Expert Consensus Document: An Algorithm for the Care and Treatment of Patients with Constipation Based on Ultrasonographic Findings in the Rectum
Abstract
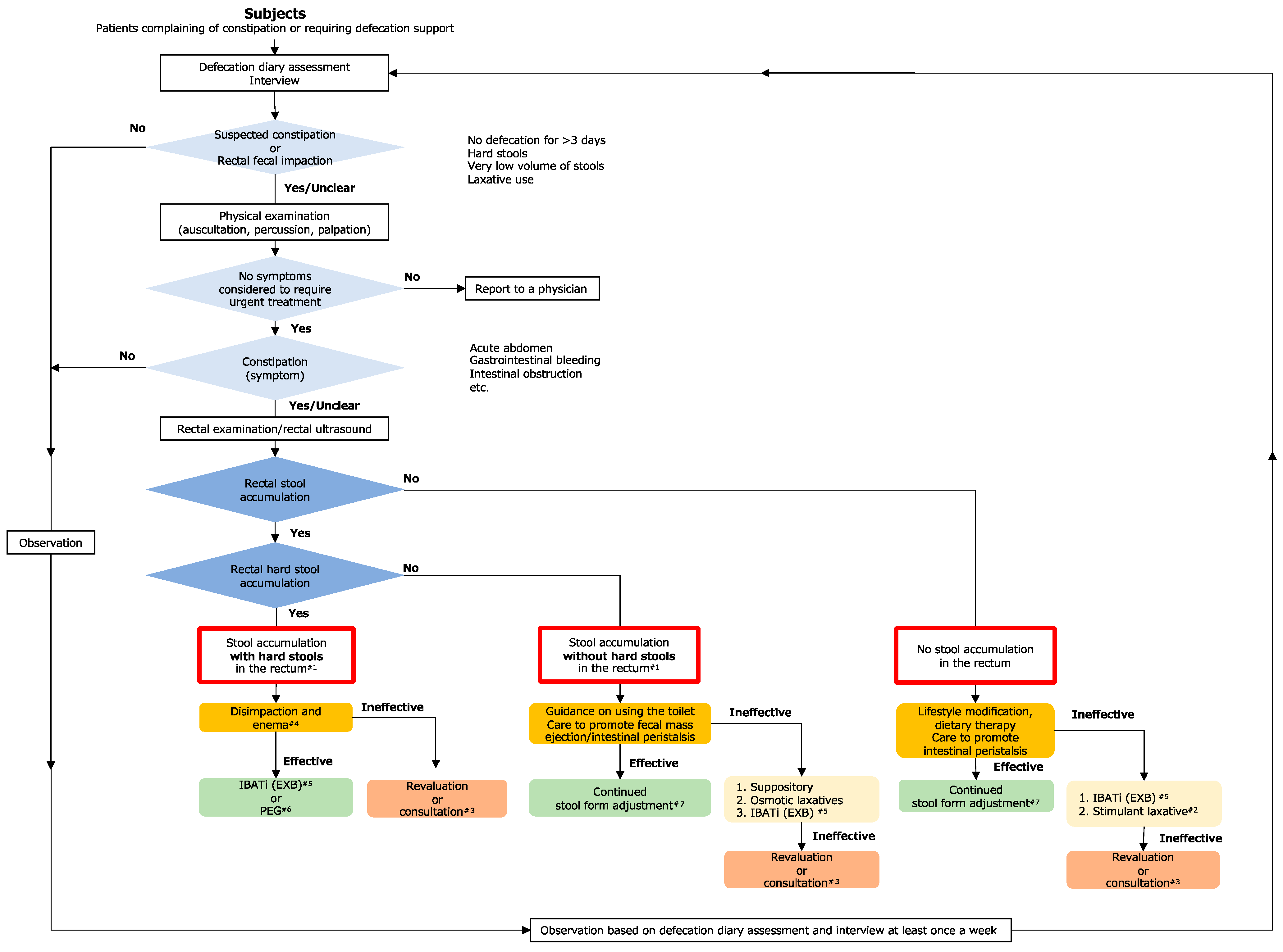
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Rectal Ultrasonography Protocol
2.3. Methods and Classification
2.4. Assessment-Based Selection of Nursing Care Interventions
2.4.1. Care to Promote Stool Mass Discharge
- Fecal disimpaction
- Enema, suppository
- Biofeedback
- Pelvic floor muscle exercises
- Forced defecation method
2.4.2. Care to Promote Intestinal Peristalsis
- Lifestyle improvements
2.4.3. Dietary and Medication Adjustments
- Dietary treatment
- Probiotics
- Pharmacotherapy using laxatives
- Osmotic laxatives
- (i)
- Magnesium oxideOsmotic laxatives increase defecation frequency by inducing intestinal water secretion. Magnesium oxide (MgO) is a common osmotic laxative, and although it is inexpensive and its long-term administration has been reported to be safe, electrolyte abnormalities can cause dehydration and bradycardia, and overdoses can, therefore, be especially problematic in patients with renal and cardiac failure [27]. Oral MgO administration has been reported to increase serum magnesium levels in patients with renal failure [28], and the “Guidelines for Safe Pharmacotherapy of the Elderly 2015” published by the Japanese Geriatrics Society recommend that older patients with renal dysfunction should not receive MgO because of the increased risk of hypermagnesemia [29]. The reported risk factors for hypermagnesemia include age ≥ 68 years, renal dysfunction, MgO dosage > 1650 mg/day, and administration of MgO for >36 days [30]. Additionally, as gastric juices convert MgO into magnesium salts, their effectiveness is generally reduced to less than 50% in patients who have undergone total gastrectomy or are taking acid-secretion inhibitors [31]. When MgO is administered in daily practice, care should be taken regarding surgical and medical histories, especially the administration of proton pump inhibitors and histamine 2 blockers. Caution should also be exercised with regard to the concomitant administration of medications such as acid secretion inhibitors, bisphosphonates, non-steroidal anti-inflammatory drugs, and antiepileptic drugs, as it reduces their efficacy by inhibiting their absorption. Thus, although MgO is widely used, it should be used with caution and after ensuring that renal function will not be impaired.
- (ii)
- Polyethylene glycolThe osmotic effect of polyethylene glycol promotes water secretion into the intestinal tract and exerts a laxative effect [32].
- (iii)
- LactuloseLactulose reaches the lower gastrointestinal tract without being digested or absorbed, increases the osmotic pressure in the intestine to promote water secretion, and is metabolized by intestinal bacteria to produce organic acids that increase intestinal peristalsis and have a laxative effect. It was reported to be significantly effective in a randomized, double-blind, placebo-controlled study in patients with chronic constipation in Japan [33].
- Stimulant laxativesStimulant laxatives include anthraquinones such as sennosides and aloe, and diphenyls such as sodium picosulfate. Both are hydrolyzed to their active forms by intestinal bacteria and enzymes in the digestive tract, which then act on the intermuscular plexus of the colon to promote high-amplitude propagated contractions (HAPCs), inhibit water absorption from the intestinal tract, and produce purgative effects [25,34]. Anthraquinone-based stimulant laxatives are widely used in Japan; however, no randomized controlled trials have investigated their efficacy in the treatment of chronic constipation. In contrast, the diphenyls sodium picosulfate and bisacodyl have been shown to be effective [35,36,37]. As anthraquinones can cause intractable constipation owing to the emergence of tolerance after long-term continuous use, they should be used only when necessary, under supervision, and for short durations or rescue use [15].
- Intestinal secretagogues
- (i)
- Lubiprostone activates the CIC-2 chloride channel on the luminal side of the small intestine and stimulates osmotic secretion of water into the intestinal tract, softening stool and promoting defecation [38]; side effects include nausea and diarrhea. It has been shown to significantly improve the symptoms of OIC and was well tolerated by patients with chronic non-cancer pain [39]. Another advantage is its capsule-based formulation; it is administered once or twice daily after meals and reduces the burden on nurses and caregivers.
- (ii)
- Linaclotide improves gastrointestinal tract hypersensitivity by increasing cyclic guanosine monophosphate levels in intestinal epithelial cells and promoting intestinal fluid secretion and defecation. It has been shown to be effective in treating constipation-type irritable bowel syndrome [40].
- IBAT inhibitorsElobixibat inhibits IBAT expressed on epithelial cells in the terminal portion of the ileum, increasing the amount of bile acid entering the colon, which in turn increases water and electrolyte secretion into the intestinal tract, enhances intestinal peristalsis and promotes defecation [41]. Regarding constipation in cancer patients, an increase in the frequency of spontaneous bowel movements has also been observed, and elobixibat treatment has been shown to be unaffected by the amount of food consumed. Therefore, it is suggested that elobixibat may be used by cancer patients regardless of dietary intake [42]. The decrease in rectal sensory thresholds due to the increase in bile acid levels has restorative effects on the desire to defecate [43,44]. Thus, besides promoting water secretion and intestinal peristalsis, it also recovers the LODD and, therefore, has a triple-action effect.
- Peripherally acting μ-opioid receptor antagonists (PAMORAs)Naldemedine, a PAMORA, is covered by insurance only for OIC in Japan [45]. OIC is caused by decreased water retention, decreased intestinal peristalsis, and contraction of the anal sphincter, and the mechanism of action of naldemedine suggests that it can ameliorate these effects. Until now, OIC has mainly been treated using MgO; however, in a randomized controlled trial in which either MgO or naldemedine was administered simultaneously with opioid initiation as a prophylactic treatment for OIC, naldemedine showed no worsening of the Japanese version of the Patient Assessment of Constipation Quality of Life (JPAC-QOL) score after 2 weeks compared with MgO; additionally, complete spontaneous bowel movements were significantly higher, and the incidence of nausea was significantly lower [46]. We have also investigated the changes in defecation frequency and quality of life after administering various laxatives to patients with OIC to determine which laxatives are effective in treating OIC. The results showed no difference in defecation frequency among conventional and novel laxatives and naldemedine. However, compared with conventional laxatives, naldemedine and the novel laxatives elobixibat and lubiprostone significantly improved defecation-related quality of life; additionally, naldemedine and elobixibat improved defecation-related symptoms. Thus, the prevention and treatment of OIC are expected to transition towards naldemedine. According to recent Japanese guidelines, if symptoms of constipation are observed, the first priority is to deal with drug-induced constipation, especially OIC. Therefore, if there is a possibility of OIC, naldemedine is recommended [15]; notably, it can be used simultaneously with both weak and strong opioids [26].
3. Recommended Care and Treatments Based on Rectal Constipation Ultrasonography and Discussion
3.1. No Fecal Retention in the Rectum
- Ileal bile acid transporter inhibitor (elobixibat, [Goofice®]).
- Stimulant laxative (Sodium picosulfate®), rescue use only.
3.2. Fecal Retention in the Rectum, but No Hard Fecal Impaction
- Suppository (sodium bicarbonate/anhydrous sodium dihydrogen phosphate suppository [New lecicarbon®]).
- Osmotic laxatives (MgO or PEG [Movicol®]).
- IBAT inhibitor (elobixibat [Goofice®]) [preferred, especially in cases of LODD].
3.3. Hard Fecal Retention in the Rectum
- IBAT inhibitor (elobixibat [Goofice®]) [preferred, especially in cases of LODD].
- Osmotic laxatives (polyethylene glycol [Movicol®]).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bharucha, A.E.; Pemberton, J.H.; Locke, G.R., 3rd. American Gastroenterological Association Technical Review on constipation. Gastroenterology 2013, 144, 218–238. [Google Scholar] [CrossRef]
- Bouras, E.P.; Tangalos, E.G. Chronic constipation in the elderly. Gastroenterol. Clin. North. Am. 2009, 38, 463–480. [Google Scholar] [CrossRef]
- Camilleri, M.; Ford, A.C.; Mawe, G.M.; Dinning, P.G.; Rao, S.S.; Chey, W.D.; Simrén, M.; Lembo, A.; Young-Fadok, T.M.; Chang, L. Chronic constipation. Nat. Rev. Dis. Primers 2017, 3, 17095. [Google Scholar] [CrossRef]
- Suares, N.C.; Ford, A.C. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 1582–1591. [Google Scholar] [CrossRef]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and risk of death and cardiovascular events. Atherosclerosis 2019, 281, 114–120. [Google Scholar] [CrossRef]
- Honkura, K.; Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Watanabe, T.; Zhang, S.; Suawara, Y.; Tsuji, I. Defecation frequency and cardiovascular disease mortality in Japan: The Ohsaki cohort study. Atherosclerosis 2016, 246, 251–256. [Google Scholar] [CrossRef]
- Aziz, I.; Whitehead, W.E.; Palsson, O.S.; Törnblom, H.; Simrén, M. An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 39–46. [Google Scholar] [CrossRef]
- Seltzer, R. Evaluation and diagnosis of constipation. Gastroenterol. Nurs. 2012, 35, 343–348. [Google Scholar] [CrossRef]
- Remes-Troche, J.M.; Rao, S.S.C. Diagnostic testing in patients with chronic constipation. Curr. Gastroenterol. Rep. 2006, 8, 416–424. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Ozturk, R.; Laine, L. Clinical utility of diagnostic tests for constipation in adults: A systematic review. Am. J. Gastroenterol. 2005, 100, 1605–1615. [Google Scholar] [CrossRef]
- Berger, M.Y.; Tabbers, M.M.; Kurver, M.J.; Boluyt, N.; Benninga, M.A. Value of abdominal radiography, colonic transit time, and rectal ultrasound scanning in the diagnosis of idiopathic constipation in children: A systematic review. J. Pediatr. 2012, 161, 44–50. [Google Scholar] [CrossRef]
- Perniola, G.; Shek, C.; Chong, C.C.W.; Chew, S.; Cartmill, J.; Dietz, H.P. Defecation proctography and translabial ultrasound in the investigation of defecatory disorders. Ultrasound Obstet. Gynecol. 2008, 31, 567–571. [Google Scholar] [CrossRef]
- Matsumoto, M.; Misawa, N.; Tsuda, M.; Manabe, N.; Kessoku, T.; Tamai, N.; Kawamoto, A.; Sugama, J.; Tanaka, H.; Kato, M.; et al. Expert consensus document: Diagnosis for chronic constipation with faecal retention in the rectum using ultrasonography. Diagnostics 2022, 12, 300. [Google Scholar] [CrossRef]
- Japan Academy of Nursing Science. Clinical Practice Guidelines for Colonic Fecal Retention Assessment during Constipation for Nursing Care. 2023. Available online: https://www.jans.or.jp/modules/en/index.php?content_id=102 (accessed on 8 August 2023).
- Japan Gastroenterological Association. Guidelines for Abnormal Bowel Movements 2023-Chronic Constipation, Nanzan-do.
- Knowles, C.H.; Grossi, U.; Chapman, M.; Mason, J.; NIHR CapaCiTY Working Group; Pelvic floor Society. Surgery for constipation: Systematic review and practice recommendations: Results I: Colonic resection. Colorectal Dis. 2017, 3, 17–36. [Google Scholar] [CrossRef]
- Obokhare, I. Fecal impaction: A cause for concern? Clin. Colon. Rectal Surg. 2012, 25, 53. [Google Scholar] [CrossRef]
- Portalatin, M.; Winstead, N. Medical management of constipation. Clin. Colon. Rectal Surg. 2012, 25, 12–19. [Google Scholar] [CrossRef]
- Heymen, S.; Jones, K.R.; Scarlett, Y.; Whitehead, W.E. Biofeedback treatment of constipation: A critical review. Dis. Colon. Rectum 2003, 46, 1208. [Google Scholar] [CrossRef]
- Chiarioni, G.; Salandini, L.; Whitehead, W.E. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005, 129, 86. [Google Scholar] [CrossRef]
- Gosselink, M.P.; Darby, M.; Zimmerman, D.D.; Smits, A.A.; van Kessel, I.; Hop, W.C.; Briel, J.W.; Schouten, W.R. Long-term follow-up of retrograde colonic irrigation for defaecation disturbances. Color. Dis. 2005, 7, 65. [Google Scholar] [CrossRef]
- Gao, R.; Tao, Y.; Zhou, C.; Li, J.; Wang, X.; Chen, L.; Li, F. Exercise therapy in patients with constipation: A systematic review and meta-analysis of randomized controlled trials. Scand. J. Gastroenterol. 2019, 54, 169–177. [Google Scholar] [CrossRef]
- Hosono, K.; Horioka, T.; Hisamitsu, M. Effect of lower abdominal warming on bowel movement in elderly patients with a low level of consciousness—Analysis based on the Bristol Stool Scale and the Japanese version of the Constipation Assessment Scale-Short Term. Jpn. J. Nurs. Art. Sci. 2013, 11, 28–34. [Google Scholar]
- Kira, I. Random control trial of hot compresses for women those who used laxatives on severity of constipation and quality of life. Jpn. J. Nurs. Sci. 2016, 13, 95–104. [Google Scholar] [CrossRef]
- Andresen, V.; Banerji, V.; Hall, G.; Lass, A.; Emmanuel, A.V. The patient burden of opioid-induced constipation: New insights from a large multinational survey in five European countries. United Eur. Gastroenterol. J. 2018, 6, 1254–1266. [Google Scholar] [CrossRef]
- Kessoku, T.; Misawa, N.; Ohkubo, H.; Nakajima, A. Current treatment practices for adult patients with constipation in Japan. Digestion 2024, 105, 40–48. [Google Scholar] [CrossRef]
- Lembo, A.; Camilleri, M. Chronic constipation. N. Engl. J. Med. 2003, 349, 1360–1368. [Google Scholar] [CrossRef]
- Nyberg, C.; Hendel, J.; Nielsen, O.H. The safety of osmotically acting cathartics in colonic cleansing. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 557–564. [Google Scholar] [CrossRef]
- The Japan Geriatrics Society. Guidelines for Safe Medication for the Elderly; The Japan Geriatrics Society: Tokyo, Japan, 2015. [Google Scholar]
- Wakai, E.; Ikemura, K.; Sugimoto, H.; Iwamoto, T.; Okuda, M. Risk factors for the development of hypermagnesemia in patients prescribed magnesium oxide: A retrospective cohort study. J. Pharm. Health Care Sci. 2019, 5, 4. [Google Scholar] [CrossRef]
- Yamasaki, M.; Funakoshi, S.; Matsuda, S.; Imazu, T.; Takeda, Y.; Murakami, T.; Maeda, Y. Interaction of magnesium oxide with gastric acid secretion inhibitors in clinical pharmacotherapy. Eur. J. Clin. Pharmacol. 2014, 70, 921–924. [Google Scholar] [CrossRef]
- Nakajima, A.; Shinbo, K.; Oota, A.; Kinoshita, Y. Polyethylene glycol 3350 plus electrolytes for chronic constipation: A 2-week, randomized, double-blind, placebo-controlled study with a 52-week open-label extension. J. Gastroenterol. 2019, 54, 792–803. [Google Scholar] [CrossRef]
- Kasugai, K.; Iwai, H.; Kuboyama, N.; Yoshikawa, A.; Fukudo, S. Efficacy and safety of a crystalline lactulose preparation (SK-1202) in Japanese patients with chronic constipation: A randomized, double-blind, placebo-controlled, dose-finding study. J. Gastroenterol. 2019, 54, 530–540. [Google Scholar] [CrossRef]
- Bassotti, G.; Chiarioni, G.; Germani, U.; Battaglia, E.; Vantini, I.; Morelli, A. Endoluminal instillation of bisacodyl in patients with severe (slow transit type) constipation is useful to test residual colonic propulsive activity. Digestion 1999, 60, 69–73. [Google Scholar] [CrossRef]
- Kienzle-Horn, S.; Vix, J.M.; Schuijt, C.; Peil, H.; Jordan, C.C.; Kamm, M.A. Efficacy and safety of bisacodyl in the acute treatment of constipation: A double-blind, randomized, placebo-controlled study. Aliment. Pharmacol. Ther. 2006, 23, 1479–1488. [Google Scholar] [CrossRef]
- Kamm, M.A.; Mueller-Lissner, S.; Wald, A.; Richter, E.; Swallow, R.; Gessner, U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin. Gastroenterol. Hepatol. 2011, 9, 577–583. [Google Scholar] [CrossRef]
- Mueller-Lissner, S.; Kamm, M.A.; Wald, A.; Hinkel, U.; Koehler, U.; Richter, E. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am. J. Gastroenterol. 2010, 105, 897–903. [Google Scholar] [CrossRef]
- Johanson, J.F.; Morton, D.; Geenen, J.; Ueno, R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am. J. Gastroenterol. 2008, 103, 170–177. [Google Scholar] [CrossRef]
- Jamal, M.M.; Adams, A.B.; Jansen, J.P.; Webster, L.R. A randomized placebo-controlled trial of lubiprostone for opioid-induced constipation in chronic noncancer pain. Am. J. Gastroenterol. 2015, 110, 725–732. [Google Scholar] [CrossRef]
- Lembo, A.J.; Schneier, H.A.; Shiff, S.J.; Kurtz, C.B.; MacDougall, J.E.; Jia, X.D.; Shao, J.Z.; Lavins, B.J.; Currie, M.G.; Fitch, D.A.; et al. Two randomized trials of linaclotide for chronic constipation. N. Engl. J. Med. 2011, 365, 527–536. [Google Scholar] [CrossRef]
- Nakajima, A.; Seki, M.; Taniguchi, S.; Ohta, A.; Gillberg, P.G.; Mattsson, J.P.; Camilleri, M. Safety and efficacy of elobixibat for chronic constipation: Results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 537–547. [Google Scholar] [CrossRef]
- Ozaki, A.; Kessoku, T.; Kasai, Y.; Takeda, Y.; Okubo, N.; Iwaki, M.; Kobayashi, T.; Yoshihara, T.; Honda, Y.; Fuyukiet, A.; et al. Elobixibat effectively relieves chronic constipation in patients with cancer regardless of the amount of food intake. Oncologist 2021, 26, e1862–e1869. [Google Scholar] [CrossRef]
- Ishikawa, T. Efficacy of elobixibat on defecation desire in patients with chronic constipation: A single center, retrospective, observational study. Shinryo Shinyaku 2021, 58, 865–872. [Google Scholar]
- Manabe, N.; Umeyama, M.; Ishizaki, S.; Ota, T.; Kuratani, S.; Katsumata, R.; Fujita, M.; Haruma, K.; Camilleri, M. Elobixibat improves rectal sensation in patients with chronic constipation aged ≥60 years: A randomised placebo-controlled study. BMJ Open Gastroenterol. 2023, 10, e001257. [Google Scholar] [CrossRef]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized phase III and extension studies of naldemedine in patients with opioid-induced constipation and cancer. J. Clin. Oncol. 2017, 35, 3859–3866. [Google Scholar] [CrossRef]
- Ozaki, A.; Kessoku, T.; Tanaka, K.; Yamamoto, A.; Takahashi, K.; Takeda, Y.; Kasai, Y.; Iwaki, M.; Kobayashi, T.; Yoshihara, T.; et al. Effectiveness of naldemedine compared with magnesium oxide in preventing opioid-induced constipation: A randomized controlled trial. Cancers 2022, 14, 2112. [Google Scholar] [CrossRef]
- Bampton, P.A.; Dinning, P.G.; Kennedy, M.L.; Lubowski, D.Z.; Cook, I.J. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G443–G449. [Google Scholar] [CrossRef]
- Matsuura, M.; Inamori, M.; Endo, H.; Matsuura, T.; Kanoshima, K.; Inoh, Y.; Fujita, Y.; Umezawa, S.; Fuyuki, A.; Uchiyama, S.; et al. Lubiprostone decreases the small bowel transit time by capsule endoscopy: An exploratory, randomised, double-blind, placebo-controlled 3-way crossover study. Gastroenterol. Res. Pract. 2014, 2014, 879595. [Google Scholar] [CrossRef][Green Version]
- Cirocchi, R.; Randolph, J.; Panata, L.; Verdelli, A.M.; Mascagni, D.; Mingoli, A.; Zago, M.; Chiarugi, M.; Lancia, M.; Fedeli, P.; et al. The tip of the iceberg of colorectal perforation from enema: A systematic review and meta-analysis. Tech. Coloproctol. 2020, 24, 1109–1119. [Google Scholar] [CrossRef]
- Bekkali, N.L.H.; van den Berg, M.M.; Dijkgraaf, M.G.W.; van Wijk, M.P.; Bongers, M.E.; Liem, O.; Benninga, M.A. Rectal fecal impaction treatment in childhood constipation: Enemas versus high doses oral PEG. Pediatrics 2009, 124, e1108–e1115. [Google Scholar] [CrossRef]
- de Geus, A.; Koppen, I.J.N.; Flint, R.B.; Benninga, M.A.; Tabbers, M.M. An update of pharmacological management in children with functional constipation. Paediatr. Drugs 2023, 25, 343–358. [Google Scholar] [CrossRef]


| Classification | Sub-classification | Common name | Product Name | Form | Compliance (times/day) | Administration timing | Dosage | Adaptation | Mechanism of action | Water secretion action | Peristaltic stimulant action | Recovery of defecation desire | Low nursing and caregiving burden | Contraindications | Notes |
| Bulking agents | □ | Polycarbophil calcium | Coronel® Porifuru® | Tablet Powder | 1–3 | After meals | 1.5–3.0 g/day | Stool abnormality (diarrhea/constipation) and gastrointestinal symptoms in irritable bowel syndrome | Include large amounts of water in stools | ○ | □ | □ | □ | Acute abdomen Postoperative ileus Hypercalcemia Renal failure, renal stones | □ |
| Carmellose sodium | Carmellose sodium | Powder | 1–3 | After meals | 1.5–6.0 g/day Adjustable according to age and symptoms. | Constipation | Absorbs water in the intestine, expands to form a gel, increases the volume of the fecal mass, and physically stimulates the intestinal wall. | ○ | □ | □ | □ | Acute abdomen Severe indurated stool | □ | ||
| Osmotic laxatives Note: Select polyethylene glycol in cases of elderly patients, renal dysfunction, patients on acid secretion inhibitors, and a prior total gastrectomy procedure | Sugar-based laxative | Lactulose | Monilak® | Syrup | 1–3 | After meals | Adult: 19.5–39.0 g/day Children: 0.33–1.30 mg/kg Adjustable according to age and symptoms. | Constipation (limited to children), Chronic constipation (except organic disease), stool abnormality after obstetric/gynecologic surgery | It reaches the large intestine without being degraded or absorbed in the small intestine, and the osmotic effect of the lactulose unchanged form promotes the movement of water into the intestine. | ○ | □ | □ | ○ | Patients with lactose/galactosemia | □ |
| Ragnos NF® | Jelly | 1–3 | Two packets at a time (max. 6 packets) Adjustable according to age and symptoms. | ||||||||||||
| Salt-based laxatives | Magnesium oxide | Magnesium oxide | Tablet Powder | 1–3 | After meals Before bedtime | 2 g/day Adjustable according to age and symptoms. | Constipation, gastric/duodenal ulcer Abnormal upper gastrointestinal function | The osmotic pressure of magnesium salts promotes water transfer into the intestinal tract | ○ | □ | □ | ○ | □ | Be aware of hypermagnesemia # (especially in cases of impaired renal function). Some deaths have occurred. Prone to causing hypermagnesemia with concomitant use of active vitamin D preparations. Many concomitant medications * should be used with caution. Weak effect if acid secretion inhibitors are taken orally or after total gastrectomy. | |
| □ | Polyethylene glycol | Movicol® | Powder | 1–3 | After meals Before bedtime | Infants 2 to 7 years: 6.85 g/day –27.4 g/day Children 7 to 12 years: 13.7 g/day –27.4 g/day Children over 12 years and adults: 13.7 g/day –41.1 g/day Adjustable according to age and symptoms. | Chronic constipation (excluding organic diseases) | The osmotic pressure of polyethylene glycol promotes water transfer into the intestinal tract | ○ | □ | □ | □ | Intestinal obstruction, intestinal perforation, severe inflammatory bowel disease | Less dependent and addictive. Large amount of powder. No pills. No contraindications or precautions. | |
| Contact laxative | Casanthranol/Dioctyl sodium sulfosuccinate | Vemas® combination tablet | Tablet | 1–3 | After meals Before bedtime | 2–3 tablets once or 5–6 tablets once before bedtime Adjustable according to age and symptoms | constipation, elimination of intestinal contents during abdominal organ examination or before and after surgery | Surfactant action lowers the surface tension of stools, making them moist and soft | ○ | ○ | □ | □ | Acute abdomen, severely indurated stool | Yellowish brown or red urine | |
| Intestinal secretagogues | Chloride channel activator | Lubiprostone | Amitiza® | Capsule | 1–2 | After meals | 12–48 μg/day Can be adjusted according to age and symptoms | Chronic constipation (excluding organic diseases) | Activates ClC-2 chloride ion channels, thereby promoting water secretion into the intestinal tract | ○ | □ | □ | ○ | Intestinal obstruction, pregnancy | Nausea is common in young women. |
| Guanylate cyclase C receptor agonists | Linaclotide | Linzess® | Tablet | 1 | Before meals | 0.25–0.5 mg/day Adjustable according to age and symptoms | Chronic constipation (excluding organic diseases), irritable bowel syndrome constipation type | Acts on guanylate cyclase C receptors on the surface of intestinal epithelial cells to promote water secretion into the intestinal tract | ○ | □ | □ | □ | Intestinal obstruction | Effective for abdominal pain in irritable bowel syndrome. No contraindications or precautions related to concomitant medications. | |
| Ileal bile acid transporter inhibitors | □ | Elobixibat | Goofice® | Tablet | 1 | Before meals | 5–15 mg/day Adjustable according to age and symptoms | Chronic constipation (excluding organic diseases) | Inhibits reabsorption of bile acids in the ileum | ○ | ○ | ○ | □ | Intestinal obstruction | Effective for both water transfer and peristalsis promotion. Effective in improving bowel movements. |
| Small bowel stimulant laxatives | □ | Castor oil | Castor oil | Liquid | □ | On demand | Adults: 15–30 mL per dose (maximum: 60 mL), Children: 5 to 15 mL per dose, Infants: 1 to 5 mL per dose, Adjustable according to age and symptoms. | Constipation, elimination of intestinal contents before and after surgery | It is broken down into glycerol and ricinoleic acid by the action of lipases in the small intestine. Ricinoleic acid stimulates the small intestine to stimulate defecation. | □ | ○ | □ | □ | Acute abdomen, severely indurated stool | □ |
| Large intestine stimulant laxatives Note: For rescue use only. | Anthraquinone | Senna/Sennoside | Plusenide® Sennoside® | Tablet | 1 | Before bedtime | 12–48 mg/times Adjustable according to age and symptoms | Constipation | Improves peristalsis of the large intestine by producing rainanthrone through the action of intestinal bacteria | □ | ○ | □ | ○ | Acute abdomen, severely indurated stools, electrolyte imbalance (especially hypokalemia) | Colonic melanosis. Dependent and addictive. Yellowish brown or red urine. |
| Alosenn® | Powder | 1–2 | Before meals After meals | 0.5–1.0 g/ times Adjustable according to age and symptoms | □ | □ | □ | □ | □ | □ | □ | □ | |||
| Diphenyl | Sodium picosulfate | Laxoberon® Sodium picosulfate® | Liquid Tablet | 1 | After meals Before bedtime | Adults: 2.5 mg–7.5 mg (10–15 drops) 7–15 years: 5 mg (10 drops) 4–6 years: 3.5 mg (7 drops) 1–3 years: 3 mg (6 drops) 7–12 months: 1.5 mg (3 drops) 6 months and younger: 1 mg (2 drops) Adjustable according to age and symptoms | Constipation, postoperative defecation aid, elimination of intestinal contents prior to colonoscopy (radiography and endoscopy) and surgery | Diphenyl bodies generated by allylsulfatase derived from intestinal bacteria stimulate the colonic mucosa and inhibit water absorption in the large intestine | □ | ○ | □ | ○ | Acute abdomen | No colonic melanosis. Less addictive. Acceptable for young children, pregnant women, and the elderly. | |
| External agents/procedures | Suppository | Bisacodyl suppository | Teleminsoft® | Suppository | 1–2 | On demand | 1–2 per day Adjustable according to age and symptoms | Constipation, elimination of intestinal contents during gastrointestinal examination or before and after surgery | Selectively acts on the colon and rectal mucosa to promote peristalsis | □ | ○ | □ | □ | Acute abdomen | □ |
| Sodium□bicarbonate/anhydrous sodium□dihydrogen phosphate suppository | New lecicarbon® | Suppository | On demand | On demand | 1–3 per day | Constipation | Produces carbon dioxide gas, which stimulates the rectal mucosa and dilates the rectum to promote defecation through the diastolic reflex | □ | ○ | □ | □ | □ | Can be used for pregnant and parturient women. | ||
| Enema | Glycerin enema | Glycerin | Enema | On demand | On demand | 30–60 mL per application | Constipation, defecation during bowel disease | The concentrated liquid stimulates the intestines and promotes bowel movement. It also penetrates the stool and softens it | ○ | ○ | □ | □ | Intra-intestinal hemorrhage, intra-abdominal inflammation, intestinal perforation | Urine color (hemolysis) | |
| Peripherally acting μ-opioid receptor antagonist | □ | Naldemedine | Symproic® | Tablet | 1 | After meals | 0.2 mg/day | Opioid-induced constipation | Antagonizes intestinal μ-opioid receptors | ○ | ○ | □ | ○ | Gastrointestinal obstruction | □ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessoku, T.; Matsumoto, M.; Misawa, N.; Tsuda, M.; Miura, Y.; Uchida, A.; Toriumi, Y.; Onodera, T.; Arima, H.; Kawamoto, A.; et al. Expert Consensus Document: An Algorithm for the Care and Treatment of Patients with Constipation Based on Ultrasonographic Findings in the Rectum. Diagnostics 2024, 14, 1510. https://doi.org/10.3390/diagnostics14141510
Kessoku T, Matsumoto M, Misawa N, Tsuda M, Miura Y, Uchida A, Toriumi Y, Onodera T, Arima H, Kawamoto A, et al. Expert Consensus Document: An Algorithm for the Care and Treatment of Patients with Constipation Based on Ultrasonographic Findings in the Rectum. Diagnostics. 2024; 14(14):1510. https://doi.org/10.3390/diagnostics14141510
Chicago/Turabian StyleKessoku, Takaomi, Masaru Matsumoto, Noboru Misawa, Momoko Tsuda, Yuka Miura, Ayaka Uchida, Yuki Toriumi, Tomoyuki Onodera, Hiromi Arima, Atsuo Kawamoto, and et al. 2024. "Expert Consensus Document: An Algorithm for the Care and Treatment of Patients with Constipation Based on Ultrasonographic Findings in the Rectum" Diagnostics 14, no. 14: 1510. https://doi.org/10.3390/diagnostics14141510
APA StyleKessoku, T., Matsumoto, M., Misawa, N., Tsuda, M., Miura, Y., Uchida, A., Toriumi, Y., Onodera, T., Arima, H., Kawamoto, A., Sugama, J., Matsushima, M., Kato, M., Manabe, N., Tamai, N., Sanada, H., & Nakajima, A. (2024). Expert Consensus Document: An Algorithm for the Care and Treatment of Patients with Constipation Based on Ultrasonographic Findings in the Rectum. Diagnostics, 14(14), 1510. https://doi.org/10.3390/diagnostics14141510






