Markers of Epithelial–Mesenchymal Transition and Mucinous Histology Are Significant Predictors of Disease Severity and Tumor Characteristics in Early-Onset Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Immunohistochemical Analysis
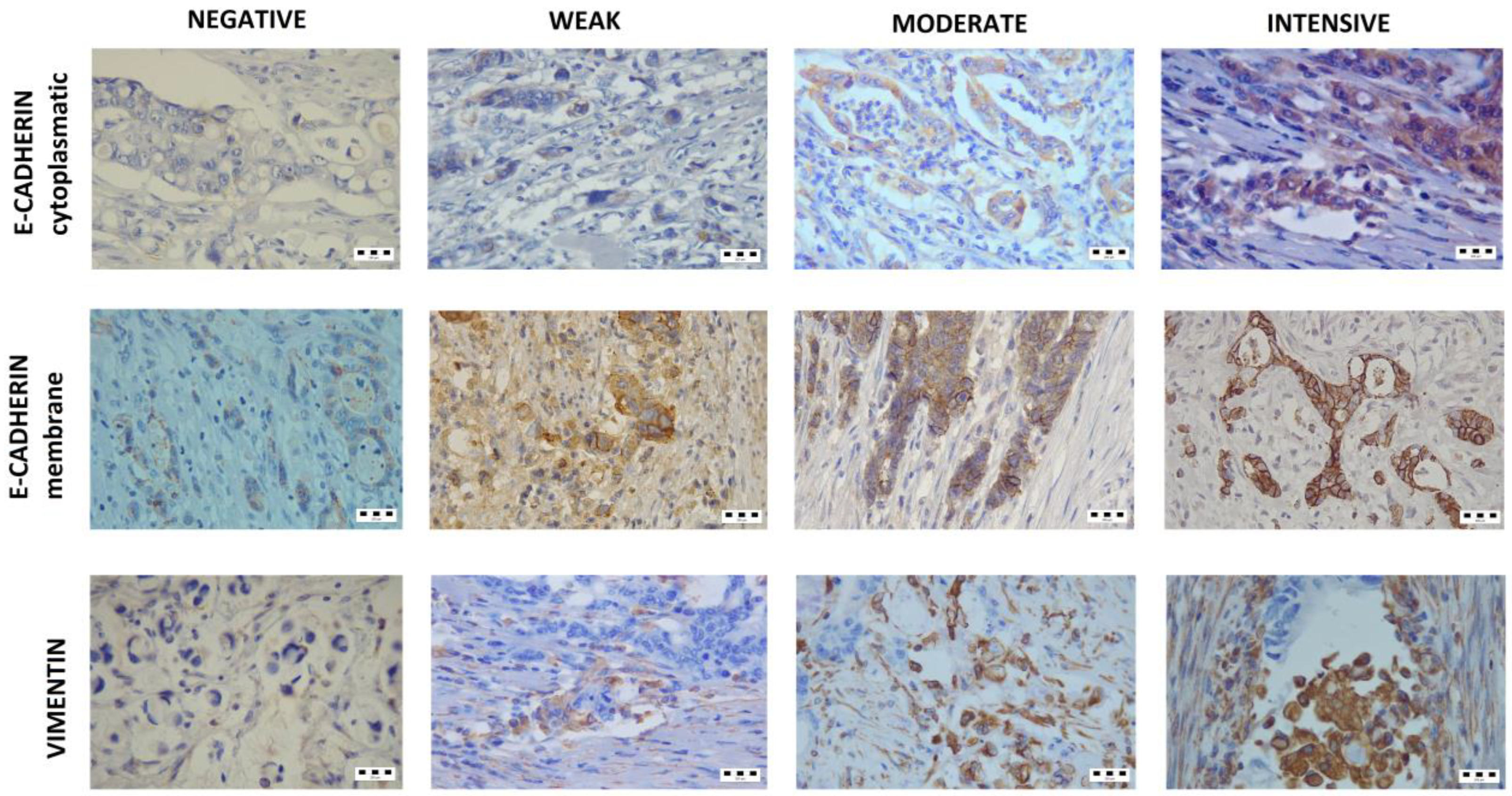
2.3. Evaluation of E-Cadherin Expression
2.4. Evaluation of Vimentin Expression
2.5. Evaluation of Mucin-1 Expression
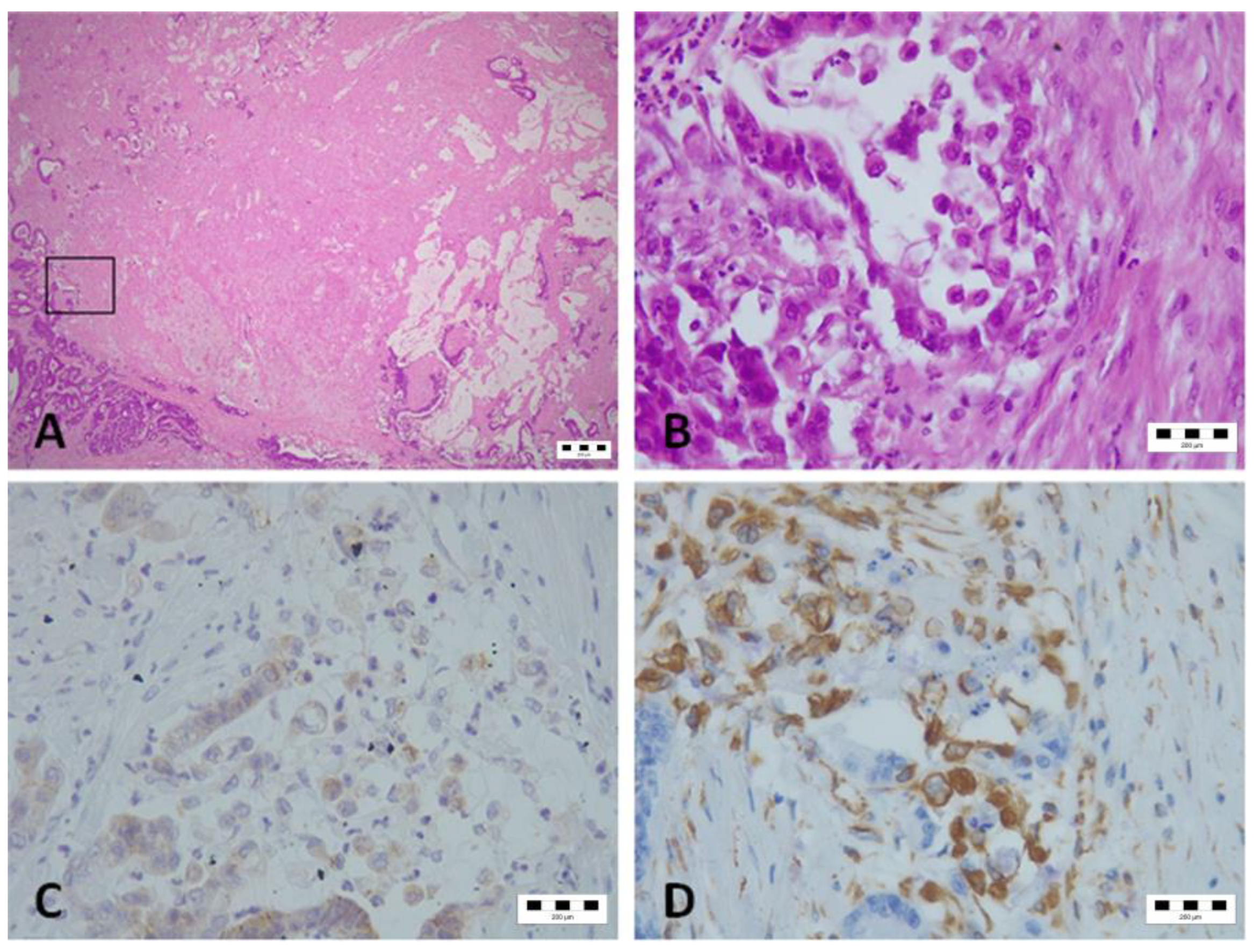
2.6. Pancytokeratin Staining and Tumor Budding Evaluation
2.7. Statistical Analysis
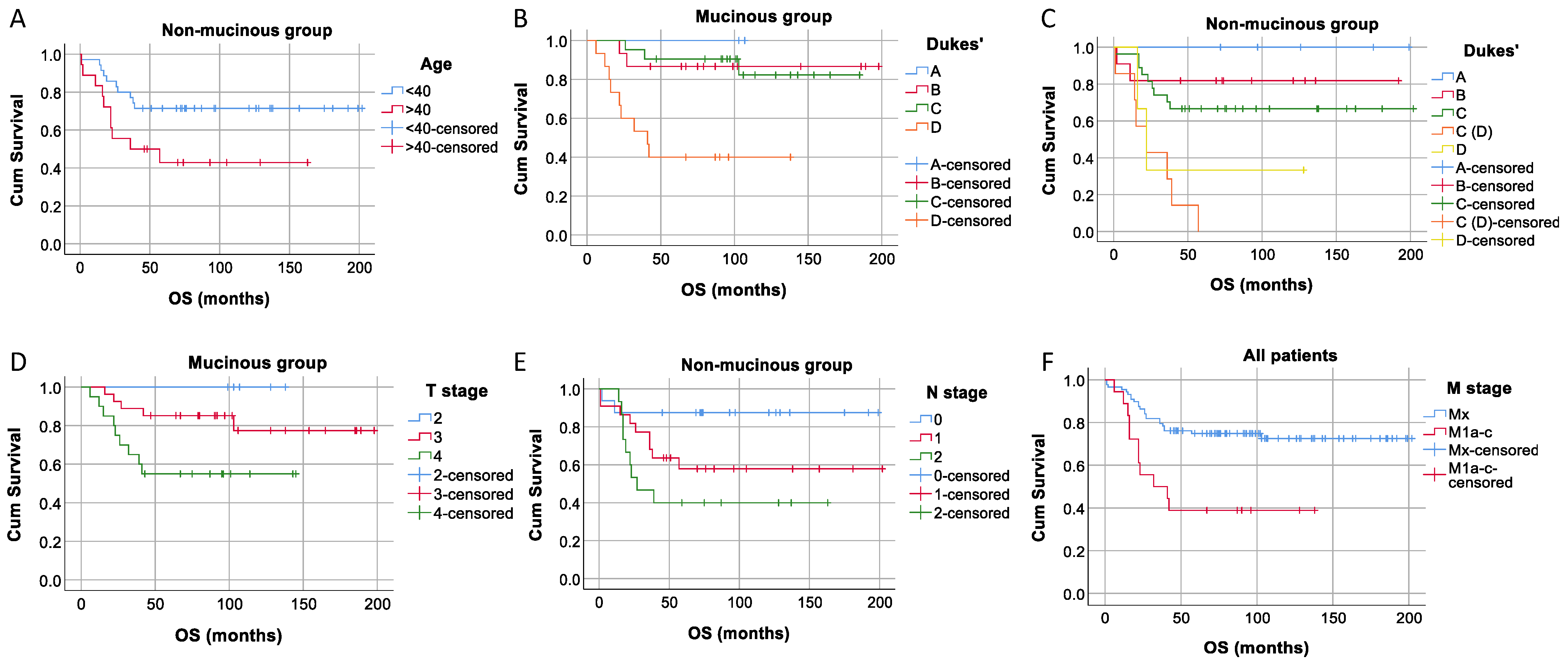
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.G.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, S.; Sussman, D.A.; Deshpande, A. US Preventive Services Task Force Recommendation Statement on Screening for Colorectal Cancer. JAMA 2021, 326, 1328. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bharti, B.; Ahnen, D.J.; Buchanan, D.D.; Cheng, I.C.; Cotterchio, M.; Figueiredo, J.C.; Gallinger, S.J.; Haile, R.W.; Jenkins, M.A.; et al. Potential impact of family history-based screening guidelines on the detection of early-onset colorectal cancer. Cancer 2020, 126, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Stigliano, V.; Sanchez-Mete, L.; Martayan, A.; Anti, M. Early-onset colorectal cancer: A sporadic or inherited disease? World J. Gastroenterol. 2014, 20, 12420–12430. [Google Scholar] [CrossRef] [PubMed]
- Cavestro, G.M.; Mannucci, A.; Zuppardo, R.A.; Di Leo, M.; Stoffel, E.; Tonon, G. Early onset sporadic colorectal cancer: Worrisome trends and oncogenic features. Dig. Liver Dis. 2018, 50, 521–532. [Google Scholar] [CrossRef]
- Willauer, A.N.; Liu, Y.; Pereira, A.A.L.; Lam, M.; Morris, J.S.; Raghav, K.P.S.; Morris, V.K.; Menter, D.; Broaddus, R.; Meric-Bernstam, F.; et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019, 125, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, B.A.; Marshall, J.L. Colon Cancer in Young Adults: Trends and Their Implications. Curr. Oncol. Rep. 2019, 21, 3. [Google Scholar] [CrossRef]
- Yeo, H.; Betel, D.; Abelson, J.S.; Zheng, X.E.; Yantiss, R.; Shah, M.A. Early-onset Colorectal Cancer is Distinct From Traditional Colorectal Cancer. Clin. Color. Cancer 2017, 16, 293–299.e296. [Google Scholar] [CrossRef]
- Luo, C.; Cen, S.; Ding, G.; Wu, W. Mucinous colorectal adenocarcinoma: Clinical pathology and treatment options. Cancer Commun. 2019, 39, 13. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Zhou, B.; Zhong, Y.; Jin, H.; Wang, X. Survival of patients with resected primary colorectal mucinous adenocarcinoma: A competing risk nomogram analysis. Oncol. Lett. 2019, 18, 6594–6604. [Google Scholar] [CrossRef] [PubMed]
- Niknami, Z.; Muhammadnejad, A.; Ebrahimi, A.; Harsani, Z.; Shirkoohi, R. Significance of E-cadherin and Vimentin as epithelial-mesenchymal transition markers in colorectal carcinoma prognosis. Excli J. 2020, 19, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Muller, K.E.; Pattabiraman, D.R. Quantifying the Epithelial-to-Mesenchymal Transition (EMT) from Bench to Bedside. Cancers 2022, 14, 1138. [Google Scholar] [CrossRef] [PubMed]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, M.P.; Seshacharyulu, P.; Lakshmanan, I.; Vaz, A.P.; Chugh, S.; Batra, S.K. Emerging role of mucins in epithelial to mesenchymal transition. Curr. Cancer Drug Targets 2013, 13, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; You, C.; Dou, J. Role of transmembrane glycoprotein mucin 1 (MUC1) in various types of colorectal cancer and therapies: Current research status and updates. Biomed. Pharmacother. 2018, 107, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, T.; Yin, L.; Zuo, D.; Lin, Y.; Wang, L. Prognostic and clinicopathological value of MUC1 expression in colorectal cancer: A meta-analysis. Medecine 2019, 98, e14659. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: Greer, SC, USA, 2017; Volume 1024. [Google Scholar]
- Mori, T. A comparison of the new (planned) TNM classification and Japanese general rule for staging colorectal cancer. Cancer Investig. 2010, 28, 387–392. [Google Scholar] [CrossRef]
- Banias, L.; Jung, I.; Chiciudean, R.; Gurzu, S. From Dukes-MAC Staging System to Molecular Classification: Evolving Concepts in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 9455. [Google Scholar] [CrossRef]
- Wick, M.R. The hematoxylin and eosin stain in anatomic pathology-An often-neglected focus of quality assurance in the laboratory. Semin. Diagn. Pathol. 2019, 36, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Elzagheid, A.; Kuopio, T.; Ilmen, M.; Collan, Y. Prognostication of invasive ductal breast cancer by quantification of E-cadherin immunostaining: The methodology and clinical relevance. Histopathology 2002, 41, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Elzagheid, A.; Algars, A.; Bendardaf, R.; Lamlum, H.; Ristamaki, R.; Collan, Y.; Syrjanen, K.; Pyrhonen, S. E-cadherin expression pattern in primary colorectal carcinomas and their metastases reflects disease outcome. World J. Gastroenterol. 2006, 12, 4304–4309. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Yasuda, H.; Saigusa, S.; Tanaka, K.; Inoue, Y.; Goel, A.; Kusunoki, M. Increased expression of Slug and Vimentin as novel predictive biomarkers for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis 2013, 34, 2548–2557. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A.; Siodła, E.; Andrzejewska, M.; Szmeja, J.; Seraszek-Jaros, A.; Cofta, S.; Szaflarski, W. Differential expression of mucin 1 and mucin 2 in colorectal cancer. World J. Gastroenterol. 2018, 24, 4164–4177. [Google Scholar] [CrossRef] [PubMed]
- Manne, U.; Weiss, H.L.; Grizzle, W.E. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin. Cancer Res. 2000, 6, 4017–4025. [Google Scholar] [PubMed]
- Duncan, T.J.; Watson, N.F.; Al-Attar, A.H.; Scholefield, J.H.; Durrant, L.G. The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World J. Surg. Oncol. 2007, 5, 31. [Google Scholar] [CrossRef]
- Yamagishi, H.; Imai, Y.; Okamura, T.; Fukuda, K.; Ono, Y.; Ban, S.; Inoue, T.; Ueda, Y. Aberrant cytokeratin expression as a possible prognostic predictor in poorly differentiated colorectal carcinoma. J. Gastroenterol. Hepatol. 2013, 28, 1815–1822. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rauth, S.; Ganguly, K.; Zhang, C.; Lakshmanan, I.; Batra, S.K.; Ponnusamy, M.P. Mucins reprogram stemness, metabolism and promote chemoresistance during cancer progression. Cancer Metastasis Rev. 2021, 40, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Gurzu, S.; Bara, T.; Molnar, C.; Butiurca, V.; Beres, H.; Savoji, S.; Jung, I. The epithelial-mesenchymal transition induces aggressivity of mucinous cystic neoplasm of the pancreas with neuroendocrine component: An immunohistochemistry study. Pathol. Res. Pract. 2019, 215, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, R.; Uesugi, N.; Yamada, N.; Osakabe, M.; Fujita, Y.; Eizuka, M.; Kato, R.; Ishida, K.; Obara, W.; Nagashima, Y.; et al. Sarcomatoid change associated with epithelial-mesenchymal transition in mucinous tubular and spindle cell carcinoma of the kidney: A case report. Int. J. Clin. Exp. Pathol. 2019, 12, 2767–2771. [Google Scholar] [PubMed]
- Bhuyan, G.; Arora, R.; Ahluwalia, C.; Sharma, P. Epithelial-mesenchymal transition in serous and mucinous epithelial tumors of the ovary. J. Cancer Res. Ther. 2019, 15, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Silla, I.O.; Rueda, D.; Rodríguez, Y.; García, J.L.; de la Cruz Vigo, F.; Perea, J. Early-onset colorectal cancer: A separate subset of colorectal cancer. World J. Gastroenterol. 2014, 20, 17288–17296. [Google Scholar] [CrossRef]
- Archambault, A.N.; Su, Y.R.; Jeon, J.; Thomas, M.; Lin, Y.; Conti, D.V.; Win, A.K.; Sakoda, L.C.; Lansdorp-Vogelaar, I.; Peterse, E.F.P.; et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer. Gastroenterology 2020, 158, 1274–1286.e1212. [Google Scholar] [CrossRef] [PubMed]
- Archambault, A.N.; Lin, Y.; Jeon, J.; Harrison, T.A.; Bishop, D.T.; Brenner, H.; Casey, G.; Chan, A.T.; Chang-Claude, J.; Figueiredo, J.C.; et al. Nongenetic Determinants of Risk for Early-Onset Colorectal Cancer. JNCI Cancer Spectr. 2021, 5, pkab029. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Jung, A.; Reu, S.; Porzner, M.; Hlubek, F.; Kunz-Schughart, L.A.; Knuechel, R.; Kirchner, T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA 2001, 98, 10356–10361. [Google Scholar] [CrossRef] [PubMed]
- Rosivatz, E.; Becker, I.; Bamba, M.; Schott, C.; Diebold, J.; Mayr, D.; Höfler, H.; Becker, K.F. Neoexpression of N-cadherin in E-cadherin positive colon cancers. Int. J. Cancer 2004, 111, 711–719. [Google Scholar] [CrossRef]
- Ye, X.; Weinberg, R.A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef]
- Tsanou, E.; Peschos, D.; Batistatou, A.; Charalabopoulos, A.; Charalabopoulos, K. The E-cadherin adhesion molecule and colorectal cancer. A global literature approach. Anticancer. Res. 2008, 28, 3815–3826. [Google Scholar] [PubMed]
- Bruun, J.; Kolberg, M.; Nesland, J.M.; Svindland, A.; Nesbakken, A.; Lothe, R.A. Prognostic Significance of β-Catenin, E-Cadherin, and SOX9 in Colorectal Cancer: Results from a Large Population-Representative Series. Front. Oncol. 2014, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Hugo, H.J.; Gunasinghe, N.P.A.D.; Hollier, B.G.; Tanaka, T.; Blick, T.; Toh, A.; Hill, P.; Gilles, C.; Waltham, M.; Thompson, E.W. Epithelial requirement for in vitro proliferation and xenograft growth and metastasis of MDA-MB-468 human breast cancer cells: Oncogenic rather than tumor-suppressive role of E-cadherin. Breast Cancer Res. 2017, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Inamura, K.; Yamauchi, M.; Nishihara, R.; Mima, K.; Sukawa, Y.; Li, T.; Yasunari, M.; Morikawa, T.; Fitzgerald, K.C.; et al. Loss of CDH1 (E-cadherin) expression is associated with infiltrative tumour growth and lymph node metastasis. Br. J. Cancer 2016, 114, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Choi, S.Y.; Kim, W.J.; Ji, M.; Lee, T.G.; Son, B.R.; Yoon, S.M.; Sung, R.; Lee, E.J.; Youn, S.J.; et al. Combined aberrant expression of E-cadherin and S100A4, but not β-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn. Pathol. 2013, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, Q.; Zhang, Y.; Lu, M.; Liu, Y.; Zheng, T.; Feng, S.; Hao, M.; Shi, H. MUC1 Predicts Colorectal Cancer Metastasis: A Systematic Review and Meta-Analysis of Case Controlled Studies. PLoS ONE 2015, 10, e0138049. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Pathangey, L.B.; Bradley, J.B.; Tinder, T.L.; Basu, G.D.; Akporiaye, E.T.; Gendler, S.J. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine 2007, 25, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Cen, Q.; Lei, H. A review on development of MUC1-based cancer vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef]
- Abualkhair, W.H.; Zhou, M.; Ahnen, D.; Yu, Q.; Wu, X.C.; Karlitz, J.J. Trends in Incidence of Early-Onset Colorectal Cancer in the United States Among Those Approaching Screening Age. JAMA Netw. Open 2020, 3, e1920407. [Google Scholar] [CrossRef]
- Gausman, V.; Dornblaser, D.; Anand, S.; Hayes, R.B.; O’Connell, K.; Du, M.; Liang, P.S. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 2752–2759.e2752. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; Board, W.C.o.T.E. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.Q.; Li, Q.G.; Ma, Y.L.; Peng, J.J.; Cai, S. Mucinous Adenocarcinomas Histotype Can Also be a High-Risk Factor for Stage II Colorectal Cancer Patients. Cell Physiol. Biochem. 2018, 47, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, M.; Okabayashi, K.; Hasegawa, H.; Tsuruta, M.; Kitagawa, Y. The Worse Prognosis of Right-Sided Compared with Left-Sided Colon Cancers: A Systematic Review and Meta-analysis. J. Gastrointest. Surg. 2016, 20, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.M.; Yeh, C.Y.; Changchien, C.R.; Chen, J.S.; Tang, R.; Chen, J.R. Mucinous adenocarcinoma showing different clinicopathological and molecular characteristics in relation to different colorectal cancer subgroups. Int. J. Color. Dis. 2010, 25, 941–947. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, Z.; Jia, M.; Zhao, X. Downregulated E-cadherin expression indicates worse prognosis in Asian patients with colorectal cancer: Evidence from meta-analysis. PLoS ONE 2013, 8, e70858. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Sugai, T.; Eizuka, M.; Tsuchida, K.; Sugimoto, R.; Mue, Y.; Suzuki, M.; Osakabe, M.; Uesugi, N.; Ishida, K.; et al. Tumor budding at the invasive front of colorectal cancer may not be associated with the epithelial-mesenchymal transition. Hum. Pathol. 2017, 60, 151–159. [Google Scholar] [CrossRef]
- Zlobec, I.; Lugli, A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: Tumor budding as oncotarget. Oncotarget 2010, 1, 651–661. [Google Scholar] [CrossRef]
- Galván, J.A.; Helbling, M.; Koelzer, V.H.; Tschan, M.P.; Berger, M.D.; Hädrich, M.; Schnüriger, B.; Karamitopoulou, E.; Dawson, H.; Inderbitzin, D.; et al. TWIST1 and TWIST2 promoter methylation and protein expression in tumor stroma influence the epithelial-mesenchymal transition-like tumor budding phenotype in colorectal cancer. Oncotarget 2015, 6, 874–885. [Google Scholar] [CrossRef]
- Bronsert, P.; Enderle-Ammour, K.; Bader, M.; Timme, S.; Kuehs, M.; Csanadi, A.; Kayser, G.; Kohler, I.; Bausch, D.; Hoeppner, J.; et al. Cancer cell invasion and EMT marker expression: A three-dimensional study of the human cancer-host interface. J. Pathol. 2014, 234, 410–422. [Google Scholar] [CrossRef] [PubMed]
| Non-Mucionous (n = 53) | Mucinous (n = 53) | p Value | |
|---|---|---|---|
| Age, years | 36.9 ± 5.2 | 35.7 ± 7.2 | 0.619 |
| Male gender, % | 66.0 | 66.0 | 1.000 |
| Tumor localization, % | |||
| rectum + left colon | 81.1 | 60.4 | 0.039 * |
| right colon | 18.9 | 39.6 | |
| T stadium, % | |||
| T2 | 13.2 | 11.3 | 0.587 |
| T3 | 58.5 | 50.9 | |
| T4 | 28.3 | 37.8 | |
| N stadium, % | |||
| N0 | 32.0 | 30.2 | 0.429 |
| N1 | 30.2 | 41.5 | |
| N2 | 37.8 | 28.3 | |
| TNM stage, % | |||
| I | 9.4 | 3.8 | 0.027 * |
| IIA | 13.2 | 20.8 | |
| IIB | 3.8 | 3.8 | |
| IIC | 3.8 | 3.8 | |
| IIIA | 3.8 | 0 | |
| IIIB | 43.4 | 28.3 | |
| IIIC | 16.9 | 11.3 | |
| IVA | 3.8 | 9.4 | |
| IVB | 1.9 | 0 | |
| IVC | 0 | 18.8 | |
| Tumor grade, % | |||
| G1 + G2 | 94.3 | 73.6 | 0.007 * |
| G3 | 5.7 | 26.4 | |
| Tumor budding, % | |||
| 1 | 26.4 | 37.8 | 0.450 |
| 2 | 20.8 | 18.9 | |
| 3 | 52.8 | 43.3 | |
| L positive, % | 73.1 | 84.8 | 0.218 |
| V positive, % | 51.9 | 54.2 | 0.844 |
| Mucin-1 score | 2.07 ± 0.96 | 3.15 ± 0.64 | <0.001 * |
| E-cadherin score | |||
| total | 1.84 ± 1.46 | 2.17 ± 1.09 | 0.112 |
| membrane | 0.84 ± 0.96 | 0.95 ± 0.89 | 0.206 |
| cytoplasmatic | 1.00 ± 0.62 | 1.21 ± 0.42 | 0.043 * |
| Vimentin score | 0.85 ± 1.73 | 0.62 ± 1.47 | 0.473 |
| Epithelial (n = 51) | Mesenchymal (n = 55) | p Value | |
|---|---|---|---|
| Age, years | 37.1 ± 7.2 | 35.6 ± 6.0 | 0.143 |
| Male gender, % | 70.6 | 61.8 | 0.413 |
| Tumor localization, % | |||
| rectum + left colon | 72.5 | 65.5 | 0.726 |
| right colon | 27.5 | 34.5 | |
| T stadium, % | |||
| T2 | 15.7 | 9.1 | 0.231 |
| T3 | 58.8 | 50.9 | |
| T4 | 25.5 | 40.0 | |
| N stadium, % | |||
| N0 | 39.2 | 23.6 | 0.091 |
| N1 | 37.3 | 34.5 | |
| N2 | 23.5 | 41.9 | |
| TNM stage, % | |||
| I | 9.8 | 3.6 | 0.198 |
| IIA | 21.6 | 12.7 | |
| IIB | 2.0 | 5.4 | |
| IIC | 5.8 | 1.9 | |
| IIIA | 2.0 | 1.9 | |
| IIIB | 37.2 | 34.5 | |
| IIIC | 7.8 | 20.0 | |
| IVA | 2.0 | 10.9 | |
| IVB | 0 | 1.9 | |
| IVC | 11.8 | 7.2 | |
| Tumor grade, % | |||
| G1 + G2 | 92.2 | 76.4 | 0.034 * |
| G3 | 7.8 | 23.6 | |
| Tumor budding, % | |||
| 1 | 43.1 | 21.8 | <0.001 * |
| 2 | 31.4 | 9.1 | |
| 3 | 25.5 | 69.1 | |
| L positive, % | 73.9 | 82.7 | 0.331 |
| V positive, % | 44.7 | 60.4 | 0.160 |
| Mucin-1 score | 2.56 ± 0.98 | 2.64 ± 0.98 | 0.716 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djikic Rom, A.; Dragicevic, S.; Jankovic, R.; Radojevic Skodric, S.; Sabljak, P.; Markovic, V.; Stojkovic, J.R.; Barisic, G.; Nikolic, A. Markers of Epithelial–Mesenchymal Transition and Mucinous Histology Are Significant Predictors of Disease Severity and Tumor Characteristics in Early-Onset Colorectal Cancer. Diagnostics 2024, 14, 1512. https://doi.org/10.3390/diagnostics14141512
Djikic Rom A, Dragicevic S, Jankovic R, Radojevic Skodric S, Sabljak P, Markovic V, Stojkovic JR, Barisic G, Nikolic A. Markers of Epithelial–Mesenchymal Transition and Mucinous Histology Are Significant Predictors of Disease Severity and Tumor Characteristics in Early-Onset Colorectal Cancer. Diagnostics. 2024; 14(14):1512. https://doi.org/10.3390/diagnostics14141512
Chicago/Turabian StyleDjikic Rom, Aleksandra, Sandra Dragicevic, Radmila Jankovic, Sanja Radojevic Skodric, Predrag Sabljak, Velimir Markovic, Jovana Rosic Stojkovic, Goran Barisic, and Aleksandra Nikolic. 2024. "Markers of Epithelial–Mesenchymal Transition and Mucinous Histology Are Significant Predictors of Disease Severity and Tumor Characteristics in Early-Onset Colorectal Cancer" Diagnostics 14, no. 14: 1512. https://doi.org/10.3390/diagnostics14141512
APA StyleDjikic Rom, A., Dragicevic, S., Jankovic, R., Radojevic Skodric, S., Sabljak, P., Markovic, V., Stojkovic, J. R., Barisic, G., & Nikolic, A. (2024). Markers of Epithelial–Mesenchymal Transition and Mucinous Histology Are Significant Predictors of Disease Severity and Tumor Characteristics in Early-Onset Colorectal Cancer. Diagnostics, 14(14), 1512. https://doi.org/10.3390/diagnostics14141512










