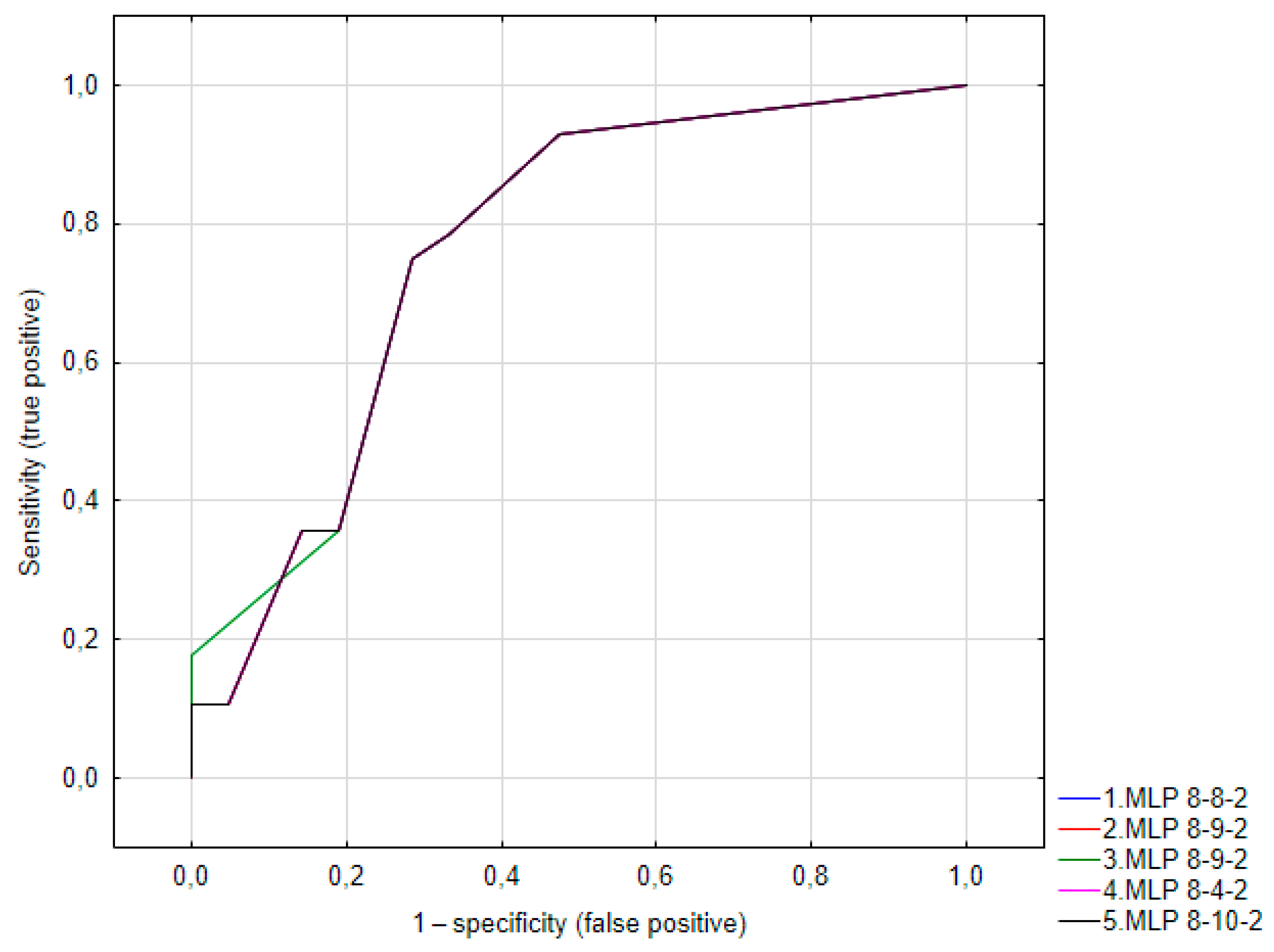
| Network ID | Quality (Learning) | Quality (Testing) | Quality (Validation) | Learning Algorithm | Function Error | Activation (Hidden) | Activation (Output) |
|---|---|---|---|---|---|---|---|
| MLP 8-8-2 | 74.28571 | 85.71429 | 71.42857 | BFGS 4 | Entropy | Linear | Softmax |
| MLP 8-9-2 | 74.28571 | 85.71429 | 71.42857 | BFGS 4 | SOS | Logistic | Linear |
| MLP 8-9-2 | 74.28571 | 85.71429 | 71.42857 | BFGS 2 | Entropy | Linear | Softmax |
| MLP 8-4-2 | 74.28571 | 85.71429 | 71.42857 | BFGS 3 | Entropy | Linear | Softmax |
| MLP 8-10-2 | 74.28571 | 85.71429 | 71.42857 | BFGS 2 | SOS | Tanh | Logistic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipowicz, K.; Turkowski, P.; Zdolińska-Malinowska, I. Classification-Predictive Model Based on Artificial Neural Network Validated by Histopathology and Direct Immunofluorescence for the Diagnosis of Oral Lichen Planus. Diagnostics 2024, 14, 1525. https://doi.org/10.3390/diagnostics14141525
Osipowicz K, Turkowski P, Zdolińska-Malinowska I. Classification-Predictive Model Based on Artificial Neural Network Validated by Histopathology and Direct Immunofluorescence for the Diagnosis of Oral Lichen Planus. Diagnostics. 2024; 14(14):1525. https://doi.org/10.3390/diagnostics14141525
Chicago/Turabian StyleOsipowicz, Katarzyna, Piotr Turkowski, and Izabela Zdolińska-Malinowska. 2024. "Classification-Predictive Model Based on Artificial Neural Network Validated by Histopathology and Direct Immunofluorescence for the Diagnosis of Oral Lichen Planus" Diagnostics 14, no. 14: 1525. https://doi.org/10.3390/diagnostics14141525
APA StyleOsipowicz, K., Turkowski, P., & Zdolińska-Malinowska, I. (2024). Classification-Predictive Model Based on Artificial Neural Network Validated by Histopathology and Direct Immunofluorescence for the Diagnosis of Oral Lichen Planus. Diagnostics, 14(14), 1525. https://doi.org/10.3390/diagnostics14141525




