Differentiation of Acute Internal Carotid Artery Occlusion Etiology on Computed Tomography Angiography: Diagnostic Tree for Preparing Endovascular Treatment
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Imaging Protocol
2.3. CTA Analysis
2.4. DSA Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Malhotra, K.; Goyal, N.; Tsivgoulis, G. Internal carotid artery occlusion: Pathophysiology, diagnosis, and management. Curr. Atheroscler. Rep. 2017, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Gornbein, J.; Saver, J.L. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: A review. Front. Neurol. 2017, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Paciaroni, M.; Inzitari, D.; Agnelli, G.; Caso, V.; Balucani, C.; Grotta, J.C.; Sarraj, A.; Sung-Il, S.; Chamorro, A.; Urra, X.; et al. Intravenous thrombolysis or endovascular therapy for acute ischemic stroke associated with cervical internal carotid artery occlusion: The ICARO-3 study. J. Neurol. 2015, 262, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.; Cerejo, R.; Williamson, R.; Malhotra, K. Internal carotid artery occlusion: Management. Curr. Neurol. Neurosci. Rep. 2022, 22, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, V.; Pusceddu, F.; Lattanzi, S.; Scaggiante, J.; Sallustio, F.; Marrama, F.; di Poggio, M.B.; Toscano, G.; Di Giuliano, F.; Rolla-Bigliani, C.; et al. Endovascular treatment of patients with acute ischemic stroke and tandem occlusion due to internal carotid artery dissection: A multicenter experience. Neuroradiol. J. 2023, 36, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, V.; Scaggiante, J.; Pitocchi, F.; Sallustio, F.; Lattanzi, S.; Umana, G.E.; Chaurasia, B.; di Poggio, M.B.; Toscano, G.; Bigliani, C.R.; et al. Mechanical thrombectomy in acute ischemic stroke with tandem occlusions: Impact of extracranial carotid lesion etiology on endovascular management and outcome. Neurosurg. Focus 2021, 51, E6. [Google Scholar] [CrossRef]
- Ter Schiphorst, A.; Peres, R.; Dargazanli, C.; Blanc, R.; Gory, B.; Richard, S.; Marnat, G.; Sibon, I.; Guillon, B.; Bourcier, R.; et al. Endovascular treatment of ischemic stroke due to isolated internal carotid artery occlusion: ETIS registry data analysis. J. Neurol. 2022, 269, 4383–4395. [Google Scholar] [CrossRef] [PubMed]
- Vo, K.D.; Yoo, A.J.; Gupta, A.; Qiao, Y.; Vagal, A.; Hirsch, J.; Yousem, D.; Lum, C. Multimodal diagnostic imaging for hyperacute stroke. AJNR Am. J. Neuroradiol. 2015, 36, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Marquering, H.A.; Nederkoorn, P.J.; Beenen, L.F.; Nijeholt, G.J.L.; van den Berg, R.; Roos, Y.B.; Majoie, C.B. Carotid pseudo-occlusion on CTA in patients with acute ischemic stroke: A concerning observation. Clin. Neurol. Neurosurg. 2013, 115, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Dillon, W.P.; Glastonbury, C.M.; Provenzale, J.M.; Wintermark, M. Sixty-four-section multidetector CT angiography of carotid arteries: A systematic analysis of image quality and artifacts. AJNR Am. J. Neuroradiol. 2010, 31, 91–99. [Google Scholar] [CrossRef]
- Grossberg, J.A.; Haussen, D.C.; Cardoso, F.B.; Rebello, L.C.; Bouslama, M.; Anderson, A.M.; Frankel, M.R.; Nogueira, R.G. Cervical carotid pseudo-occlusions and false dissections: Intracranial Occlusions Masquerading as Extracranial Occlusions. Stroke 2017, 48, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Prakkamakul, S.; Pitakvej, N.; Dumrongpisutikul, N.; Lerdlum, S. Mid-cervical flame-shaped pseudo-occlusion: Diagnostic performance of mid-cervical flame-shaped extracranial internal carotid artery sign on computed tomographic angiography in hyperacute ischemic stroke. Neuroradiology 2017, 59, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Kappelhof, M.; Marquering, H.A.; Berkhemer, O.A.; Borst, J.; van der Lugt, A.; van Zwam, W.H.; Vos, J.A.; Lycklama, À.; Nijeholt, G.; Majoie, C.B.L.M.; et al. Accuracy of CT angiography for differentiating pseudo-occlusion from true occlusion or high-grade stenosis of the extracranial ICA in acute ischemic stroke: A retrospective MR CLEAN substudy. AJNR Am. J. Neuroradiol. 2018, 39, 892–898. [Google Scholar] [CrossRef]
- Flis, C.M.; Jäger, H.R.; Sidhu, P.S. Carotid and vertebral artery dissections: Clinical aspects, imaging features and endovascular treatment. Eur. Radiol. 2007, 17, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Rodallec, M.H.; Marteau, V.; Gerber, S.; Desmottes, L.; Zins, M. Craniocervical arterial dissection: Spectrum of imaging findings and differential diagnosis. RadioGraphics 2008, 28, 1711–1728. [Google Scholar] [CrossRef]
- Akpınar, S.; Gelener, P.; Yilmaz, G. Aetiologies of internal carotid artery pseudo-occlusions in acute stroke patients: What neurointerventionalists can expect. Br. J. Radiol. 2017, 90, 20160352. [Google Scholar] [CrossRef]
- Diouf, A.; Fahed, R.; Gaha, M.; Chagnon, M.; Khoury, N.; Kotowski, M.; Guilbert, F.; Landry, D.; Raymond, J.; Roy, D.; et al. Cervical internal carotid occlusion versus pseudo-occlusion at CT angiography in the context of acute stroke: An accuracy, interobserver, and intraobserver agreement study. Radiology 2018, 286, 1008–1015. [Google Scholar] [CrossRef]
- Siddiq, F.; Chaudhry, S.A.; Das, P.; Khatri, R.; Rodriguez, G.; Qureshi, A.I. Occurrence and prognostic significance of cervical pseudodissection phenomenon associated with acute intracranial internal carotid artery occlusion. J. Neuroimaging 2013, 23, 384–390. [Google Scholar] [CrossRef]
- Rha, J.H.; Saver, J.L. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke 2007, 38, 967–973. [Google Scholar] [CrossRef]
- Ng, F.C.; Choi, P.M.C.; Datta, M.; Gilligan, A. Perfusion-derived dynamic 4D CT angiography identifies carotid pseudo-occlusion in hyperacute stroke. J. Neuroimaging 2016, 26, 588–591. [Google Scholar] [CrossRef] [PubMed]



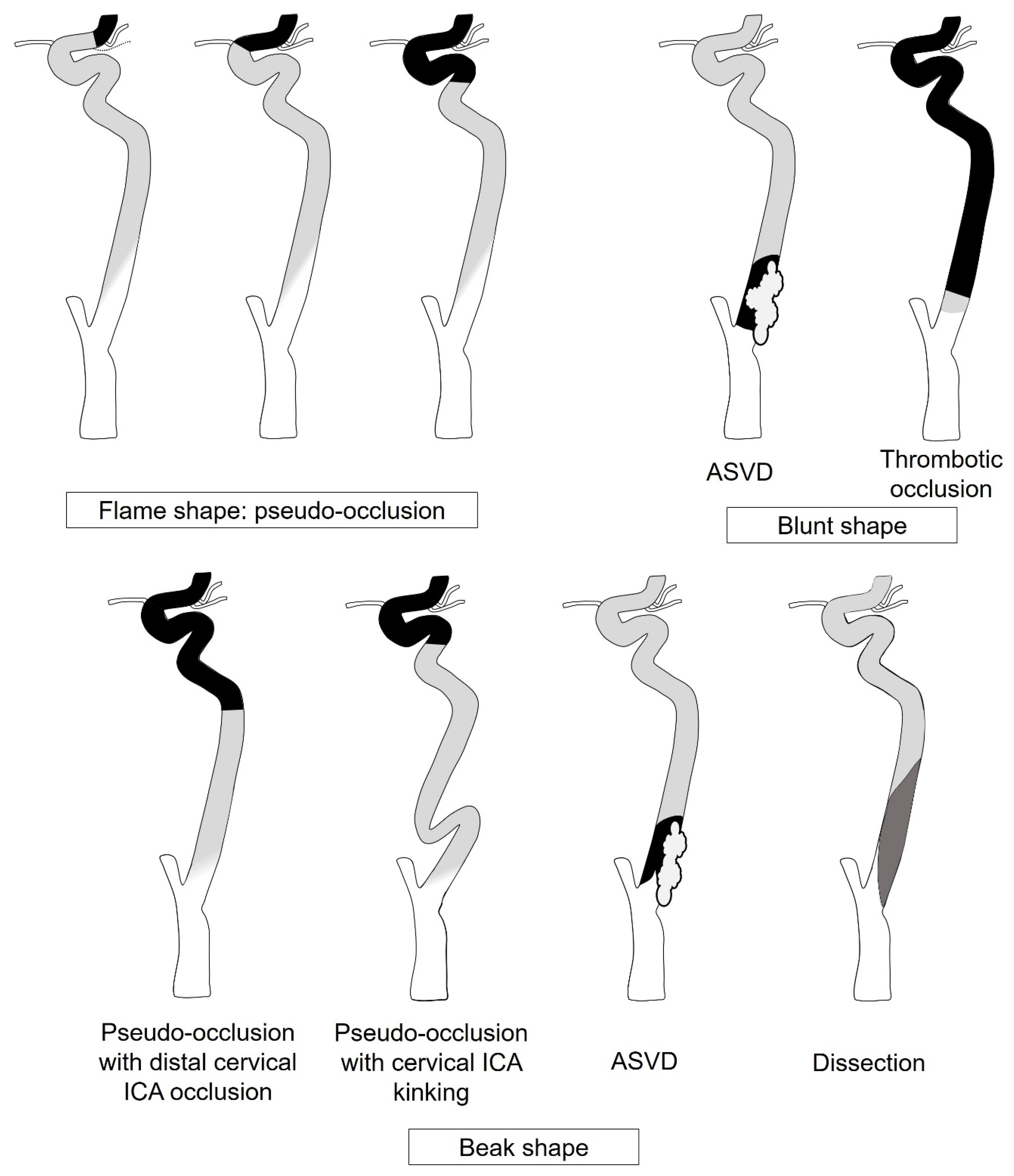
| Diagnosis | Pseudo-Occlusion (n = 51) | ASVD (n = 27) | Thrombotic Occlusion (n = 9) | Dissection (n = 6) | Apparent ICAO (n = 21) | p-Value |
|---|---|---|---|---|---|---|
| Shape | <0.001 | |||||
| Flame | 45 (88.2%) | 0 (0%) | 0 (0%) | 1 (16.7%) | ||
| Beak | 6 (11.8%) | 17 (63.0%) | 2 (22.2%) | 5 (83.3%) | ||
| Blunt | 0 (0%) | 10 (37.0%) | 7 (77.8%) | 0 (0%) | ||
| Tubular | 21 (100%) | |||||
| Location | <0.001 | |||||
| Dependent | 50 (98.0%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | n.a. | |
| Non-dependent | 1 (2.0%) | 27 (100%) | 9 (100.0%) | 5 (83.3%) | n.a. | |
| Margin | <0.001 | |||||
| Ill-defined | 50 (98.0%) | 2 (7.4%) | 3 (33.3%) | 1 (16.7%) | n.a. | |
| Circumscribed | 1 (2.0%) | 25 (92.6%) | 6 (66.7%) | 5 (83.3%) | n.a. | |
| Hounsfield Unit | 117.6 ± 24.0 | 59.3 ± 22.6 | 84.7 ± 32.6 | 88.4 ± 30.9 | n.a. | <0.001 |
| Calcification | <0.001 | |||||
| Present | 10 (19.6%) | 23 (85.2%) | 3 (33.3%) | 4 (66.7%) | n.a. | |
| Absent | 41 (81.4%) | 4 (14.8%) | 6 (66.7%) | 2 (33.3%) | n.a. | |
| Degree of calcification | <0.001 | |||||
| Mild | 4 (7.8%) | 4 (14.8%) | 0 (0.0%) | 4 (66.7%) | n.a. | |
| Moderate | 6 (11.8%) | 7 (25.9%) | 3 (33.3%) | 0 (0.0%) | n.a. | |
| Severe | 0 (0.0%) | 12 (44.4%) | 0 (0.0%) | 0 (0.0%) | n.a. | |
| Curvilinear hypoattenuated stripe | 0.096 | |||||
| Present | 6 (11.8%) | 1 (3.7%) | 1 (11.1%) | 1 (16.7%) | n.a. | |
| Absent | 45 (88.2%) | 26 (96.3%) | 8 (88.9%) | 5 (83.3%) | n.a. | |
| Etiology | Occlusion Site on DSA | ICA Shape on CTA | Number | Remarks |
|---|---|---|---|---|
| Pseudo- occlusion (n = 51) | Distal cervical ICA | Beak | 5 (9.8%) | |
| Petro-cavernous ICA | Flame | 32 (62.7%) | ||
| Beak | 1 (2.0%) | Kinking of proximal cervical ICA | ||
| Ophthalmic ICA | Flame | 5 (9.8%) | ||
| Communicating ICA | Flame | 3 (5.9%) | Spontaneous recanalization after IV tPA injection (2/3) | |
| Hypoplastic posterior communicating artery (1/3) | ||||
| Terminal ICA | Flame | 1 (2.0%) | Spontaneous recanalization after IV tPA injection | |
| MCA | Flame | 4 (7.8%) | Spontaneous recanalization after IV tPA injection | |
| Apparent ICAO (n = 21) | Ophthalmic ICA | 7 (33.3%) | ||
| Communicating ICA | 13 (61.9%) | |||
| Terminal ICA | 1 (4.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.K.; Kim, B.; You, S.-H. Differentiation of Acute Internal Carotid Artery Occlusion Etiology on Computed Tomography Angiography: Diagnostic Tree for Preparing Endovascular Treatment. Diagnostics 2024, 14, 1524. https://doi.org/10.3390/diagnostics14141524
Kim BK, Kim B, You S-H. Differentiation of Acute Internal Carotid Artery Occlusion Etiology on Computed Tomography Angiography: Diagnostic Tree for Preparing Endovascular Treatment. Diagnostics. 2024; 14(14):1524. https://doi.org/10.3390/diagnostics14141524
Chicago/Turabian StyleKim, Bo Kyu, Byungjun Kim, and Sung-Hye You. 2024. "Differentiation of Acute Internal Carotid Artery Occlusion Etiology on Computed Tomography Angiography: Diagnostic Tree for Preparing Endovascular Treatment" Diagnostics 14, no. 14: 1524. https://doi.org/10.3390/diagnostics14141524
APA StyleKim, B. K., Kim, B., & You, S.-H. (2024). Differentiation of Acute Internal Carotid Artery Occlusion Etiology on Computed Tomography Angiography: Diagnostic Tree for Preparing Endovascular Treatment. Diagnostics, 14(14), 1524. https://doi.org/10.3390/diagnostics14141524






