Role of Myostatin in Rheumatoid Arthritis: A Review of the Clinical Impact
Abstract
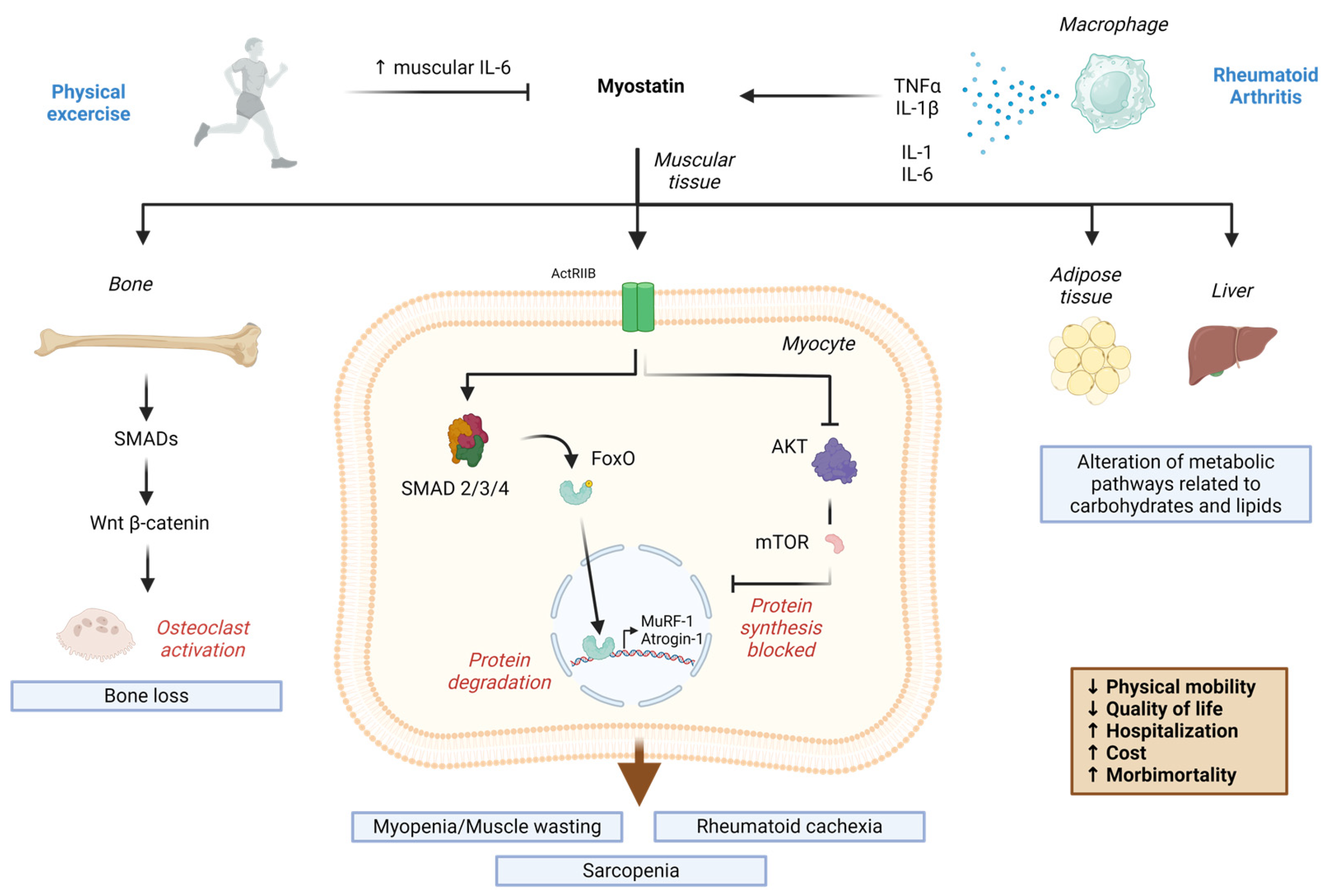
1. Introduction
2. Myokines in Rheumatoid Arthritis: Relevance of Myostatin
| Myokine | Role | Reference |
|---|---|---|
| Myostatin/GDF-8 | Muscle growth suppressor. Cachexia, bone inflammation mediated by TNF-α, activation of osteoclast formation mediated by RANKL in RA. | Akash, 2023 [54] |
| Follistatin | Antagonist of TGF-β ligands. Positively correlates with muscle mass. Has improved synovitis and inhibited the erosion of proteoglycans in an experimental study. | Yamada, 2014 [55] |
| Activin A | Member of the TGF-β superfamily. Negative regulator of muscle growth. Same pathway as myostatin, but cohort-based evidence is still lacking. Contributes to synovial inflammation and pain. | Dong, 2014 [56] |
| GDF-15 | Member of the TGF-β superfamily. It is associated with joint involvement and atherosclerosis in RA. | Tanrikulu, 2017 [57] |
| Irisin | Responsible for the darkening of white adipose tissue. Low titers are associated with high disease activity, disability, and subclinical atherosclerosis in RA. | Samar, 2002 [58] |
3. Myostatin in Rheumatoid Arthritis
3.1. Serum Myostatin Levels in RA Patients Compared to Healthy Controls
3.2. Effects of Exercise on Serum Myostatin Levels in Patients with RA
3.3. Radiographic Progression Is Associated with Serum Myostatin Levels in RA Patients
3.4. Appendicular Skeletal Muscle Mass Index Associated with Serum Myostatin Levels in RA Patients
3.5. Myostatin Cutoff
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [PubMed]
- Mageriu, V.; Manole, E.; Bastian, A.E.; Staniceanu, F. Role of Myokines in Myositis Pathogenesis and Their Potential to be New Therapeutic Targets in Idiopathic Inflammatory Myopathies. J. Immunol. Res. 2020, 2020, 9079083. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Exercise-induced myokines and their role in chronic diseases. Brain Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef]
- Groner, L.K.; Green, D.B.; Weisman, S.V.; Legasto, A.C.; Toy, D.; Gruden, J.F.; Escalon, J.G. Thoracic Manifestations of Rheumatoid Arthritis. RadioGraphics 2021, 41, 32–55. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Dixey, J.; Cox, N.; Davies, P.; Devlin, J.; Emery, P.; Gallivan, S.; Gough, A.; James, D.; Prouse, P.; et al. How does functional disability in early rheumatoid arthritis (RA) affect patients and their lives? Results of 5 years of follow-up in 732 patients from the Early RA Study (ERAS). Rheumatology 2000, 39, 603–611. [Google Scholar] [CrossRef]
- Almutairi, K.B.; Inderjeeth, C.A.; Preen, D.B.; Keen, H.I.; Nossent, J.C. Mortality Trends Among Patients with Rheumatoid Arthritis in Western Australia. Rheumatol. Ther. 2023, 10, 1021–1037. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Thomson, P.J.; Jones, M.E.; Walker, J.G.; Macfarlane, J.G.; Smith, M.D.; Ahern, M.J. Stochastic processes in the causation of rheumatic disease. J. Rheumatol. 2002, 29, 2628–2634. [Google Scholar]
- Macgregor, A.J.; Snieder, H.; Rigby, A.S.; Koskenvuo, M.; Kaprio, J.; Aho, K.; Silman, A.J. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000, 43, 30–37. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Terao, C. The impact of cigarette smoking on risk of rheumatoid arthritis: A narrative review. Cells 2020, 9, 475. [Google Scholar] [CrossRef]
- Mehri, F.; Jenabi, E.; Bashirian, S.; Shahna, F.G.; Khazaei, S. The association between occupational exposure to silica and risk of developing rheumatoid arthritis: A meta-analysis. Saf. Health Work. 2020, 11, 136–142. [Google Scholar] [CrossRef]
- Jin, J.; Li, J.; Gan, Y.; Liu, J.; Zhao, X.; Chen, J.; Zhang, R.; Zhong, Y.; Chen, X.; Wu, L.; et al. Red meat intake is associated with early onset of rheumatoid arthritis: A cross-sectional study. Sci. Rep. 2021, 11, 5681. [Google Scholar]
- Pattison, D.J.; Harrison, R.A.; Symmons, D.P.M. The role of diet in susceptibility to rheumatoid arthritis: A systematic review. J. Rheumatol. 2004, 31, 1310–1319. [Google Scholar]
- Karlson, E.W.; Mandl, L.A.; Aweh, G.N.; Grodstein, F. Coffee consumption and risk of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 3055–3060. [Google Scholar] [CrossRef]
- Feder, H.M.J.; Johnson, B.J.B.; O’Connell, S.; Shapiro, E.D.; Steere, A.C.; Wormser, G.P. A critical appraisal of “chronic lyme disease”. N. Engl. J. Med. 2009, 357, 1422–1430. [Google Scholar] [CrossRef]
- Perricone, C.; Ceccarelli, F.; Saccucci, M.; di Carlo, G.; Bogdanos, D.P.; Lucchetti, R.; Pilloni, A.; Valesini, G.; Polimeni, A.; Conti, F. Porphyromonas gingivalis and rheumatoid arthritis. Curr. Opin. Rheumatol. 2019, 31, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Balandraud, N.; Roudier, J. Epstein-Barr virus and rheumatoid arthritis. Jt. Bone Spine 2018, 85, 165–170. [Google Scholar] [CrossRef]
- Bandinelli, F.; Pagano, M.; Vallecoccia, M.S. Post-COVID-19 and Post-COVID-19 Vaccine Arthritis, Polymyalgia Rheumatica and Horton’s Arteritis: A Single-Center Assessment of Clinical, Serological, Genetic, and Ultrasonographic Biomarkers. J. Clin. Med. 2023, 12, 7563. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Kandimalla, R.; Kalra, R.S.; Valupadas, C.; Vallamkondu, J.; Kolli, V.; Dey Ray, S.; Reddy, A.P.; Reddy, P.H. COVID-19 and Rheumatoid Arthritis Crosstalk: Emerging Association, Therapeutic Options and Challenges. Cells 2021, 10, 3291. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of rheumatoid arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- Curran, A.M.; Naik, P.; Giles, J.T.; Darrah, E. PAD enzymes in rheumatoid arthritis: Pathogenic effectors and autoimmune targets. Nat. Rev. Rheumatol. 2020, 16, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Van-Drongelen, V.; Holoshitz, J. HLA-disease associations in rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2017, 43, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Stavnezer, J.; Guikema, J.E.J.; Schrader, C.E. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008, 26, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Ingegnoli, F.; Castelli, R.; Gualtierotti, R. Rheumatoid factors: Clinical applications. Dis. Markers 2013, 35, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.C.; Lu, M.C. The roles of anti-citrullinated protein antibodies in the immunopathogenesis of rheumatoid arthritis. Tzu-Chi Med. J. 2019, 31, 5–10. [Google Scholar]
- Croia, C.; Bursi, R.; Sutera, D.; Petrelli, F.; Alunno, A.; Puxeddu, I. One year in review 2019: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2019, 37, 347–357. [Google Scholar] [PubMed]
- Gao, Y.; Zhang, Y.; Liu, X. Rheumatoid arthritis: Pathogenesis and therapeutic advances. MedComm 2024, 5, e509. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; de Maio, M.C.; Barbalho, S.M.; Guiguer, E.L.; Araujo, A.C.; de Alvares-Goulart, R.; Flato, U.A.P.; Júnior, E.B.; Detregiachi, C.R.P.; Dos Santos Haber, J.F.; et al. Organokines in Rheumatoid Arthritis: A Critical Review. Int. J. Mol. Sci. 2022, 23, 6193. [Google Scholar] [CrossRef]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Vrabie, C.D.; Tanasescu, R. Extra-articular Manifestations in Rheumatoid Arthritis. Maedica 2010, 5, 286–291. [Google Scholar]
- Crostein, B.N. Interleukin-6—A key mediator of systemic and local symptoms in rheumatoid arthritis. Bull. NYU Hosp. J. Dis. 2007, 65, S11–S15. [Google Scholar]
- Cimmino, M.A.; Salvarani, C.; Macchioni, P. Extra-articular manifestations in 587 Italian patients with rheumatoid arthritis. Rheumatol. Int. 2000, 19, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Masuko, K. Rheumatoid cachexia revisited: A metabolic co-morbidity in rheumatoid arthritis. Front. Nutr. 2014, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zou, Y.W.; Zhu, Y.Y.; Lin, J.Z.; Wu, T.; Yang, Z.H.; Zhang, X.P.; Zhang, Q.; Zheng, H.W.; He, X.L.; et al. Muscle mass loss is associated with physical dysfunction in patients with early rheumatoid arthritis. Front. Nutr. 2022, 9, 1007184. [Google Scholar] [CrossRef] [PubMed]
- Walsmith, J.; Roubenoff, R. Cachexia in rheumatoid arthritis. Int. J. Cardiol. 2002, 85, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Ladang, A.; Beaudart, C.; Reginster, J.Y.; Al-Daghri, N.; Bruyère, O.; Burlet, N.; Cesari, M.; Cherubini, A.; da Silva, M.C.; Cooper, C.; et al. Biochemical Markers of Musculoskeletal Health and Aging to be Assessed in Clinical Trials of Drugs Aiming at the Treatment of Sarcopenia: Consensus Paper from an Expert Group Meeting Organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the Centre Académique de Recherche et d‘Expérimentation en Santé (CARES SPRL), Under the Auspices of the World Health Organization Collaborating Center for the Epidemiology of Musculoskeletal Conditions and Aging. Calcif. Tissue Int. 2023, 112, 197–217. [Google Scholar]
- Bruyère, O.; Beaudart, C.; Ethgen, O.; Reginster, J.Y.; Locquet, M. The health economics burden of sarcopenia: A systematic review. Maturitas 2019, 119, 61–69. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health outcomes of sarcopenia: A systematic review and meta-analysis. PLoS ONE 2017, 12, e169548. [Google Scholar] [CrossRef]
- Moschou, D.; Krikelis, M.; Georgakopoulos, C.; Mole, E.; Chronopoulos, E.; Tournis, S.; Mavragani, C.; Makris, K.; Dontas, I.; Gazi, S. Sarcopenia in Rheumatoid arthritis. A narrative review. J. Frailty Sarcopenia Falls 2023, 8, 44–52. [Google Scholar] [CrossRef]
- Atkinson, A.J.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.; Downing, G.; Hoth, D.; Oates, J.; Peck, C.; Schooley, R.; Spilker, B.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological aspects of selected myokines in skeletal muscle: Focus on aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Delanaye, P.; Bataille, S.; Quinonez, K.; Buckinx, F.; Warling, X.; Krzesinski, J.M.; Pottel, H.; Burtey, S.; Bruyère, O.; Cavalier, E. Myostatin and insulin-like growth factor 1 are biomarkers of muscle strength, muscle mass, and mortality in patients on Hemodialysis. J. Ren. Nutr. 2019, 29, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Fennen, M.; Weinhage, T.; Kracke, V.; Intemann, J.; Varga, G.; Wehmeyer, C.; Foell, D.; Korb-Pap, A.; Pap, T.; Dankbar, B. A myostatin-CCL20-CCR6 axis regulates Th17 cell recruitment to inflamed joints in experimental arthritis. Sci. Rep. 2021, 11, 14145. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Hu, S.L.; Sun, Y.; Zhao, J.; Dai, C.; Wang, L.; Xu, G.; Tang, C.H. Myostatin induces tumor necrosis factor-α expression in rheumatoid arthritis synovial fibroblasts through the PI3K-Akt signaling pathway. J. Cell Physiol. 2019, 234, 9793–9801. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.Y.; Ho Lim, J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609, Erratum in: Endocr. Rev. 2021, 42, 97–99. [Google Scholar] [CrossRef]
- Zakharova, A.N.; Kironenko, T.A.; Milovanova, K.G.; Orlova, A.A.; Dyakova, E.Y.; Kalinnikova, Y.G.; Kabachkova, A.V.; Chibalin, A.V.; Kapilevich, L.V. Treadmill Training Effect on the Myokines Content in Skeletal Muscles of Mice with a Metabolic Disorder Model. Front. Physiol. 2021, 12, 709039. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Williams, N.G.; Interlichia, J.P.; Jackson, M.F.; Hwang, D.; Cohen, P.; Rodgers, B.D. Endocrine actions of myostatin: Systemic regulation of the IGF and IGF binding protein axis. Endocrinology 2011, 152, 172–180. [Google Scholar] [CrossRef]
- Pistilli, E.E.; Bogdanovich, S.; Goncalves, M.D.; Ahima, R.S.; Lachey, J.; Seehra, J.; Khurana, T. Targeting the activin type IIB receptor to improve muscle mass and function in the mdx mouse model of Duchenne muscular dystrophy. Am. J. Pathol. 2011, 178, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.N.; Cohn, R.D. Role of TGF-beta signaling in inherited and acquired myopathies. Skelet. Muscle 2011, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Q.; Zhou, X.; Mitch, W.E.; Goldberg, A.L. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 2013, 45, 2333–2347. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Qaisar, R.; Bose, B.; Sudheer, S.P. The elusive role of myostatin signaling for muscle regeneration and maintenance of muscle and bone homeostasis. Osteoporos. Sarcopenia 2023, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Tsuji, K.; Miyatake, K.; Matsukura, Y.; Abula, K.; Inoue, M.; Sekiya, I.; Muneta, T. Follistatin alleviates synovitis and articular cartilage degeneration induced by carrageenan. Int. J. Inflam. 2014, 20, 959271. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; He, X. Activin A: A potential therapeutic target for characterizing and stopping joint pain early in rheumatoid arthritis patients. Inflammation 2014, 37, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Tanrıkulu, O.; Sarıyıldız, M.A.; Batmaz, I.; Yazmalar, L.; Polat, N.; Kaplan, İ.; Çevik, R. Serum GDF-15 level in rheumatoid arthritis: Relationship with disease activity and subclinical atherosclerosis. Acta Reumatol. Port. 2017, 42, 66–72. [Google Scholar] [PubMed]
- Soliman, S.A.; Gad, R.; Aliaa, M.H.; Elshereef, R. Serum irisin level in rheumatoid arthritis patients: Relationship to disease activity, subclinical atherosclerosis, and cardiovascular risk factors. Egypt. Rheumatol. 2022, 44, 109–114. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Ebenbichler, G.; Föeger-Samwald, U.; Leiss, H.; Gesslbauer, C.; Herceg, M.; Stummvoll, G.; Marculescu, R.; Crevenna, R.; Pietschmann, P. Rheumatoid arthritis in remission: Decreased myostatin and increased serum levels of periostin. Wien. Klin. Wochenschr. 2019, 131, 1–7. [Google Scholar] [CrossRef]
- Brühlmann, P.; Stucki, G.; Michel, B.A. Evaluation of a German version of the physical dimensions of the Health Assessment Questionnaire in patients with rheumatoid arthritis. J. Rheumatol. 1994, 21, 1245–1249. [Google Scholar]
- Murillo-Saich, J.D.; Vazquez-Villegas, M.L.; Ramirez-Villafaña, M.; Saldaña-Cruz, A.M.; Aceves-Aceves, J.A.; Gonzalez-Lopez, L.; Guma, M.; Gamez-Nava, J.I. Association of myostatin, a cytokine released by muscle, with inflammation in rheumatoid arthritis: A cross-sectional study. Medicine 2021, 100, e24186. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Ma, J.D.; Yang, L.J.; Zou, Y.W.; Zhang, X.P.; Pan, J.; Li, Q.H.; Li, H.G.; Yang, Z.H.; Wu, T.; et al. Myokine myostatin is a novel predictor of one-year radiographic progression in patients with rheumatoid arthritis: A prospective cohort study. Front. Immunol. 2022, 13, 1005161. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ponce, F.; Gamez-Nava, J.I.; Gomez-Ramirez, E.E.; Ramirez-Villafaña, M.; Jacobo-Cuevas, H.; Rodriguez-Jimenez, N.A.; Olivas-Flores, E.M.; Esparza-Guerrero, Y.; Martelli-García, A.; Santiago-Garcia, A.P.; et al. Myostatin Levels and the Risk of Myopenia and Rheumatoid Cachexia in Women with Rheumatoid Arthritis. J. Immunol. Res. 2022, 2022, 7258152. [Google Scholar] [CrossRef] [PubMed]
- Andonian, B.J.; Bartlett, D.B.; Huebner, J.L.; Willis, L.; Hoselton, A.; Kraus, V.B.; Huffman, K.M. Effect of high-intensity interval training on muscle remodeling in rheumatoid arthritis compared to prediabetes. Arthritis Res. Ther. 2018, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Rovenský, J.; Payer, J. Total Sharp score (TSS). In Dictionary of Rheumatology, 1st ed.; Springer: Vienna, Austria, 2009; pp. 215–216. [Google Scholar]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Fries, J.F.; Spitz, P.; Kraines, R.G.; Holman, H.R. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980, 23, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymstleld, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.; Brennan, S.L.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Total and appendicular lean mass reference ranges for Australian men and women: The Geelong osteoporosis study. Calcif. Tissue Int. 2014, 94, 363–372. [Google Scholar] [CrossRef]
- Engvall, I.L.; Elkan, A.C.; Tengstrand, B.; Cederholm, T.; Brismar, K.; Hafstrom, I. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand. J. Rheumatol. 2008, 37, 321–328. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van-Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 3, 44–48. [Google Scholar] [CrossRef]
- Fransen, J.; Stucki, G.; van-Riel, P. Rheumatoid arthritis measures: Disease Activity Score (DAS), Disease Activity Score-28(DAS28), Rapid Assessment of Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis Disease Activity Index (RADAI). Arthritis Rheum. 2003, 49, S214–S224. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 2020, 41, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Chang, A.C.; Huang, C.C.; Tsai, C.H.; Lin, C.C.; Tang, C.H. Myostatin Promotes Interleukin-1β Expression in Rheumatoid Arthritis Synovial Fibroblasts through Inhibition of miR-21-5p. Front. Immunol. 2017, 8, 1747. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Cipriani, P.; Carubbi, F.; Liakouli, V.; Zazzeroni, F.; Di Benedetto, P.; Berardicurti, O.; Alesse, E.; Giacomelli, R. The role of IL-1beta in the bone loss during rheumatic diseases. Mediat. Inflamm. 2015, 2015, 782382. [Google Scholar] [CrossRef] [PubMed]
- Eastgate, J.A.; Symons, J.A.; Wood, N.C.; Grinlinton, F.M.; di Giovine, F.S.; Duff, G.W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet 1988, 2, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The effect of exercise on cytokines: Implications for musculoskeletal health: A narrative review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Lenk, K.; Erbs, S.; Höllriegel, R.; Beck, E.; Linke, A.; Gielen, S.; Winkler, S.M.; Sandri, M.; Hambrecht, R.; Schuler, G.; et al. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur. J. Prev. Cardiol. 2012, 19, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Jang, H.Y.; Ahn, J.M.; Hwang, S.H.; Chung, J.W.; Choi, Y.S.; Kim, J.W.; Jang, E.S.; Choi, G.H.; Jeong, S.H. The association of the serum levels of myostatin, follistatin, and interleukin-6 with sarcopenia, and their impacts on survival in patients with hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 492–505. [Google Scholar] [CrossRef]
- Philip, B.; Lu, Z.; Gao, Y. Regulation of GDF-8 signaling by the p38 MAPK. Cell Signal. 2005, 17, 365–375. [Google Scholar] [CrossRef]
- Strand, V.; Boklage, S.H.; Kimura, T.; Joly, F.; Boyapati, A.; Msihid, J. High levels of interleukin-6 in patients with rheumatoid arthritis are associated with greater improvements in health-related quality of life for sarilumab compared with adalimumab. Arthritis Res. Ther. 2020, 22, 250. [Google Scholar] [CrossRef]
- An, H.J.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Lee, K.H.; Nam, S.W.; Kim, J.S.; Yang, J.W.; Lee, J.Y.; Smith, L.; et al. Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5678. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Pereira, B.; Dutheil, F.; Giraud, C.; Courteix, D.; Sapin, V.; Frayssac, T.; Mathieu, S.; Malochet Guinamand, S.; Soubrier, M. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J. Cachexia Sarcopenia Muscle 2017, 8, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Benatti, F.; Pedersen, B. Exercise as an anti-inflammatory therapy for rheumatic diseases—Myokine regulation. Nat. Rev. Rheumatol. 2015, 11, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Steensberg, A.; Pilegaard, H.; Osada, T.; Saltin, B.; Pedersen, B.K.; Neufer, P.D. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: Influence of muscle glycogen content. FASEB J. 2001, 15, 2748–2750. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Klarlund Pedersen, B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Tocilizumab: A review in rheumatoid arthritis. Drugs 2017, 77, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.; Mancilla, J.; Endres, S.; Ghorbani, R.; Clark, S.C.; Dinarello, C.A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990, 75, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle derived interleukin-6: Possible biological effects. J. Physiol. 2001, 536, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Pedersenm, B.K.; Fischer, C.P. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 265–271. [Google Scholar] [CrossRef]
- Jørgensen, S.B.; Richter, E.A.; Wojtaszewski, J.F.P. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J. Physiol. 2006, 574, 17–31. [Google Scholar] [CrossRef]
- Ye, H.; Weng, H.; Xu, Y.; Wang, L.; Wang, Q.; Xu, G. Effectiveness and safety of aerobic exercise for rheumatoid arthritis: A systematic review and meta-analysis of randomized controlled trials. BMC Sports Sci. Med. Rehabil. 2022, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Flanagan, J.; Jasuja, R.; Kirkland, J.; Jiang, L.; Bhasin, S. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and wnt/beta-catenin signaling pathways. J. Biol. Chem. 2008, 283, 9136–9145. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Divieti Pajevic, P.; Bonewald, L.F.; Bauman, W.A.; et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017, 292, 11021–11033. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yi, Q.; Sun, W.; Huang, D.; Zhang, H.; Duan, L.; Shang, H.; Wang, D.; Xiong, J. Molecular basis and therapeutic potential of myostatin on bone formation and metabolism in orthopedic disease. Biofactors 2023, 49, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, E.; Grammatikopoulou, M.G.; Gkiouras, K.; Efthymiou, G.; Zafiriou, E.; Goulis, D.G.; Sakkas, L.I.; Bogdanos, D.P. Time to Deal with Rheumatoid Cachexia: Prevalence, Diagnostic Criteria, Treatment Effects and Evidence for Management. Mediterr. J. Rheumatol. 2022, 33, 271–290. [Google Scholar] [CrossRef]
- Tobin, J.F.; Celeste, A.J. Myostatin, a negative regulator of muscle mass: Implications for muscle degenerative diseases. Curr. Opin. Pharmacol. 2005, 5, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Summary comments. Am. J. Clin. Nutr. 1989, 50, 231–1233. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Cruz Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Santos, M.J.; Vinagre, F.; Canas Da Silva, J.; Gil, V.; Fonseca, J.E. Body composition phenotypes in systemic lupus erythematosus and rheumatoid arthritis: A comparative study of Caucasian female patients. Clin. Exp. Rheumatol. 2011, 29, 470–476. [Google Scholar] [PubMed]
- Lin, J.; Liang, J.; Ma, J.; Li, Q.; Mo, Y.; Cheng, W.; He, X.; Li, N.; Cao, M.; Xu, D.; et al. Myopenia is associated with joint damage in rheumatoid arthritis: A cross-sectional study. J. Cachexia Sarcopenia Muscle 2019, 10, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef] [PubMed]
- Bischoff Ferrari, H.A.; Borchers, M.; Gudat, F.; Dürmüller, U.; Stähelin, H.B.; Dick, W. Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. 2004, 19, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Wang, X.L.; Chu, Y.R.; Wang, J.X.; Xu, S.Q. Association of sarcopenia and vitamin D deficiency with glucocorticoid-induced osteoporosis in Chinese patients with rheumatoid arthritis. Clin. Rheumatol. 2024, 43, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Minamino, H.; Katsushima, M.; Torii, M.; Yamamoto, W.; Fujita, Y.; Ikeda, K.; Okamura, E.; Murakami, K.; Watanabe, R.; Murata, K.; et al. Serum vitamin D status inversely associates with a prevalence of severe sarcopenia among female patients with rheumatoid arthritis. Sci. Rep. 2021, 11, 20485. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lehar, A.; Sebald, S.; Liu, M.; Swaggart, K.A.; Talbot, C.C., Jr.; Pytel, P.; Barton, E.R.; McNally, E.M.; Lee, S.J. Muscle hypertrophy induced by myostatin inhibition accelerates degeneration in dysferlinopathy. Hum. Mol. Genet. 2015, 24, 5711–5719. [Google Scholar] [CrossRef]
- Abati, E.; Manini, A.; Comi, G.P.; Corti, S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol. Life Sci. 2022, 79, 374. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, K.; Choi, C.-I. Farmacogenómica de anticuerpos monoclonales para el tratamiento de la artritis reumatoide. J. Pers. Med. 2022, 12, 1265. [Google Scholar] [CrossRef]
- Suh, J.; Lee, Y.S. Myostatin Inhibitors: Panacea or Predicament for Musculoskeletal Disorders? J. Bone Metab. 2020, 27, 151–165. [Google Scholar] [CrossRef]
| Author/Country/Year | Groups | Results | Conclusions |
|---|---|---|---|
| Kerschan-Schindl K,/Austria/2019 [59] |
|
| RA patients in remission have reduced serum levels of myostatin when compared with healthy controls. Patients with higher myostatin levels had a lower grip strength in both hands and worse physical functional condition. |
| Murillo-Saich JD/Mexico/2021 [61] |
|
| Myostatin was associated with disease activity in RA patients, suggesting a mechanistic link between myostatin, muscle wasting, and inflammation in RA. |
| Lin JZ/China/2022 [62] |
|
| Serum myostatin levels are higher in patients with RA than in healthy controls |
| Gonzalez-Ponce F/Mexico/2022 [63] |
|
| Myostatin level are higher in RA female patients. |
| Author/Country/Year | Groups | Results | Conclusions |
|---|---|---|---|
| Murillo-Saich JD/Mexico/2021 [61]. | 84 female RA | Correlation of myostatin levels with
| Myostatin was inversely related to the index of skeletal muscle mass and fat-free mass. |
| Lin JZ/China/2022 [62]. | 344 RA | Myostatin levels (ng/mL) 3004 ± 1640 with myopenia vs. 3428 ± 1689 without myopenia, p-value = 0.013 ** | RA patients with myopenia had lower levels than the group without myopenia at the beginning of the study. |
| Gonzalez-Ponce F/Mexico/2022 [63]. | 161 female RA:
|
| A high myostatin level is a risk factor for low muscle mass. |
| Author/Country/Year | Groups | Results | Conclusions |
|---|---|---|---|
| Murillo-Saich JD/Mexico/2021 [61]. | 84 female RA | Myostatin cut-off (≥13 ng/mL) point associated with moderate or severe disease activity: OR = 2.938 (CI 95% 1.13–7.61, p-value 0.027) | Myostatin (≥13 ng/mL) had a sensibility of 61.3% and a specificity of 53% to detect moderate or severe disease activity in RA patients |
| Gonzalez-Ponce F/Mexico/2022 [62]. | 161 female RA | Myostatin cut-off point (≥17 ng/mL) associated with low muscle mass: OR = 3.04 (CI 95% 1.17–7.89, p-value 0.02). | Myostatin (≥17 ng/mL) had a sensibility of 43% and specificity of 77% to detect low muscle mass in RA patients, and a sensibility of 53% and specificity of 71% to detect rheumatoid cachexia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Ponce, F.; Ramirez-Villafaña, M.; Gomez-Ramirez, E.E.; Saldaña-Cruz, A.M.; Gallardo-Moya, S.G.; Rodriguez-Jimenez, N.A.; Jacobo-Cuevas, H.; Nava-Valdivia, C.A.; Avalos-Salgado, F.A.; Totsuka-Sutto, S.; et al. Role of Myostatin in Rheumatoid Arthritis: A Review of the Clinical Impact. Diagnostics 2024, 14, 1085. https://doi.org/10.3390/diagnostics14111085
Gonzalez-Ponce F, Ramirez-Villafaña M, Gomez-Ramirez EE, Saldaña-Cruz AM, Gallardo-Moya SG, Rodriguez-Jimenez NA, Jacobo-Cuevas H, Nava-Valdivia CA, Avalos-Salgado FA, Totsuka-Sutto S, et al. Role of Myostatin in Rheumatoid Arthritis: A Review of the Clinical Impact. Diagnostics. 2024; 14(11):1085. https://doi.org/10.3390/diagnostics14111085
Chicago/Turabian StyleGonzalez-Ponce, Fabiola, Melissa Ramirez-Villafaña, Eli Efrain Gomez-Ramirez, Ana Miriam Saldaña-Cruz, Sergio Gabriel Gallardo-Moya, Norma Alejandra Rodriguez-Jimenez, Heriberto Jacobo-Cuevas, Cesar Arturo Nava-Valdivia, Felipe Alexis Avalos-Salgado, Sylvia Totsuka-Sutto, and et al. 2024. "Role of Myostatin in Rheumatoid Arthritis: A Review of the Clinical Impact" Diagnostics 14, no. 11: 1085. https://doi.org/10.3390/diagnostics14111085
APA StyleGonzalez-Ponce, F., Ramirez-Villafaña, M., Gomez-Ramirez, E. E., Saldaña-Cruz, A. M., Gallardo-Moya, S. G., Rodriguez-Jimenez, N. A., Jacobo-Cuevas, H., Nava-Valdivia, C. A., Avalos-Salgado, F. A., Totsuka-Sutto, S., Cardona-Muñoz, E. G., & Valdivia-Tangarife, E. R. (2024). Role of Myostatin in Rheumatoid Arthritis: A Review of the Clinical Impact. Diagnostics, 14(11), 1085. https://doi.org/10.3390/diagnostics14111085






