Homonymous Hemiatrophy of Macular Ganglion Cell Layer as a Marker of Retrograde Neurodegeneration in Multiple Sclerosis—A Narrative Review
Abstract
1. Introduction
1.1. Visual Pathway Involvement in Multiple Sclerosis
1.2. The Role of OCT in Tracking Neurodegeneration
2. Macular OCT Changes in MS
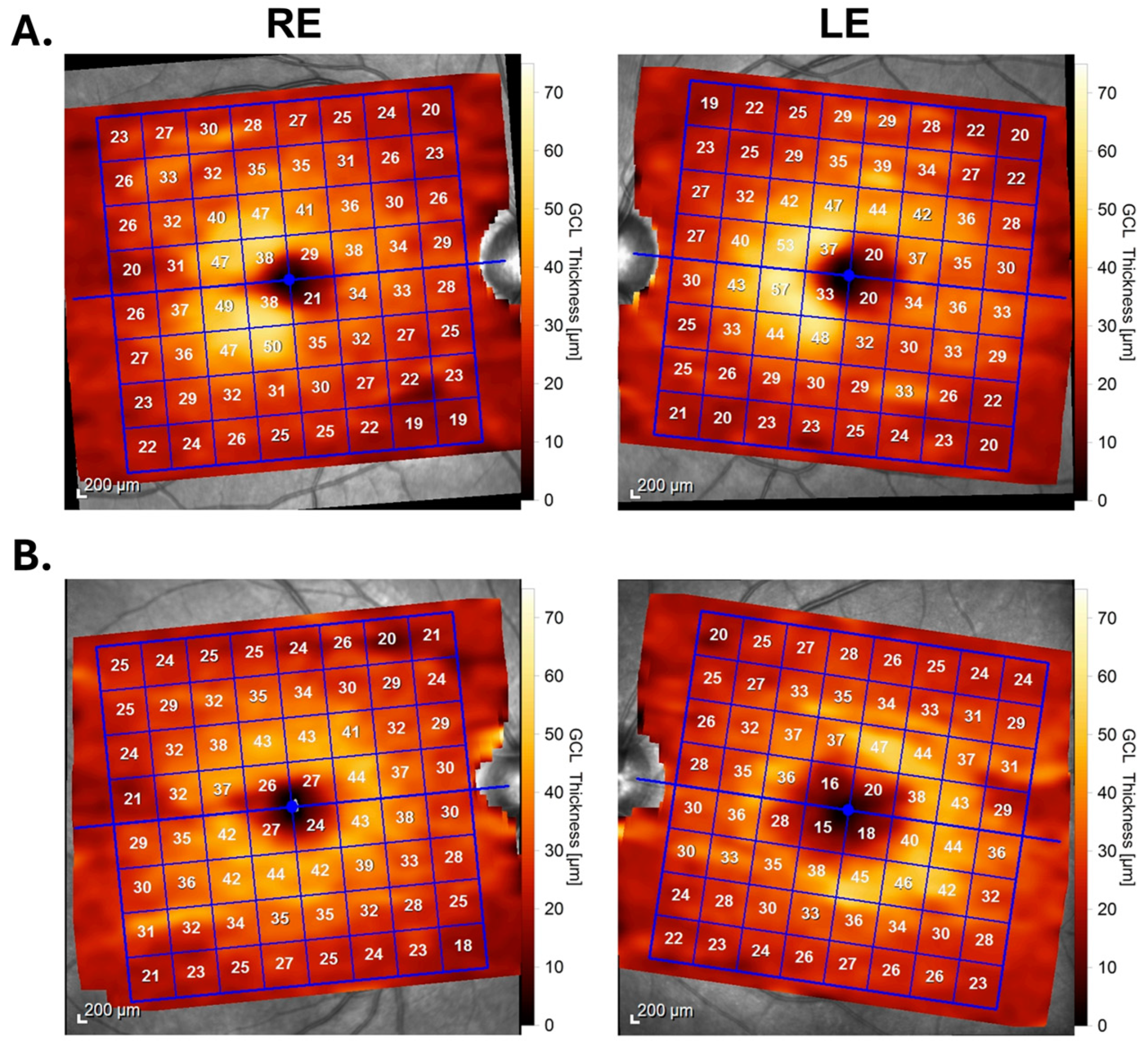
2.1. Homonymous Macular Hemiatrophy in MS
2.2. Relationship between OCT and VF
3. Discussion
3.1. OCT Findings in MS
3.2. OCT Parameters as an Outcome Measure in MS Clinical Trials
3.3. Differences in OCT Protocols for the Evaluation of Retrochiasmal Lesions in MS
3.4. Homonymous Hemiatrophy of mGC in Other Disorders
4. Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Travers, B.S.; Tsang, B.K.T.; Barton, J.L. Multiple sclerosis diagnosis, disease-modifying therapy and prognosis. Aust. J. Gen. Pract. 2022, 51, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Bisecco, A.; Bonavita, S.; Tedeschi, G. Functional plasticity of the visual system in multiple sclerosis. Front. Neurol. 2015, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Gabilondo, I.; Martínez-Lapiscina, E.H.; Martínez-Heras, E.; Fraga-Pumar, E.; Llufriu, S.; Ortiz, S.; Bullich, S.; Sepulveda, M.; Falcon, C.; Berenguer, J.; et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann. Neurol. 2014, 75, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Dinkin, M. Trans-synaptic retrograde degeneration in the human visual system: Slow, silent, and real. Curr. Neurol. Neurosci. Rep. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Rocca, M.A.; Ciccarelli, O.; De Stefano, N.; Evangelou, N.; Kappos, L.; Rovira, A.; Sastre-Garriga, J.; Tintorè, M.; Frederiksen, J.L.; et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016, 15, 292–303. [Google Scholar] [CrossRef]
- Cuna, A.; Pellegrini, F.; Interlandi, E.; Mandarà, E.; De Luca, M.; De Marco, R.; Ciabattoni, C.; Zappacosta, A.; Papayannis, A. Optical coherence tomography angiography in chiasmitis. Case Rep. Ophthalmol. 2022, 13, 517–522. [Google Scholar] [CrossRef]
- Rosenblatt, M.A.; Behrens, M.M.; Zweifach, P.H.; Forman, S.; Odel, J.G.; Duncan, C.M.; Gross, S.A. Magnetic resonance imaging of optic tract involvement in multiple sclerosis. Am. J. Ophthalmol. 1987, 104, 74–79. [Google Scholar] [CrossRef]
- Dasenbrock, H.H.; Smith, S.A.; Ozturk, A.; Farrell, S.K.; Calabresi, P.A.; Reich, D.S. Diffusion tensor imaging of the optic tracts in multiple sclerosis: Association with retinal thinning and visual disability. J. Neuroimaging 2011, 21, e41–e49. [Google Scholar] [CrossRef] [PubMed]
- Plant, G.T.; Kermode, A.G.; Turano, G.; Moseley, I.F.; Miller, D.H.; MacManus, D.G.; Halliday, A.M.; McDonald, W.I. Symptomatic retrochiasmal lesions in multiple sclerosis. Neurology 1992, 42, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kerrison, J.B.; Flynn, T.; Green, W.R. RETINAL PATHOLOGIC CHANGES IN MULTIPLE SCLEROSIS. Retina 1994, 14, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.L.; Klistorner, A. Afferent visual pathways in multiple sclerosis: A review. Clin. Exp. Ophthalmol. 2017, 45, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Gaetano, L.; Pfister, A.; Altermatt, A.; Tsagkas, C.; Morency, F.; Brandt, A.U.; Hardmeier, M.; Chakravarty, M.M.; Descoteaux, M.; et al. Damage of the lateral geniculate nucleus in MS. Neurology 2019, 92, e2240–e2249. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.M.C.; Damasceno, A.; Damasceno, B.P.; de Vasconcellos, J.P.; Reis, F.; Iyeyasu, J.N.; de Carvalho, K.M. Visual pathway abnormalities were found in most multiple sclerosis patients despite history of previous optic neuritis. Arq. Neuro-Psiquiatr. 2013, 71, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Brownell, B.; Hughes, J.T. The distribution of plaques in the cerebrum in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1962, 25, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Hornabrook, R.S.; Miller, D.H.; Newton, M.R.; MacManus, D.G.; du Boulay, G.H.; Halliday, A.M.; McDonald, W.I. Frequent involvement of the optic radiation in patients with acute isolated optic neuritis. Neurology 1992, 42, 77. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, F.; Nilsson, M.; Flodmark, O.; Jacobson, L. Damage to the immature optic radiation causes severe reduction of the retinal nerve fiber layer, resulting in predictable visual field defects. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8278–8288. [Google Scholar] [CrossRef]
- Reich, D.S.; Ozturk, A.; Calabresi, P.A.; Mori, S. Automated vs. conventional tractography in multiple sclerosis: Variability and correlation with disability. Neuroimage 2010, 49, 3047–3056. [Google Scholar] [CrossRef]
- Reich, D.S.; Smith, S.A.; Gordon-Lipkin, E.M.; Ozturk, A.; Caffo, B.S.; Balcer, L.J.; Calabresi, P.A. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch. Neurol. 2009, 66, 998–1006. [Google Scholar] [CrossRef]
- Rocca, M.A.; Mesaros, S.; Preziosa, P.; Pagani, E.; Stosic-Opincal, T.; Dujmovic-Basuroski, I.; Drulovic, J.; Filippi, M. Wallerian and trans-synaptic degeneration contribute to optic radiation damage in multiple sclerosis: A diffusion tensor MRI study. Mult. Scler. J. 2013, 19, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Costello, F. The role of optical coherence tomography in neuro-ophthalmology. Ann. Eye Sci. 2018, 3, 35. [Google Scholar] [CrossRef]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Klistorner, A.; Sriram, P.; Vootakuru, N.; Wang, C.; Barnett, M.H.; Garrick, R.; Parratt, J.; Levin, N.; Raz, N.; Van der Walt, A.; et al. Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology 2014, 82, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Mühlemann, F.; Grabe, H.; Fok, A.; Wagner, F.; Brügger, D.; Sheldon, C.A.; Abegg, M. Homonymous hemiatrophy of ganglion cell layer from retrochiasmal lesions in the visual pathway. Neurology 2020, 94, e323–e329. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.; Margolin, E. Visual fields and optical coherence tomography (OCT) in neuro-ophthalmology: Structure-function correlation. J. Neurol. Sci. 2021, 429, 118064. [Google Scholar] [CrossRef] [PubMed]
- Jindahra, P.; Petrie, A.; Plant, G.T. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain 2012, 135, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Zangerl, B.; Whatham, A.; Kim, J.; Choi, A.; Assaad, N.N.; Hennessy, M.P.; Kalloniatis, M. Reconciling visual field defects and retinal nerve fibre layer asymmetric patterns in retrograde degeneration: An extended case series. Clin. Exp. Optom. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Huang-Link, Y.-M.; Al-Hawasi, A.; Eveman, I. Retrograde degeneration of visual pathway: Hemimacular thinning of retinal ganglion cell layer in progressive and active multiple sclerosis. J. Neurol. 2014, 261, 2453–2456. [Google Scholar] [CrossRef]
- Al-Louzi, O.; Button, J.; Newsome, S.D.; Calabresi, P.A.; Saidha, S. Retrograde trans-synaptic visual pathway degeneration in multiple sclerosis: A case series. Mult. Scler. J. 2017, 23, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Lukewich, M.K.; Schlenker, M.B.; Micieli, J.A. Homonymous hemi-macular atrophy of the ganglion cell-inner plexiform layer with preserved visual function. J. Neurol. Sci. 2020, 417, 117072. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, M.; Nolan-Kenney, R.; Fatterpekar, G.; Hasanaj, L.; Serrano, L.; Joseph, B.; Wu, S.; Rucker, J.C.; Balcer, L.J.; Galetta, S.L. Role for OCT in detecting hemi-macular ganglion cell layer thinning in patients with multiple sclerosis and related demyelinating diseases. J. Neurol. Sci. 2020, 419, 117159. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, L.; Borruat, F.-X. Homonymous visual field defects in patients with multiple sclerosis: Results of computerised perimetry and optical coherence tomography. Swiss Med. Wkly. 2020, 150, w20319. [Google Scholar] [CrossRef] [PubMed]
- Hokazono, K.; Ribeiro Monteiro, M.L. Homonymous quadrantic macular ganglion cell complex loss as a sign of trans-synaptic degeneration from occipital lobe lesion. Am. J. Ophthalmol. Case Rep. 2018, 13, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Kupersmith, M.J.; Garvin, M.K.; Wang, J.-K.; Durbin, M.; Kardon, R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult. Scler. J. 2016, 22, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Gabilondo, I.; Martínez-Lapiscina, E.H.; Fraga-Pumar, E.; Ortiz-Perez, S.; Torres-Torres, R.; Andorra, M.; Llufriu, S.; Zubizarreta, I.; Saiz, A.; Sanchez-Dalmau, B.; et al. Dynamics of retinal injury after acute optic neuritis. Ann. Neurol. 2015, 77, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Chua, S.Y.L.; Khawaja, A.P.; Keane, P.A.; Khaw, P.T.; Reisman, C.; Dhillon, B.; Strouthidis, N.G.; Foster, P.J.; Patel, P.J.; et al. Retinal asymmetry in multiple sclerosis. Brain 2021, 144, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Balk, L.J.; Coric, D.; Knier, B.; Zimmermann, H.G.; Behbehani, R.; Alroughani, R.; Martinez-Lapiscina, E.H.; Brandt, A.U.; Sánchez-Dalmau, B.; Vidal-Jordana, A.; et al. Retinal inner nuclear layer volume reflects inflammatory disease activity in multiple sclerosis; a longitudinal OCT study. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319871582. [Google Scholar] [CrossRef]
- Saidha, S.; Sotirchos, E.S.; Ibrahim, M.A.; Crainiceanu, C.M.; Gelfand, J.M.; Sepah, Y.J.; Ratchford, J.N.; Oh, J.; Seigo, M.A.; Newsome, S.D.; et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: A retrospective study. Lancet Neurol. 2012, 11, 963–972. [Google Scholar] [CrossRef]
- Knier, B.; Schmidt, P.; Aly, L.; Buck, D.; Berthele, A.; Mühlau, M.; Zimmer, C.; Hemmer, B.; Korn, T. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain 2016, 139, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M.; Nolan, R.; Schwartz, D.M.; Graves, J.; Green, A.J. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 2012, 135 Pt 6, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; McQuaid, S.; Hauser, S.L.; Allen, I.V.; Lyness, R. Ocular pathology in multiple sclerosis: Retinal atrophy and inflammation irrespective of disease duration. Brain 2010, 133 Pt 6, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Gouravani, M.; Salehi, M.A.; Arevalo, J.F.; Galetta, S.L.; Harandi, H.; Frohman, E.M.; Frohman, T.C.; Saidha, S.; Sattarnezhad, N.; et al. Optical coherence tomography angiography measurements in multiple sclerosis: A systematic review and meta-analysis. J. Neuroinflamm. 2023, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Murphy, O.C.; Kwakyi, O.; Iftikhar, M.; Zafar, S.; Lambe, J.; Pellegrini, N.; Sotirchos, E.S.; Gonzalez-Caldito, N.; Ogbuokiri, E.; Filippatou, A.; et al. Alterations in the retinal vasculature occur in multiple sclerosis and exhibit novel correlations with disability and visual function measures. Mult. Scler. J. 2020, 26, 815–828. [Google Scholar] [CrossRef]
- Park, J.J.; Soetikno, B.T.; Fawzi, A.A. Characterization of the middle capillary plexus using optical coherence tomography angiography in healthy and diabetic eyes. Retina 2016, 36, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Perumal, J.; Fox, R.J.; Balabanov, R.; Balcer, L.J.; Galetta, S.; Makh, S.; Santra, S.; Hotermans, C.; Lee, L. Outcomes of natalizumab treatment within 3 years of relapsing-remitting multiple sclerosis diagnosis: A prespecified 2-year interim analysis of STRIVE. BMC Neurol. 2019, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Bermel, R.A.; Fedler, J.K.; Kaiser, P.; Novalis, C.; Schneebaum, J.; Klingner, E.A.; Williams, D.; Yankey, J.W.; Ecklund, D.J.; Chase, M.; et al. Optical coherence tomography outcomes from SPRINT-MS, a multicenter, randomized, double-blind trial of ibudilast in progressive multiple sclerosis. Mult. Scler. J. 2021, 27, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, H.; Lambe, J.; Moussa, H.; Vasileiou, E.S.; Kalaitzidis, G.; Murphy, O.C.; Filippatou, A.G.; Pellegrini, N.; Douglas, M.; Davis, S.; et al. Effects of ibudilast on retinal atrophy in progressive multiple sclerosis subtypes: Post hoc analyses of the SPRINT-MS Trial. Neurology 2023, 101, E1014–E1024. [Google Scholar] [CrossRef]
- Ingwersen, J.; Masanneck, L.; Pawlitzki, M.; Samadzadeh, S.; Weise, M.; Aktas, O.; Meuth, S.G.; Albrecht, P. Real-world evidence of ocrelizumab-treated relapsing multiple sclerosis cohort shows changes in progression independent of relapse activity mirroring phase 3 trials. Sci. Rep. 2023, 13, 15003. [Google Scholar] [CrossRef]
- Pérez Del Palomar, A.; Cegoñino, J.; Montolío, A.; Orduna, E.; Vilades, E.; Sebastián, B.; Pablo, L.E.; Garcia-Martin, E. Swept source optical coherence tomography to early detect multiple sclerosis disease. The use of machine learning techniques. PLoS ONE 2019, 14, e0216410. [Google Scholar] [CrossRef] [PubMed]
- Cujba, L.; Stan, C.; Samoila, O.; Drugan, T.; Benedec Cutas, A.; Nicula, C. Identifying Optical Coherence Tomography Markers for Multiple Sclerosis Diagnosis and Management. Diagnostics 2023, 13, 2077. [Google Scholar] [CrossRef]
- Viladés, E.; Cordón, B.; Pérez-Velilla, J.; Orduna, E.; Satue, M.; Polo, V.; Sebastian, B.; Larrosa, J.M.; Pablo, L.; García-Martin, E. Evaluation of multiple sclerosis severity using a new OCT tool. PLoS ONE 2023, 18, e0288581. [Google Scholar] [CrossRef]
- Meier, P.G.; Maeder, P.; Borruat, F.X. Transsynaptic Retrograde Degeneration: Clinical Evidence with Homonymous RGCL Loss on OCT. Klin. Monbl. Augenheilkd. 2016, 233, 396–398. [Google Scholar] [CrossRef]
- Blanch, R.J.; Micieli, J.A.; Oyesiku, N.M.; Newman, N.J.; Biousse, V. Optical coherence tomography retinal ganglion cell complex analysis for the detection of early chiasmal compression. Pituitary 2018, 21, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Banc, A.; Biousse, V.; Newman, N.J.; Kedar, S. Ocular optical coherence tomography in the evaluation of sellar and parasellar masses: A review. Neurosurgery 2023, 92, 42–67. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Oliveira, C.; Tsiouris, A.J.; Dinkin, M.J. Corresponding ganglion cell atrophy in patients with postgeniculate homonymous visual field loss. J. Neuro-Ophthalmol. 2015, 35, 353–359. [Google Scholar] [CrossRef]
- Yamashita, T.; Miki, A.; Goto, K.; Araki, S.; Takizawa, G.; Ieki, Y.; Kiryu, J.; Tabuchi, A.; Iguchi, Y.; Kimura, K.; et al. Evaluation of significance maps and the analysis of the longitudinal time course of the macular ganglion cell complex thicknesses in acquired occipital homonymous hemianopia using spectral-domain optical coherence tomography. Neuro-Ophthalmology 2019, 44, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Sánchez-Dalmau, B.F.; Villoslada, P. Lesions in the posterior visual pathway promote trans-synaptic degeneration of retinal ganglion cells. PLoS ONE 2014, 9, e97444. [Google Scholar] [CrossRef]
| Authors, Year of Publication | Number of Patients | Prior Episodes of Optic Neuritis (Number of Eyes) | VF Findings | mGC Thinning Pattern | pRNFL Findings | MRI Findings |
|---|---|---|---|---|---|---|
| Huang-Link et al., 2014 [30] | Active MS: 2 | 0 | Right homonymous hemianopia | Left homonymous mGC atrophy | Bilateral pRNFL thinning (most marked temporally) | Demyelinating lesion in the left OR |
| 1 | Normal | Right eye: nasal mGC thinning Left eye: diffusely thinned mGC | Slightly thinned pRNFL in the right eye and markedly thinned pRNFL in the left eye (most marked temporally) | Demyelinating lesion in the left OR | ||
| Al-Louzi et al., 2017 [31] | RRMS: 6 | 0 | 4 pts: homonymous hemianopia | 1 pt: left homonymous mGC loss 3 pts: right homonymous mGC reduction 2 pts: right homonymous mGC reduction | Not reported | 1 pt: left occipital white matter lesion 3 pts: right occipital white matter lesion 2 pts: right thalamic lesions |
| Lukewich et al., 2020 [32] | Demyelinating disease: 4 (MS: 3) TBI: 3 | Not reported | No significant defect at the time of evaluation | 4 pts: right homonymous mGC thinning 3 pts: left homonymous mGC thinning | Not reported | 3 pts: contralateral OT lesion 1 pt: LGN lesion |
| Ilardi et al., 2020 [33] | RRMS: 4 NMO: 1 | 1 | 1 pt: history of homonymous hemianopia | 2 pts: right homonymous mGC thinning 3 pts: lef homonymous mGC thinning | Not reported | 3 pts: bilateral demyelinating lesions of the post-geniculate visual pathway 1 pt: lesion of the OR on the opposite expected site 1 pt: no visible demyelinating lesion |
| Schmutz and Borruat, 2020 [34] | RRMS: 18 PPMS: 1 SPMS: 1 | 9 | 3 pts: homonymous VF defect 11 pts: homonymous quadrantanopia 2 pts: homonymous hemianopia | 3 pts: homonymous thinning 6 pts: diffuse unilateral or bilateral thinning (resulting from previous episodes of optic neuritis) 1 pt: normal mGC thickness | Not reported | 7 pts: an MRI-defined lesion explaining the VF defects: 5 pts—OR lesion; 1 pt—OT lesion; 1 pt—LGN lesion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cujbă, L.; Banc, A.; Drugan, T.; Coadă, C.A.; Cristea, A.-P.; Stan, C.; Nicula, C. Homonymous Hemiatrophy of Macular Ganglion Cell Layer as a Marker of Retrograde Neurodegeneration in Multiple Sclerosis—A Narrative Review. Diagnostics 2024, 14, 1255. https://doi.org/10.3390/diagnostics14121255
Cujbă L, Banc A, Drugan T, Coadă CA, Cristea A-P, Stan C, Nicula C. Homonymous Hemiatrophy of Macular Ganglion Cell Layer as a Marker of Retrograde Neurodegeneration in Multiple Sclerosis—A Narrative Review. Diagnostics. 2024; 14(12):1255. https://doi.org/10.3390/diagnostics14121255
Chicago/Turabian StyleCujbă, Larisa, Ana Banc, Tudor Drugan, Camelia Alexandra Coadă, Andreea-Petra Cristea, Cristina Stan, and Cristina Nicula. 2024. "Homonymous Hemiatrophy of Macular Ganglion Cell Layer as a Marker of Retrograde Neurodegeneration in Multiple Sclerosis—A Narrative Review" Diagnostics 14, no. 12: 1255. https://doi.org/10.3390/diagnostics14121255
APA StyleCujbă, L., Banc, A., Drugan, T., Coadă, C. A., Cristea, A.-P., Stan, C., & Nicula, C. (2024). Homonymous Hemiatrophy of Macular Ganglion Cell Layer as a Marker of Retrograde Neurodegeneration in Multiple Sclerosis—A Narrative Review. Diagnostics, 14(12), 1255. https://doi.org/10.3390/diagnostics14121255







