Anxiety and Mood Disruption in Collegiate Athletes Acutely Following Mild Traumatic Brain Injury
Abstract
1. Introduction
2. Methods
2.1. Post-mTBI Symptom Scale
2.2. Data Analysis
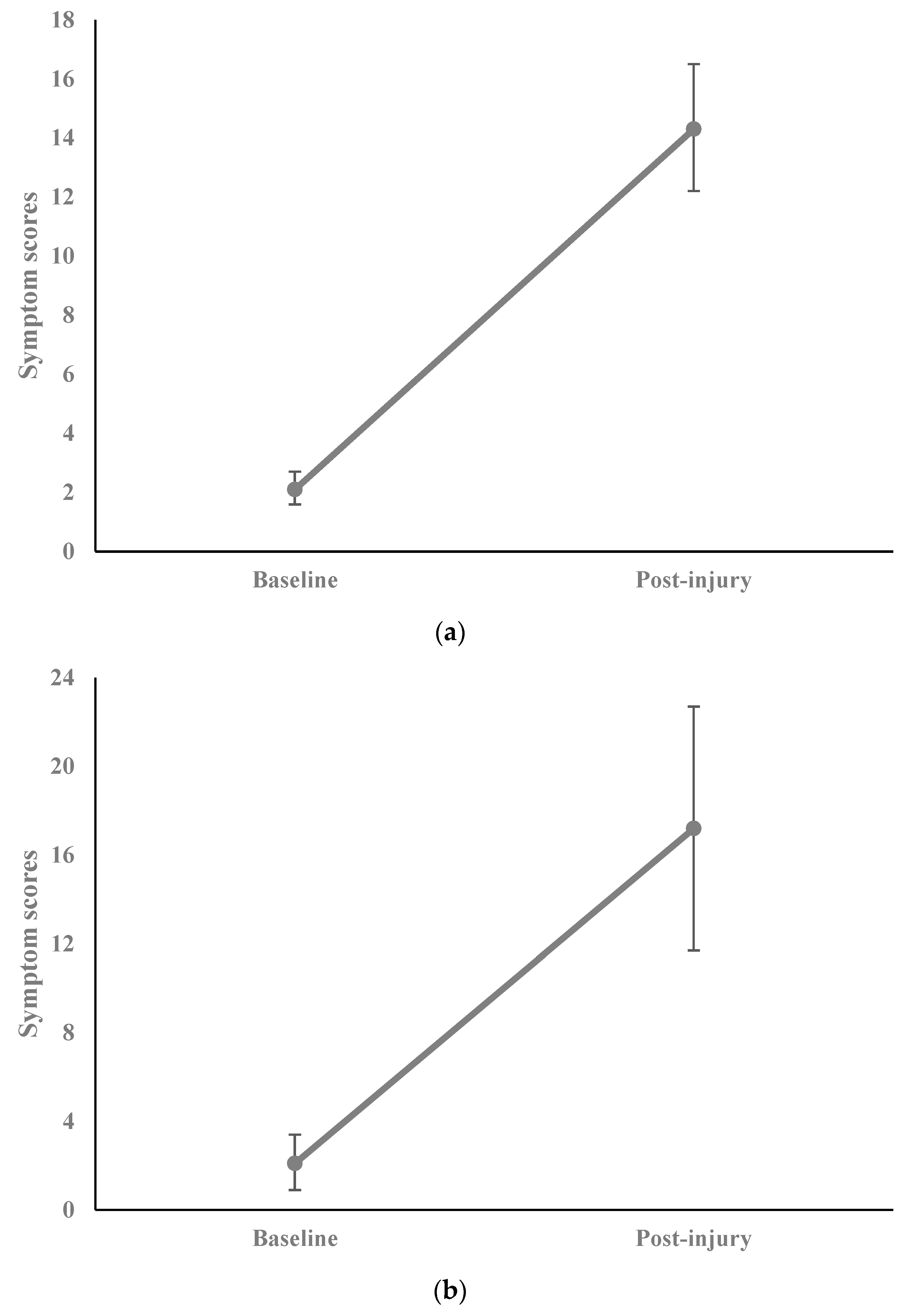
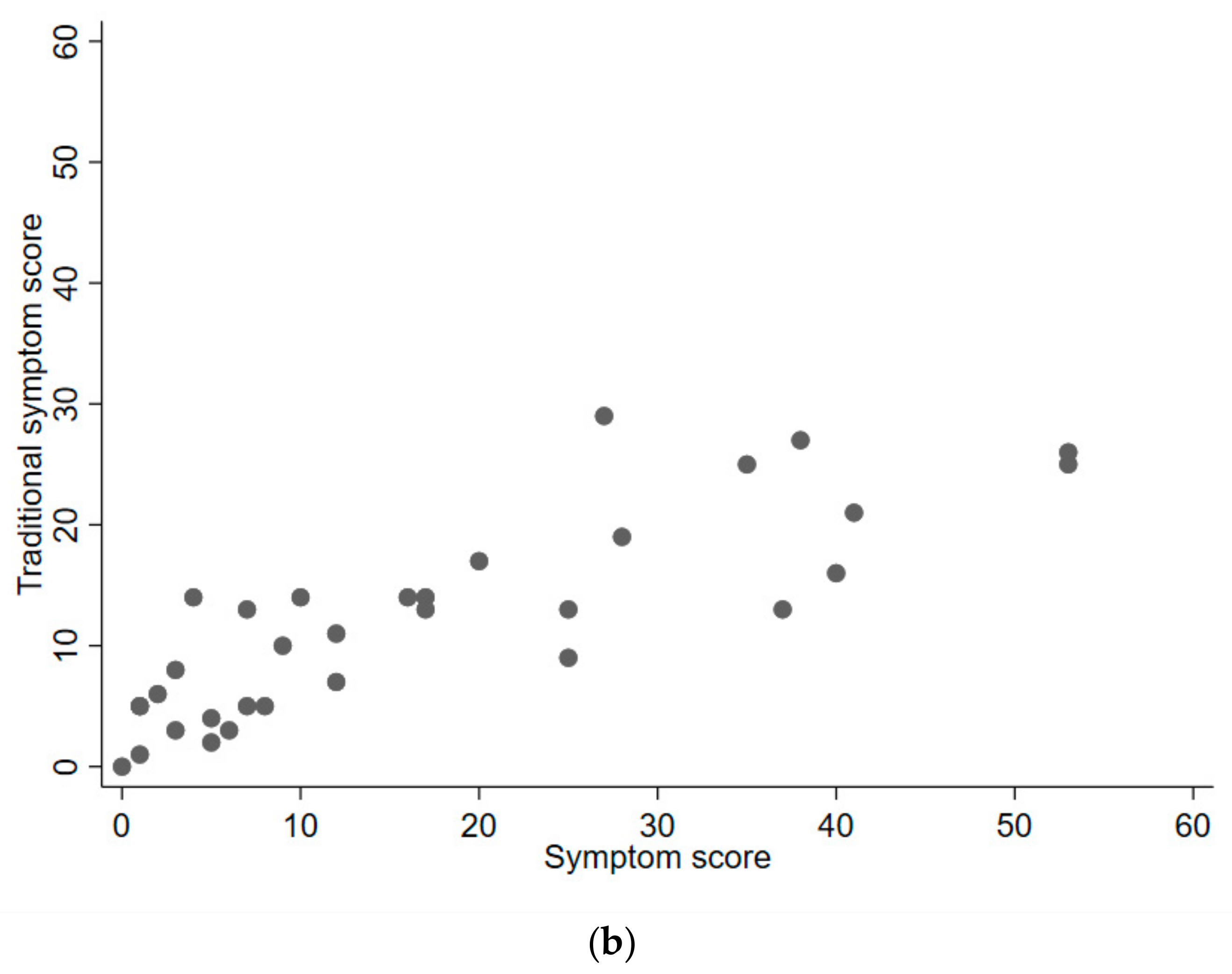
2.3. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farooq, S.A.; Muneeb, A.; Ajmal, W.; Tauni, M.A.; Mahmood, S.; Qadri, S.K.; Butt, A.Y.; Mustafa, S.F.; Sohai, S.H.; Rizvi, N. Quality of life perceptions in school-going adolescents with social anxiety. J. Child. Dev. Disord. 2017, 3, 8. [Google Scholar] [CrossRef]
- Blaze, J.; Choi, I.; Wang, Z.; Umali, M.; Mendelev, N.; Tschiffely, A.E.; Ahlers, S.T.; Elder, G.A.; Ge, Y.; Haghighi, F. Blast-related mild TBI alters anxiety-like behavior and transcriptional signatures in the rat amygdala. Front. Behav. Neurosci. 2020, 14, 160. [Google Scholar] [CrossRef]
- Shulman, L.M. Emotional traumatic brain injury. Cogn. Behav. Neurol. 2020, 33, 301–303. [Google Scholar] [CrossRef]
- Maruta, J.; Lumba-Brown, A.; Ghajar, J. Concussion subtype identification with the RIVERMEAD post-concussion symptoms questionnaire. Front. Neurol. 2018, 9, 413691. [Google Scholar] [CrossRef] [PubMed]
- Lumba-Brown, A.; Ghajar, J.; Cornwell, J.; Bloom, O.J.; Chesnutt, J.; Clugston, J.R.; Kolluri, R.; Leddy, J.J.; Teramoto, M.; Gioia, G. Representation of concussion subtypes in common postconcussion symptom-rating scales. Concussion 2019, 4, CNC65. [Google Scholar] [CrossRef] [PubMed]
- Lumba-Brown, A.; Teramoto, M.; Bloom, O.J.; Brody, D.; Chesnutt, J.; Clugston, J.R.; Collins, M.; Gioia, G.; Kontos, A.; Lal, A.; et al. Concussion guidelines step 2: Evidence for subtype classification. Neurosurgery 2019, 86, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.C.; Yaffe, K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell. Neurosci. 2015, 66, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Athletes, T.B.I. The Brain Injury Guide & Resources. Available online: http://braininjuryeducation.org/Populations/Athletes/ (accessed on 1 January 2022).
- Silverberg, N.D.; Panenka, W.J. Antidepressants for depression after concussion and traumatic brain injury are still best practice. BMC Psychiatry 2019, 19, 100. [Google Scholar] [CrossRef]
- Gorgoraptis, N.; Zaw-Linn, J.; Feeney, C.; Tenorio-Jimenez, C.; Niemi, M.; Malik, A.; Ham, T.; Goldstone, A.P.; Sharp, D.J. Cognitive impairment and health-related quality of life following traumatic brain injury. NeuroRehabilitation 2019, 44, 321–331. [Google Scholar] [CrossRef]
- Mental Health Disorders Common following Mild Head Injury. National Institutes of Health. Published 30 January 2019. Available online: https://www.nih.gov/news-events/news-releases/mental-health-disorders-common-following-mild-head-injury (accessed on 1 January 2022).
- Lin, L.; Zhang, J.; Wang, P.; Bai, X.; Sun, X.; Zhang, L. Perceived control moderates the impact of academic stress on the attention process of working memory in male college students. Stress 2020, 23, 256–264. [Google Scholar] [CrossRef]
- Holmes, A.; Chen, Z.; Yahng, L.; Fletcher, D.; Kawata, K. Return to learn: Academic effects of concussion in high school and college student-athletes. Front. Pediatr. 2020, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Wilmoth, K.; Tan, A.; Hague, C.; Tarkenton, T.; Silver, C.H.; Didehbani, N.; Rossetti, H.C.; Batjer, H.; Bell, K.R.; Cullum, C.M. Current state of the literature on psychological and social sequelae of sports-related concussion in school-aged children and adolescents. J. Exp. Neurosci. 2019, 13, 117906951983042. [Google Scholar] [CrossRef] [PubMed]
- Sandel, N.; Reynolds, E.; Cohen, P.E.; Gillie, B.L.; Kontos, A.P. Anxiety and mood clinical profile following sport-related concussion: From risk factors to treatment. Sport Exerc. Perform. Psychol. 2017, 6, 304–323. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, A.A.; Webster, R.J.; Clarke, A.E.; Fell, D.B.; Knight, B.D.; Gardner, W.; Cloutier, P.; Gray, C.; Tuna, M.; Zemek, R. Risk of Mental Health Problems in Children and Youths Following Concussion. JAMA Netw. Open 2022, 5, e221235. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Wang, J.; Lin, Y.; Cai, X.; Chen, H.; Gou, H.; Li, X.; Jia, Z. MetaEmotionNet: Spatial–Spectral–Temporal-Based Attention 3-D Dense Network with Meta-Learning for EEG Emotion Recognition. IEEE Trans. Instrum. Meas. 2024, 73, 2501313. [Google Scholar] [CrossRef]
- Petit, K.M.; Savage, J.L.; Bretzin, A.C.; Anderson, M.; Covassin, T. The Sport Concussion Assessment Tool-5 (SCAT5): Baseline Assessments in NCAA Division I Collegiate Student-Athletes. Int. J. Exerc. Sci. 2020, 13, 1143–1155. [Google Scholar] [PubMed]
- Quinn, D.K.; Mayer, A.R.; Master, C.L.; Fann, J.R. Prolonged postconcussive symptoms. Am. J. Psychiatry 2018, 175, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, M.S.; Benton, T.; Lewis, J.; Case, J.A.; Master, C.L. Mental health in the young athlete. Curr. Psychiatry Rep. 2020, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ghosh, A.; Yan, H.; Leoutsakos, J.-M.; Rao, V.; Peters, M.E.; Van Meter, T.E.; Sair, H.; Falk, H.; Korley, F.K.; et al. Prevalence and correlates of depressive symptoms within 6 months after first-time mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2022, 34, 367–377. [Google Scholar] [CrossRef]
- Brett, B.L.; Kramer, M.D.; Whyte, J.; McCrea, M.A.; Stein, M.B.; Giacino, J.T.; Sherer, M.; Markowitz, A.J.; Manley, G.T.; Nelson, L.D. Latent profile analysis of neuropsychiatric symptoms and cognitive function of adults 2 weeks after traumatic brain injury. JAMA Netw. Open 2021, 4, e213467. [Google Scholar] [CrossRef]
- Zuckerman, S.L.; Apple, R.P.; Odom, M.J.; Lee, Y.M.; Solomon, G.S.; Sills, A.K. Effect of sex on symptoms and return to baseline in sport-related concussion. J. Neurosurg. Pediatr. 2014, 13, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Knowles, C.; Shannon, S.; Prentice, G.; Breslin, G. Comparing mental health of athletes and non-athletes as they emerge from a COVID-19 pandemic lockdown. Front. Sports Act. Living 2021, 3, 612532. [Google Scholar] [CrossRef] [PubMed]
- Wolanin, A.; Hong, E.; Marks, D.; Panchoo, K.; Gross, M. Prevalence of clinically elevated depressive symptoms in college athletes and differences by gender and sport. Br. J. Sports Med. 2016, 50, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Guillén, F.; Laborde, S. Higher-order structure of mental toughness and the analysis of latent mean differences between athletes from 34 disciplines and non-athletes. Personal. Individ. Differ. 2014, 60, 30–35. [Google Scholar] [CrossRef]
- Cox, C.E.; Ross-Stewart, L.; Foltz, B.D. Investigating the prevalence and risk factors of depression symptoms among NCAA Division I collegiate athletes. J. Sports Sci. 2017, 5, 1592018. [Google Scholar] [CrossRef]
- Martinez, C.; Christopherson, Z.; Lake, A.; Myers, H.; Bytomski, J.R.; Butler, R.J.; Cook, C.E. Clinical examination factors that predict delayed recovery in individuals with concussion. Arch. Physiother. 2020, 10, 10. [Google Scholar] [CrossRef]
- Chrisman, S.P.; Rivara, F.P.; Schiff, M.A.; Zhou, C.; Comstock, R.D. Risk factors for concussive symptoms 1 week or longer in high school athletes. Brain Inj. 2012, 27, 1–9. [Google Scholar] [CrossRef]

| Variable | Frequency (%) |
|---|---|
| Sex | |
| Male | 77 (59.7) |
| Female | 52 (40.3) |
| Sport | |
| Baseball | 2 (1.6) |
| Basketball | 8 (6.2) |
| Beach Volleyball | 4 (3.1) |
| Bicycle Accident | 5 (3.9) |
| Fencing | 1 (0.8) |
| Field Hockey | 2 (1.6) |
| Football | 38 (29.5) |
| Gymnastics | 4 (3.1) |
| Lacrosse | 6 (4.7) |
| Other | 2 (1.6) |
| Sailing | 3 (2.3) |
| Soccer | 4 (3.1) |
| Softball | 4 (3.1) |
| Swimming and Diving | 3 (2.3) |
| Track and Field | 1 (0.8) |
| Volleyball | 5 (3.9) |
| Water Polo | 8 (6.2) |
| Wrestling | 16 (12.4) |
| No data | 13 (10.1) |
| Repeat instances | |
| One | 98 (76.0) |
| Two * | 24 (18.6) |
| Three ** | 7 (5.4) |
| Prolonged symptoms | |
| Yes | 33 (25.6) |
| No | 68 (52.7) |
| No data | 28 (21.7) |
| Depression | |
| Yes | 5 (3.9) |
| No | 107 (82.9) |
| No data | 17 (13.2) |
| Family history of migraine | |
| Yes | 29 (22.5) |
| No | 89 (69.0) |
| No data | 11 (8.5) |
| Age [mean (SD)] | 19.8 (1.4) |
| Baseline symptom score [mean (SD)] | 2.1 (3.3) |
| Post-injury symptom score [mean (SD)] | 14.3 (12.2) |
| Traditional symptom score [mean (SD)] | 11.0 (7.1) |
| Grouping Variable | Post-Injury Symptom Score [Mean (SD)] | p |
|---|---|---|
| Sex | 0.117 * | |
| Male (n = 77) | 12.6 (10.6) | |
| Female (n = 52) | 16.9 (14.0) | |
| Sport | 0.563 * | |
| Football (n = 38) | 13.1 (12.3) | |
| Non-football (n = 78) | 14.1 (12.5) | |
| Contact sport | 0.193 * | |
| Contact (n = 74) | 12.5 (11.7) | |
| Non-contact (n = 40) | 15.8 (13.8) | |
| Repeat instances | 0.898 ** | |
| One (n = 98) | 14.5 (12.6) | |
| Two (n = 24) | 14.3 (11.7) | |
| ≥ Three (n = 7) | 11.7 (10.0) | |
| Depression | 0.049 * | |
| Yes (n = 5) | 26.2 (14.3) | |
| No (n = 107) | 14.0 (12.3) | |
| Family history of migraine | 0.042 * | |
| Yes (n = 29) | 20.0 (14.9) | |
| No (n = 89) | 13.3 (11.3) | |
| Age (n = 128); [r (p)] | 0.083 (0.355) |
| Grouping Variable | Post-Injury Symptom Score [Mean (SD)] | p |
|---|---|---|
| Sex | 0.008 * | |
| Male (n = 19) | 10.6 (10.8) | |
| Female (n = 14) | 26.2 (17.2) | |
| Sport | 0.356 * | |
| Football (n = 10) | 12.3 (13.2) | |
| Non-football (n = 20) | 18.7 (17.1) | |
| Contact sport | 0.012 * | |
| Contact (n = 20) | 10.9 (12.2) | |
| Non-contact (n = 9) | 29.0 (17.8) | |
| Repeat instances | 0.130 ** | |
| One (n = 26) | 18.8 (16.2) | |
| Two (n = 5) | 15.2 (14.0) | |
| ≥Three (n = 2) | 1.5 (2.1) | |
| Depression | N/A | |
| Yes (n = 0) | 0.0 (0.0) | |
| No (n = 28) | 18.2 (16.0) | |
| Family history of migraine | 0.080 * | |
| Yes (n = 10) | 25.6 (16.5) | |
| No (n = 20) | 14.8 (14.9) | |
| Age (n = 33); [r (p)] | −0.130 (0.471) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Martyna, M.; Cornwell, J.; Teramoto, M.; Selfridge, M.; Brown, A.; Ghajar, J.; Lumba-Brown, A. Anxiety and Mood Disruption in Collegiate Athletes Acutely Following Mild Traumatic Brain Injury. Diagnostics 2024, 14, 1276. https://doi.org/10.3390/diagnostics14121276
Zhang R, Martyna M, Cornwell J, Teramoto M, Selfridge M, Brown A, Ghajar J, Lumba-Brown A. Anxiety and Mood Disruption in Collegiate Athletes Acutely Following Mild Traumatic Brain Injury. Diagnostics. 2024; 14(12):1276. https://doi.org/10.3390/diagnostics14121276
Chicago/Turabian StyleZhang, Rachel, Michael Martyna, Jordan Cornwell, Masaru Teramoto, Mollie Selfridge, Amanda Brown, Jamshid Ghajar, and Angela Lumba-Brown. 2024. "Anxiety and Mood Disruption in Collegiate Athletes Acutely Following Mild Traumatic Brain Injury" Diagnostics 14, no. 12: 1276. https://doi.org/10.3390/diagnostics14121276
APA StyleZhang, R., Martyna, M., Cornwell, J., Teramoto, M., Selfridge, M., Brown, A., Ghajar, J., & Lumba-Brown, A. (2024). Anxiety and Mood Disruption in Collegiate Athletes Acutely Following Mild Traumatic Brain Injury. Diagnostics, 14(12), 1276. https://doi.org/10.3390/diagnostics14121276






