Photon-Counting Computed Tomography in Atherosclerotic Plaque Characterization
Abstract
1. Introduction
2. Photon-Counting Technology and Advantages in Plaque Evaluation
3. Features of Plaque Vulnerability
4. Clinical Application of PCCT in Carotid Plaque
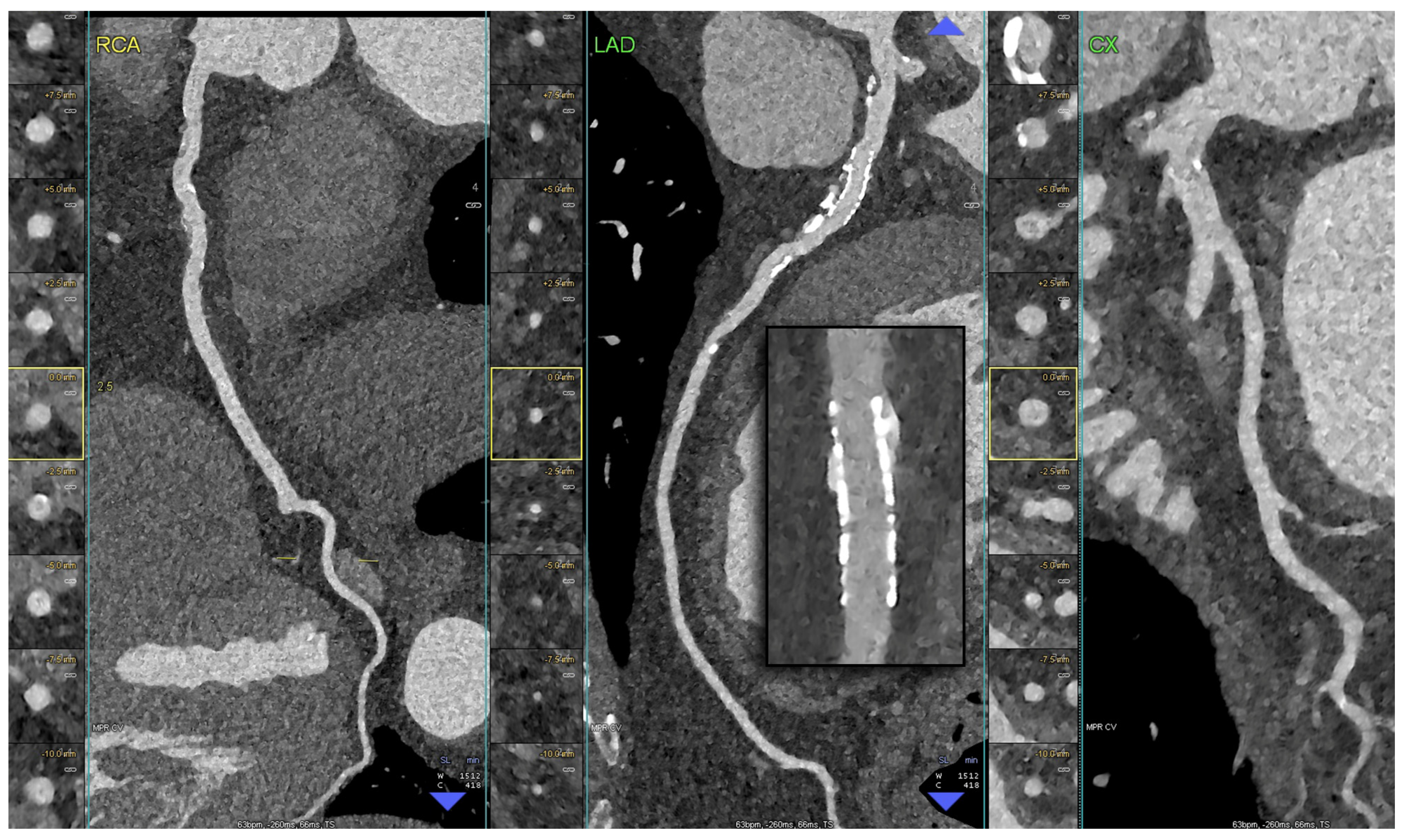
5. Clinical Application of PCCT in Coronary Plaque
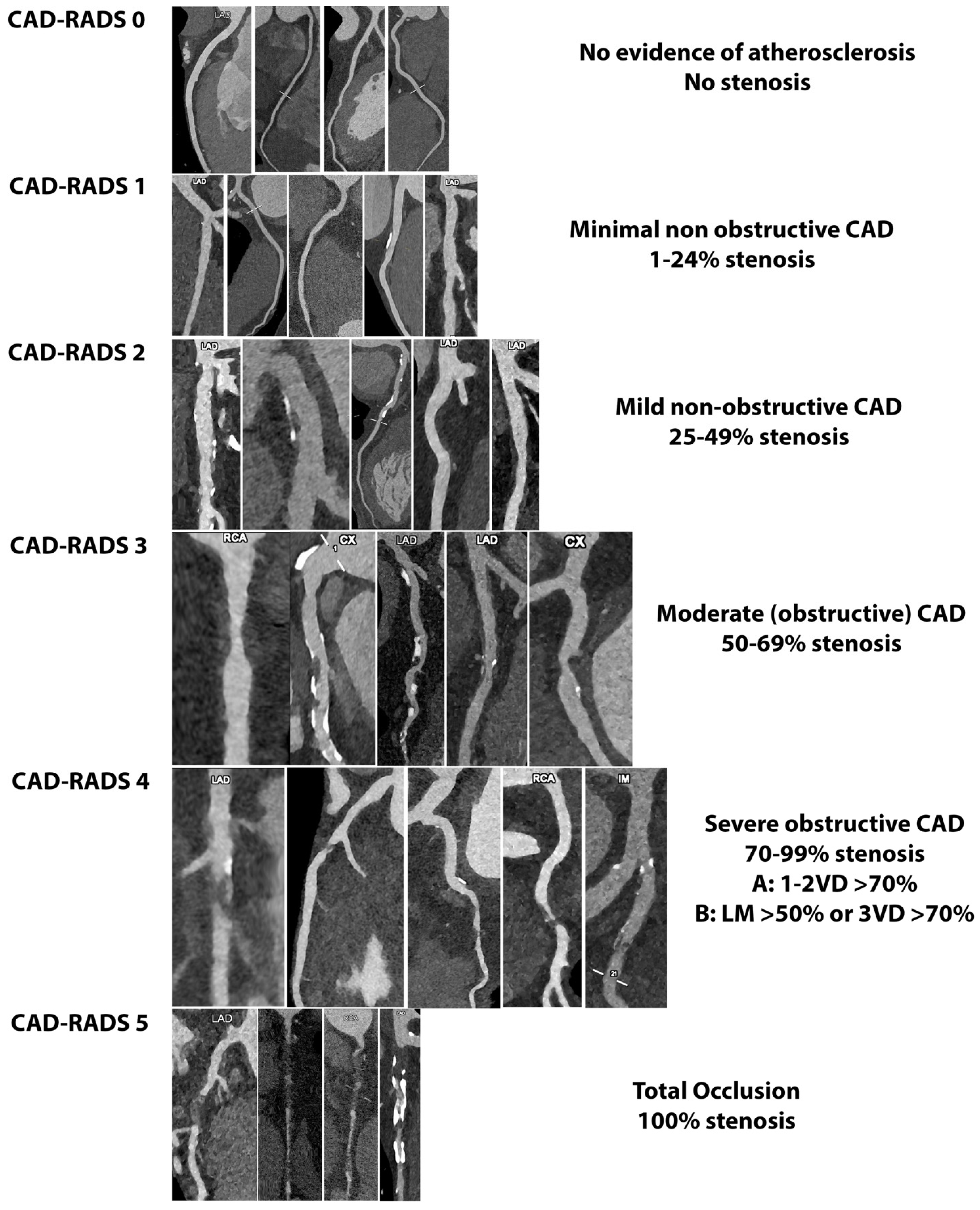
6. Potential Application of PCCT in the Light of Plaque-RADS and CAD-RADS
7. Clinical Perspective
8. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Much, A.A.; Maor, E.; Asher, E.; Younis, A.; Xu, Y.; Lu, Y.; Liu, X.; Shu, J.; Bragazzi, N.L. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990-2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J.-Qual. Care Clin. Outcomes 2022, 8, 50–60. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 8), C7–C12. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From Vulnerable Plaque to Vulnerable Patient. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef]
- Puchner, S.B.; Liu, T.; Mayrhofer, T.; Truong, Q.A.; Lee, H.; Fleg, J.L.; Nagurney, J.T.; Udelson, J.E.; Hoffmann, U.; Ferencik, M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 2014, 64, 684–692. [Google Scholar] [CrossRef]
- Saba, L.; Agarwal, N.; Cau, R.; Gerosa, C.; Sanfilippo, R.; Porcu, M.; Montisci, R.; Cerrone, G.; Qi, Y.; Balestrieri, A.; et al. Review of imaging biomarkers for the vulnerable carotid plaque. JVS Vasc. Sci. 2021, 2, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Loewe, C.; Weikert, T.; Williams, M.C.; Galea, N.; Budde, R.P.J.; Vliegenthart, R.; Velthuis, B.K.; Francone, M.; Bremerich, J.; et al. State-of-the-art CT and MR imaging and assessment of atherosclerotic carotid artery disease: The reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur. Radiol. 2023, 33, 1088–1101. [Google Scholar] [CrossRef]
- Saba, L.; Loewe, C.; Weikert, T.; Williams, M.C.; Galea, N.; Budde, R.P.J.; Vliegenthart, R.; Velthuis, B.K.; Francone, M.; Bremerich, J.; et al. State-of-the-art CT and MR imaging and assessment of atherosclerotic carotid artery disease: Standardization of scanning protocols and measurements-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur. Radiol. 2023, 33, 1063–1087. [Google Scholar] [CrossRef]
- Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; MacLean, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef]
- Saba, L.; Chen, H.; Cau, R.; Rubeis, G.D.; Zhu, G.; Pisu, F.; Jang, B.; Lanzino, G.; Suri, J.S.; Qi, Y.; et al. Impact Analysis of Different CT Configurations of Carotid Artery Plaque Calcifications on Cerebrovascular Events. AJNR. Am. J. Neuroradiol. 2022, 43, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Cau, R.; Spinato, G.; Suri, J.S.; Melis, M.; De Rubeis, G.; Antignani, P.; Gupta, A. Carotid Stenosis and Cryptogenic Stroke: The Evidence from the Imaging-based Studies Carotid stenosis and Cryptogenic Stroke. J. Vasc. Surg. 2024, 79, 1119–1131. [Google Scholar] [CrossRef]
- Farzaneh-Far, A.; Steigner, M.; Kwong, R.Y. Applications and limitations of cardiac computed tomography in the evaluation of coronary artery disease. Coron. Artery Dis. 2013, 24, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, F.; Meloni, A.; Pistoia, L.; Degiorgi, G.; Clemente, A.; De Gori, C.; Positano, V.; Celi, S.; Berti, S.; Emdin, M.; et al. Dual Source Photon-Counting Computed Tomography—Part II: Clinical Overview of Neurovascular Applications. J. Clin. Med. 2023, 12, 3626. [Google Scholar] [CrossRef]
- Wehrse, E.; Ziener, C.H.; Sawall, S. Photon-counting detectors in computed tomography: From quantum physics to clinical practice. Der Radiol. 2021, 61, 1. [Google Scholar] [CrossRef]
- Sandfort, V.; Persson, M.; Pourmorteza, A.; Noël, P.B.; Fleischmann, D.; Willemink, M.J. Spectral photon-counting CT in cardiovascular imaging. J. Cardiovasc. Comput. Tomogr. 2021, 15, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Bruesewitz, M.; Tao, S.; Rajendran, K.; Halaweish, A.F.; Campeau, N.G.; Fletcher, J.G.; McCollough, C.H. Photon-counting detector CT: System design and clinical applications of an emerging technology. Radiographics 2019, 39, 729–743. [Google Scholar] [CrossRef]
- Esquivel, A.; Ferrero, A.; Mileto, A.; Baffour, F.; Horst, K.; Rajiah, P.S.; Inoue, A.; Leng, S.; McCollough, C.; Fletcher, J.G. Photon-counting detector CT: Key points radiologists should know. Korean J. Radiol. 2022, 23, 854. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; Iglio, I.D.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112. [Google Scholar] [CrossRef]
- Leng, S.; Rajendran, K.; Gong, H.; Zhou, W.; Halaweish, A.F.; Henning, A.; Kappler, S.; Baer, M.; Fletcher, J.G.; McCollough, C.H. 150-μm spatial resolution using photon-counting detector computed tomography technology: Technical performance and first patient images. Investig. Radiol. 2018, 53, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ferda, J.; Vendiš, T.; Flohr, T.; Schmidt, B.; Henning, A.; Ulzheimer, S.; Pecen, L.; Ferdová, E.; Baxa, J.; Mírka, H. Computed tomography with a full FOV photon-counting detector in a clinical setting, the first experience. Eur. J. Radiol. 2021, 137, 109614. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.A.; Sigovan, M.; Hsu, J.C.; Tatard-Leitman, V.; Chalabreysse, L.; Naha, P.C.; Garrivier, T.; Dessouky, R.; Carnaru, M.; Boussel, L. In vivo molecular K-edge imaging of atherosclerotic plaque using photon-counting CT. Radiology 2021, 300, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Si-mohamed, S.A.; Miailhes, J.; Rodesch, P.; Boccalini, S.; Lacombe, H.; Cottin, V.; Boussel, L.; Douek, P. Spectral Photon-Counting CT Technology in Chest Imaging. J. Clin. Med. 2021, 10, 5757. [Google Scholar] [CrossRef] [PubMed]
- Sawall, S.; Klein, L.; Amato, C.; Wehrse, E.; Dorn, S.; Maier, J.; Heinze, S.; Schlemmer, H.-P.; Ziener, C.H.; Uhrig, M.; et al. Iodine contrast-to-noise ratio improvement at unit dose and contrast media volume reduction in whole-body photon-counting CT. Eur. J. Radiol. 2020, 126, 108909. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Leng, S.; Kappler, S.; Hahn, K.; Li, Z.; Halaweish, A.F.; Henning, A.; McCollough, C.H. Noise performance of low-dose CT: Comparison between an energy integrating detector and a photon counting detector using a whole-body research photon counting CT scanner. J. Med. Imaging 2016, 3, 43503. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Cork, T.E.; Sahbaee, P.; Fuld, M.K.; Kappler, S.; Folio, L.R.; Bluemke, D.A.; Pourmorteza, A. Low-dose lung cancer screening with photon-counting CT: A feasibility study. Phys. Med. Biol. 2016, 62, 202. [Google Scholar] [CrossRef] [PubMed]
- Pannenbecker, P.; Huflage, H.; Grunz, J.-P.; Gruschwitz, P.; Patzer, T.S.; Weng, A.M.; Heidenreich, J.F.; Bley, T.A.; Petritsch, B. Photon-counting CT for diagnosis of acute pulmonary embolism: Potential for contrast medium and radiation dose reduction. Eur. Radiol. 2023, 33, 7830–7839. [Google Scholar] [CrossRef] [PubMed]
- Emrich, T.; O’Doherty, J.; Schoepf, U.J.; Suranyi, P.; Aquino, G.; Kloeckner, R.; Halfmann, M.C.; Allmendinger, T.; Schmidt, B.; Flohr, T.; et al. Reduced Iodinated Contrast Media Administration in Coronary CT Angiography on a Clinical Photon-Counting Detector CT System: A Phantom Study Using a Dynamic Circulation Model. Investig. Radiol. 2022, 58, 148–155. [Google Scholar] [CrossRef]
- Cormode, D.P.; Fayad, Z.A. Nanoparticle contrast agents for CT: Their potential and the challenges that lie ahead. Imaging Med. 2011, 3, 263–266. [Google Scholar] [CrossRef]
- Tao, S.; Rajendran, K.; McCollough, C.H.; Leng, S. Feasibility of multi-contrast imaging on dual-source photon counting detector (PCD) CT: An initial phantom study. Med. Phys. 2019, 46, 4105–4115. [Google Scholar] [CrossRef]
- Muenzel, D.; Bar-Ness, D.; Roessl, E.; Blevis, I.; Bartels, M.; Fingerle, A.A.; Ruschke, S.; Coulon, P.; Daerr, H.; Kopp, F.K.; et al. Spectral Photon-counting CT: Initial Experience with Dual-Contrast Agent K-Edge Colonography. Radiology 2017, 283, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Jost, G.; McDermott, M.; Gutjahr, R.; Nowak, T.; Schmidt, B.; Pietsch, H. New Contrast Media for K-Edge Imaging With Photon-Counting Detector CT. Investig. Radiol. 2023, 58, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, T.; Eberhard, M.; Rüschoff, J.H.; Pietsch, H.; Jost, G.; Nowak, T.; Schmidt, B.; Flohr, T.; Euler, A.; Alkadhi, H. Photon-counting CT with tungsten as contrast medium: Experimental evidence of vessel lumen and plaque visualization. Atherosclerosis 2020, 310, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Thim, T.; Kristensen, I.B. Atherosclerotic plaque, adventitia, perivascular fat, and carotid imaging. JACC Cardiovasc. Imaging 2009, 2, 183–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Nardi, V.; Cau, R.; Gupta, A.; Kamel, H.; Suri, J.S.; Balestrieri, A.; Congiu, T.; Butler, A.P.H.; Gieseg, S.; et al. Carotid Artery Plaque Calcifications: Lessons from Histopathology to Diagnostic Imaging. Stroke 2022, 53, 290–297. [Google Scholar] [CrossRef]
- Ehara, S.; Kobayashi, Y.; Yoshiyama, M.; Shimada, K.; Shimada, Y.; Fukuda, D.; Nakamura, Y.; Yamashita, H.; Yamagishi, H.; Takeuchi, K. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: An intravascular ultrasound study. Circulation 2004, 110, 3424–3429. [Google Scholar] [CrossRef]
- Dahal, S.; Raja, A.Y.; Searle, E.; Colgan, F.E.; Crighton, J.S.; Roake, J.; Saba, L.; Gieseg, S.; Butler, A.P.H. Components of carotid atherosclerotic plaque in spectral photon-counting CT with histopathologic comparison. Eur. Radiol. 2023, 33, 1612–1619. [Google Scholar] [CrossRef]
- Shami, A.; Sun, J.; Gialeli, C.; Markstad, H.; Edsfeldt, A.; Aurumskjöld, M.-L.; Gonçalves, I. Atherosclerotic plaque features relevant to rupture-risk detected by clinical photon-counting CT ex vivo: A proof-of-concept study. Eur. Radiol. Exp. 2024, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.; Searle, E.; Panta, R.K.; Chernoglazov, A.; Roake, J.; Butler, P.; Butler, A.; Gieseg, S.P.; Adebileje, S.A.; Alexander, S.D.; et al. Ex-vivo atherosclerotic plaque characterization using spectral photon-counting CT: Comparing material quantification to histology. Atherosclerosis 2023, 378, 117160. [Google Scholar] [CrossRef]
- Zainon, R.; Ronaldson, J.P.; Janmale, T.; Scott, N.J.; Buckenham, T.M.; Butler, A.P.H.; Butler, P.H.; Doesburg, R.M.; Gieseg, S.P.; Roake, J.A.; et al. Spectral CT of carotid atherosclerotic plaque: Comparison with histology. Eur. Radiol. 2012, 22, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.J.; Rajendran, K.; Tao, S.; Vercnocke, A.; Anderson, J.; Leng, S.; Ritman, E.; McCollough, C. A Blooming correction technique for improved vasa vasorum detection using an ultra-high-resolution photon-counting detector CT. In Medical Imaging 2020: Physics of Medical Imaging; SPIE: Bellingham, DC, USA, 2020; Volume 11312. [Google Scholar] [CrossRef]
- Rajendran, K.; Leng, S.; Jorgensen, S.M.; Abdurakhimova, D.; Ritman, E.L.; McCollough, C.H. Detection of increased vasa vasorum in artery walls: Improving CT number accuracy using image deconvolution. In Medical Imaging 2017: Physics of Medical Imaging; SPIE: Bellingham, DC, USA, 2017; Volume 10132. [Google Scholar] [CrossRef]
- Marsh, J.F.J.; Vercnocke, A.J.; Rajendran, K.; Tao, S.; Anderson, J.L.; Ritman, E.L.; Leng, S.; McCollough, C.H. Measurement of enhanced vasa vasorum density in a porcine carotid model using photon counting detector CT. J. Med. Imaging 2023, 10, 16001. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.-A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef]
- Mergen, V.; Eberhard, M.; Manka, R.; Euler, A.; Alkadhi, H. First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography. Front. Cardiovasc. Med. 2022, 9, 981012. [Google Scholar] [CrossRef]
- Rotzinger, D.C.; Racine, D.; Becce, F.; Lahoud, E.; Erhard, K. Performance of spectral photon-counting coronary CT angiography and comparison with energy-integrating-detector CT: Objective assessment with Performance of Spectral Photon-Counting Coronary CT Angiography and Comparison with Energy-Integrating-Detector. Diagnostics 2021, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Boussel, L.; Coulon, P.; Thran, A.; Roessl, E.; Martens, G.; Sigovan, M.; Douek, P. Photon counting spectral CT component analysis of coronary artery atherosclerotic plaque samples. Br. J. Radiol. 2014, 87, 20130798. [Google Scholar] [CrossRef]
- Baturin, P.; Alivov, Y.; Molloi, S. Spectral CT imaging of vulnerable plaque with two independent biomarkers. Phys. Med. Biol. 2012, 57, 4117–4138. [Google Scholar] [CrossRef]
- Vanmeter, P.; Marsh, J.; Rajendran, K.; Leng, S.; Mccollough, C. Quantification of Coronary Calcification using High-Resolution Photon-Counting-Detector CT and an Image Domain Denoising Algorithm. In Medical Imaging 2022: Physics of Medical Imaging; SPIE: Bellingham, DC, USA, 2022; pp. 1–10. [Google Scholar] [CrossRef]
- Cury, R.C.; Abbara, S.; Achenbach, S.; Agatston, A.; Berman, D.S.; Budoff, M.J.; Dill, K.E.; Jacobs, J.E.; Maroules, C.D.; Rubin, G.D.; et al. CAD-RADSTM Coronary Artery Disease–Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J. Cardiovasc. Comput. Tomogr. 2016, 10, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.C.; Leipsic, J.; Abbara, S.; Achenbach, S.; Berman, D.; Bittencourt, M.; Budoff, M.; Chinnaiyan, K.; Choi, A.D.; Ghoshhajra, B.; et al. CAD-RADSTM 2.0-2022 Coronary Artery Disease-Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America society of cardiovascular imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2022, 16, 536–557. [Google Scholar] [CrossRef] [PubMed]
- Emrich, T.; Halfmann, M.; Fink, N.; Varga-Szemes, A.; Bockius, S.; Schoepf, U.; Hell, M. Ultra-high Resolution Of Photon-Counting Detector Coronary CT Angiography Leads To Significant Stenosis Reclassification In Acute Chest Pain Patients. J. Cardiovasc. Comput. Tomogr. 2023, 17, S6. [Google Scholar] [CrossRef]
- Saba, L.; Cau, R.; Murgia, A.; Nicolaides, A.N.; Wintermark, M.; Castillo, M.; Staub, D.; Kakkos, S.; Yang, Q.; Paraskevas, K.I.; et al. Carotid Plaque-RADS, a novel stroke risk classification system. JACC Cardiovasc. Imaging 2023, 17, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Kreisler, B. Photon counting Detectors: Concept, technical Challenges, and clinical outlook. Eur. J. Radiol. 2022, 149, 110229. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Higaki, T.; Kondo, S.; Kawashita, I.; Takahashi, I.; Awai, K. An introduction to photon-counting detector CT (PCD CT) for radiologists. Jpn. J. Radiol. 2023, 41, 266–282. [Google Scholar] [CrossRef]
- Cammin, J.; Xu, J.; Barber, W.C.; Iwanczyk, J.S.; Hartsough, N.E.; Taguchi, K. A cascaded model of spectral distortions due to spectral response effects and pulse pileup effects in a photon-counting X-ray detector for CT. Med. Phys. 2014, 41, 41905. [Google Scholar] [CrossRef]
- Taguchi, K.; Iwanczyk, J.S. Vision 20/20: Single photon counting X-ray detectors in medical imaging. Med. Phys. 2013, 40, 100901. [Google Scholar] [CrossRef]
- Flohr, T.; Schmidt, B. Technical basics and clinical benefits of photon-counting CT. Investig. Radiol. 2023, 58, 441–450. [Google Scholar] [CrossRef]
- Wang, A.S.; Pelc, N.J. Spectral photon counting CT: Imaging algorithms and performance assessment. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 5, 453–464. [Google Scholar] [CrossRef]
- Vattay, B.; Szilveszter, B.; Boussoussou, M.; Vecsey-Nagy, M.; Lin, A.; Konkoly, G.; Kubovje, A.; Schwarz, F.; Merkely, B.; Maurovich-Horvat, P.; et al. Impact of virtual monoenergetic levels on coronary plaque volume components using photon-counting computed tomography. Eur. Radiol. 2023, 33, 8528–8539. [Google Scholar] [CrossRef] [PubMed]
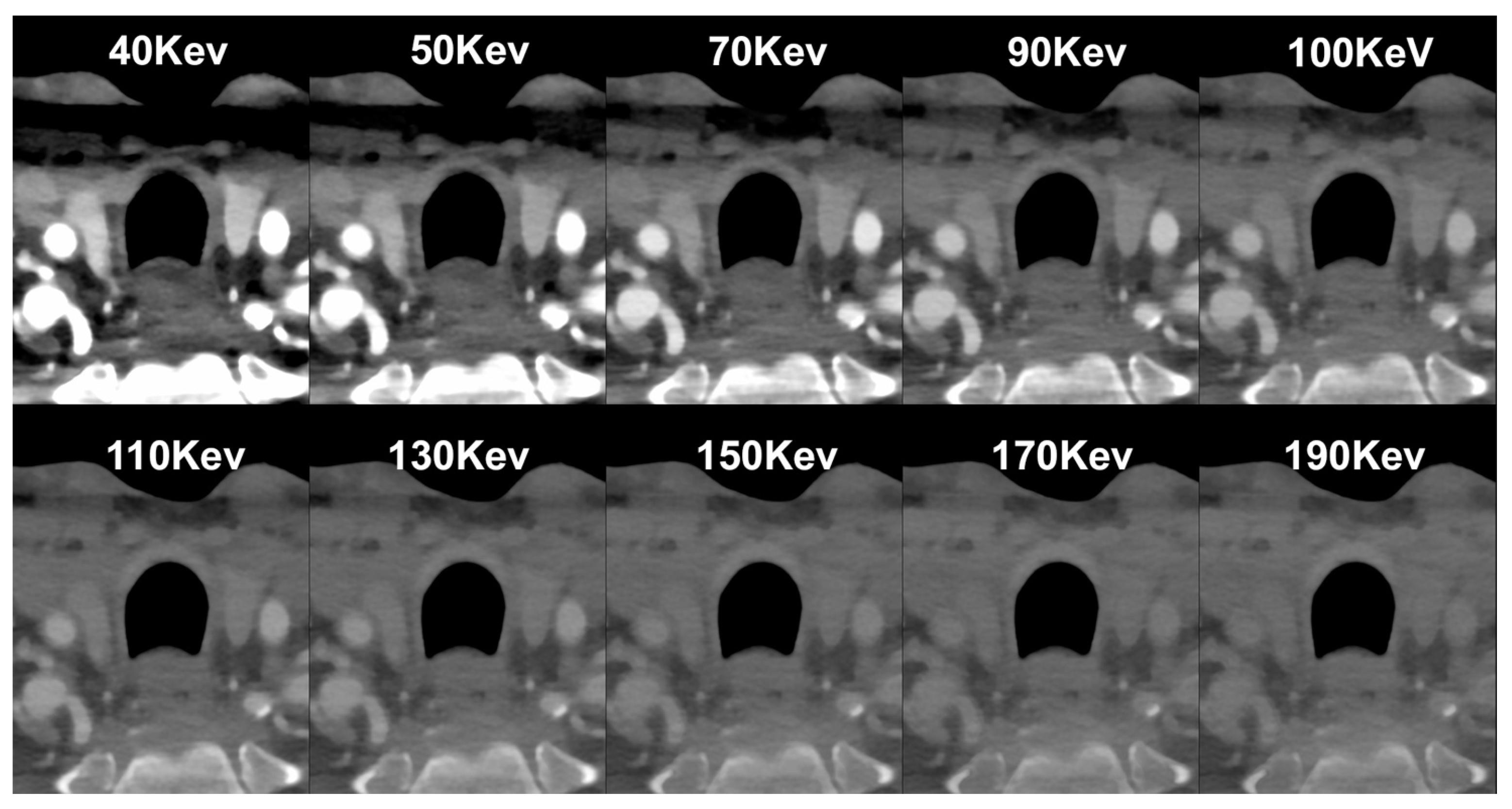

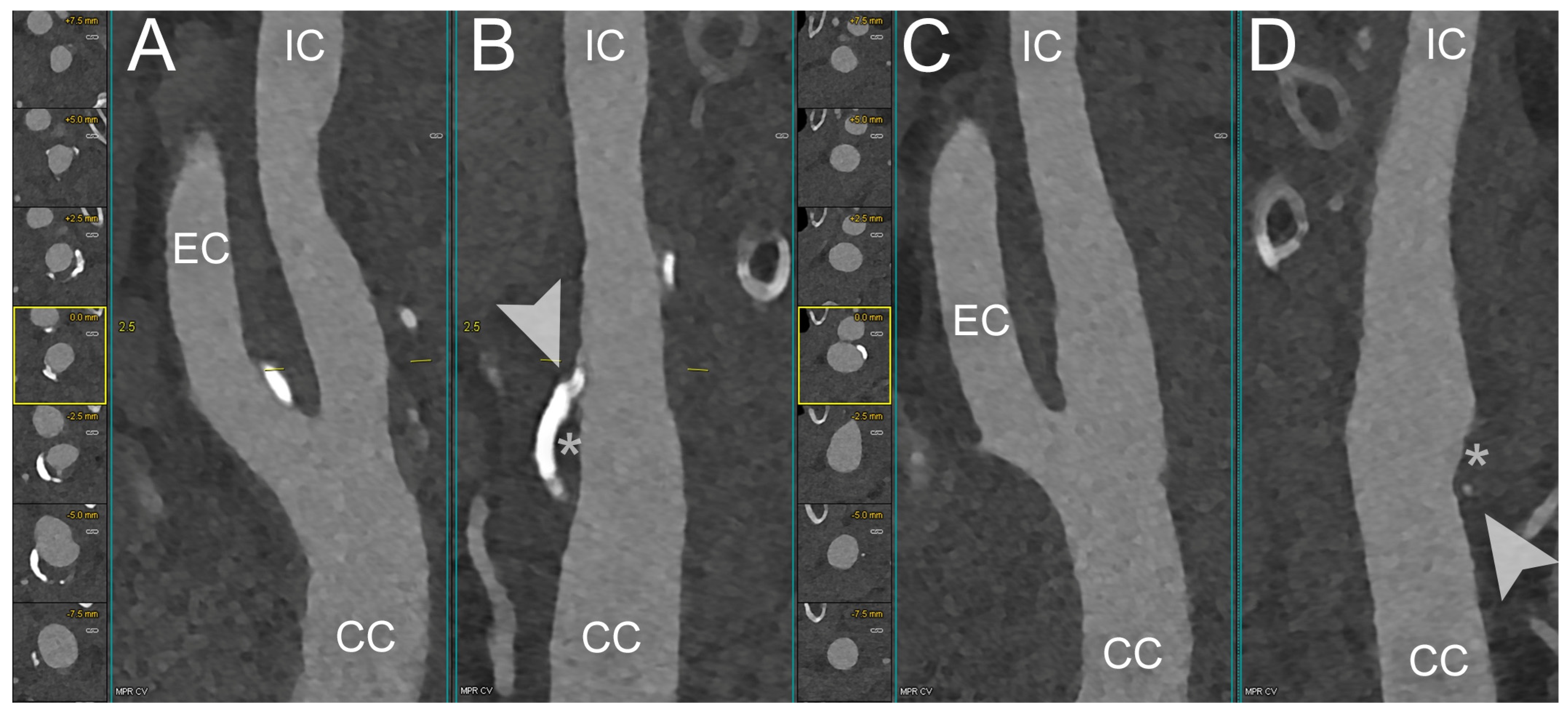
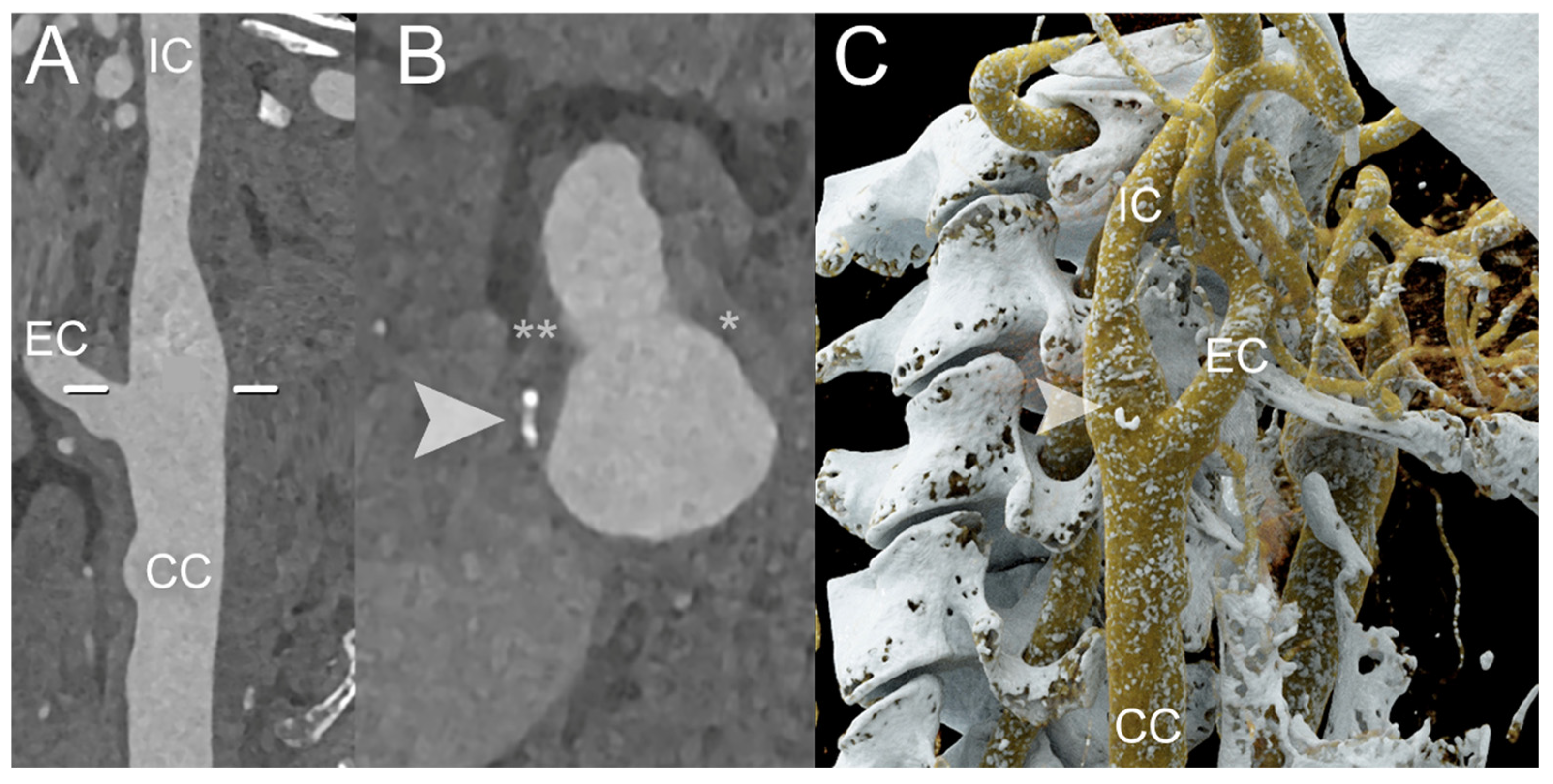
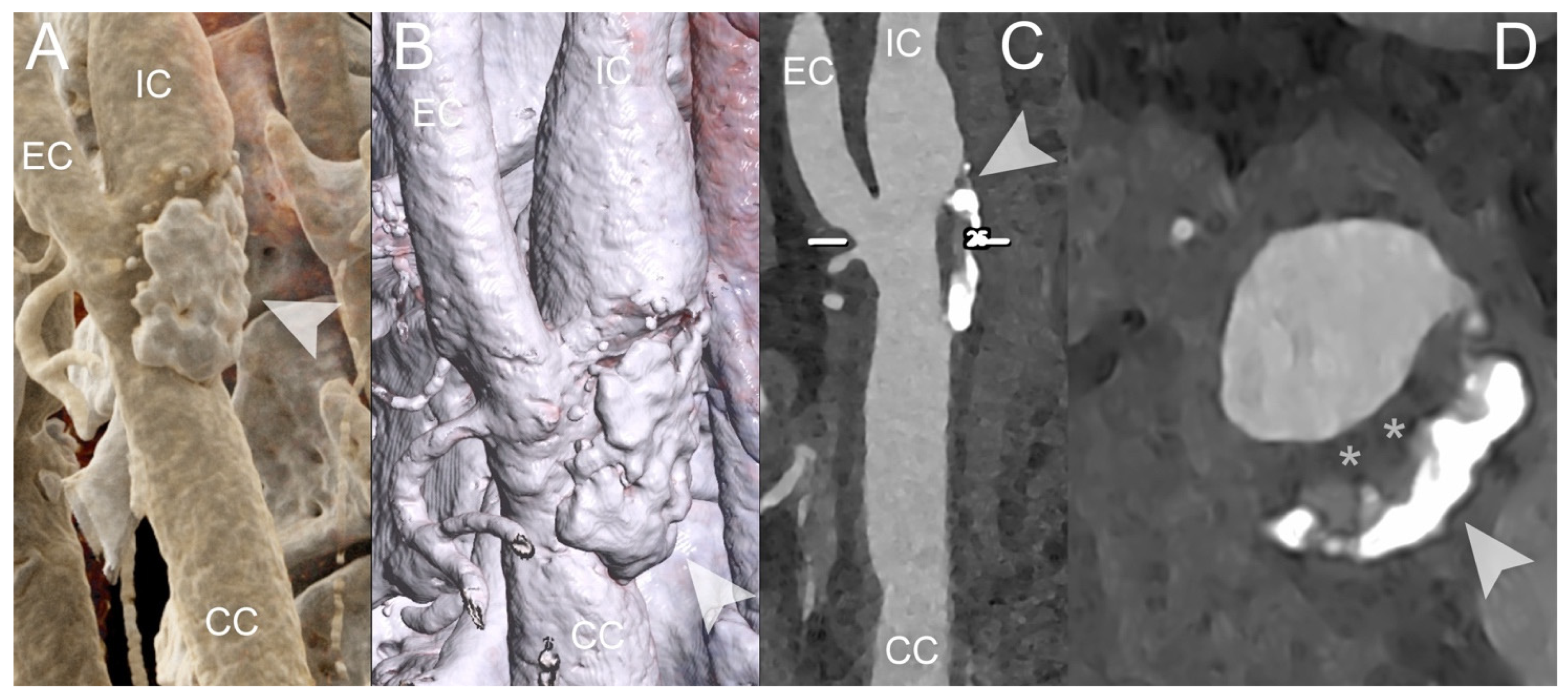

| PCCT | Conventional CT | |
|---|---|---|
| Detectors | Semi-conductive materials, such as cadmium telluride, cadmium zinc telluride, or silicon. | Energy-integrating detectors. |
| Mechanism | Direct conversion technology: X-ray photons are directly converted into an electrical signal. Each photon produces an electrical pulse, and the size (amplitude) of this pulse is directly proportional to the energy of the photon. | Indirect conversion technology: a scintillator layer first converts X-ray photons into visible light. This light is then captured by a photodiode layer and transformed into an electrical signal. |
| Detectors | Smaller detector element size without septa. | Detector element size affects spatial resolution.Thin septa between detectors are needed to prevent optical cross talk. |
| Detector element size (mm2) | 0.225 × 0.225 mm2 | 0.3–0.7 mm2 |
| Resolution | 0.2 mm (high-resolution mode)0.4 mm (multi-energy mode) | 0.6 mm |
| Signal detection | All photons are equally weighted regardless of their energy. | The detected signal represents the total energy of all photons, and high-energy photons tend to contribute more. |
| Spectral characterization | Intrinsic energy-dependent information with multi-energy acquisition. | Need for prospective selection of a dual-energy protocol (with the exception of the dual-layer detector). |
| Advantages |
|
|
| Disavantages |
|
|
| Authors | Years | Model | Number of Patients | Clinical Application | Results |
|---|---|---|---|---|---|
| Dahal et al. [40] | 2022 | Ex vivo | Carotid | PCCT enables the assessment of fibrous cap thickness and area without significant difference in comparison with histological measurements | |
| Shami et al. [41] | 2024 | Ex vivo | Carotid | PCCT was able to distinguish among well-known features of plaque vulnerability, such as intraplaque hemorrhage, as well as other plaque components including the fibrous cap, lipid, and necrosis | |
| Sartoretti et al. [34] | 2020 | Ex vivo | Carotid | A multi-energy PCCT algorithm combined with tungsten-based contrast media which enables improved visualization of the vessel lumen and vessel wall with reduced image noise | |
| Healy et al. [42] | 2023 | Ex vivo | Carotid | PCCT allows the identification of hallmarks of vulnerable plaque including a lipid-rich necrotic core, spotty calcifications, and ulcerations | |
| Zainon et al. [43] | 2012 | Phantom | Carotid | PCCT demonstrated the capability to discriminate between plaque components, providing deeper insights into features associated with plaque instability | |
| Marsh et al. [44] | 2020 | Phantom and in vivo (porcine) | Carotid | PCCT, using the blooming correction technique, enables the identification of injured artery’s vasa vasorum in comparison with control arterial walls | |
| Rajendran et al. [45] | 2017 | Phantom and in vivo (porcine) | Carotid | PCCT, using the deconvolution technique allows the identification of increased vasa vasorum density in the wall compared to normal control artery | |
| Marsh et al. [46] | 2023 | In vivo (porcine) | Carotid | PCCT is capable of detecting and quantifying the heightened perfusion observed within the carotid arterial wall due to an augmented density of vasa vasorum | |
| Si-Mohamed et al. [47] | 2022 | In vivo (human) | 14 | Coronary | The proportions of score improvement observed with PCCT in comparison to EID-CT images were 100% for coronary calcification and 45% for noncalcified plaque |
| Mergen et al. [48] | 2022 | In vivo (human) | 20 | Coronary | Ultra-high-resolution scanning with PCCT improved visualization of fibrotic and lipid-rich plaque components |
| Rotzinger et al. [49] | 2021 | In vitro | Coronary | PCCT outperformed EID-CT images in detecting non-calcified plaques (AUC ≈ 95% vs. AUC ≈ 75%) and lipid-rich atherosclerotic plaques (AUC = 85% vs. AUC = 77.5%) | |
| Boussel et al. [50] | 2014 | Ex vivo | Coronary | PCCT can identify plaque components by measuring differences in contrasting agent concentration and spectra attenuation | |
| Baturin et al. [51] | 2012 | Phantom | Coronary | Multi-energy PCCT images using iodine and gold contrast agents enable the identification of markers of plaque vulnerability, namely spotty calcification and plaque inflammation | |
| VanMeter et al. [52] | 2022 | Ex vivo | Coronary | PCCT demonstrated a reduction in calcium blooming artifacts and improvements in calcification volume quantification in comparison with EID-CT images |
| Benefits of PCCT | Limitations of PCCT |
|---|---|
| Higher spatial resolution | Technical issues (including charge sharing, pixel crosstalk, and pulse pile-up) |
| Electronic noise reduction | Lack of standardized protocol in virtual mono-energetic images |
| Improved iodine signal | High cost |
| Multi-energy acquisition | Limited availability |
| Artifact reduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cau, R.; Saba, L.; Balestrieri, A.; Meloni, A.; Mannelli, L.; La Grutta, L.; Bossone, E.; Mantini, C.; Politi, C.; Suri, J.S.; et al. Photon-Counting Computed Tomography in Atherosclerotic Plaque Characterization. Diagnostics 2024, 14, 1065. https://doi.org/10.3390/diagnostics14111065
Cau R, Saba L, Balestrieri A, Meloni A, Mannelli L, La Grutta L, Bossone E, Mantini C, Politi C, Suri JS, et al. Photon-Counting Computed Tomography in Atherosclerotic Plaque Characterization. Diagnostics. 2024; 14(11):1065. https://doi.org/10.3390/diagnostics14111065
Chicago/Turabian StyleCau, Riccardo, Luca Saba, Antonella Balestrieri, Antonella Meloni, Lorenzo Mannelli, Ludovico La Grutta, Eduardo Bossone, Cesare Mantini, Carola Politi, Jasjit S. Suri, and et al. 2024. "Photon-Counting Computed Tomography in Atherosclerotic Plaque Characterization" Diagnostics 14, no. 11: 1065. https://doi.org/10.3390/diagnostics14111065
APA StyleCau, R., Saba, L., Balestrieri, A., Meloni, A., Mannelli, L., La Grutta, L., Bossone, E., Mantini, C., Politi, C., Suri, J. S., Cavaliere, C., Punzo, B., Maffei, E., & Cademartiri, F. (2024). Photon-Counting Computed Tomography in Atherosclerotic Plaque Characterization. Diagnostics, 14(11), 1065. https://doi.org/10.3390/diagnostics14111065











