Digital Mammography (DM) vs. Dynamic Contrast Enhancement-Magnetic Resonance Imaging (DCE-MRI) in Microcalcifications Assessment: A Radiological–Pathological Comparison
Abstract
1. Introduction
- Our research offers a novel approach to correlating DM characteristics with histological and molecular subtypes of BC, enhancing the predictive accuracy of imaging methods;
- We address existing gaps in the literature by exploring the potential imaging correlations between microcalcifications and B3 lesions, expanding the understanding of these lesions’ malignant potential;
- By examining the utility of DCE-MRI in evaluating microcalcifications, our study contributes to refining diagnostic protocols and surgical planning strategies;
- Our findings underscore the importance of integrated imaging approaches in the comprehensive assessment of breast lesions, potentially leading to more personalized and effective patient care strategies.
2. Materials and Methods
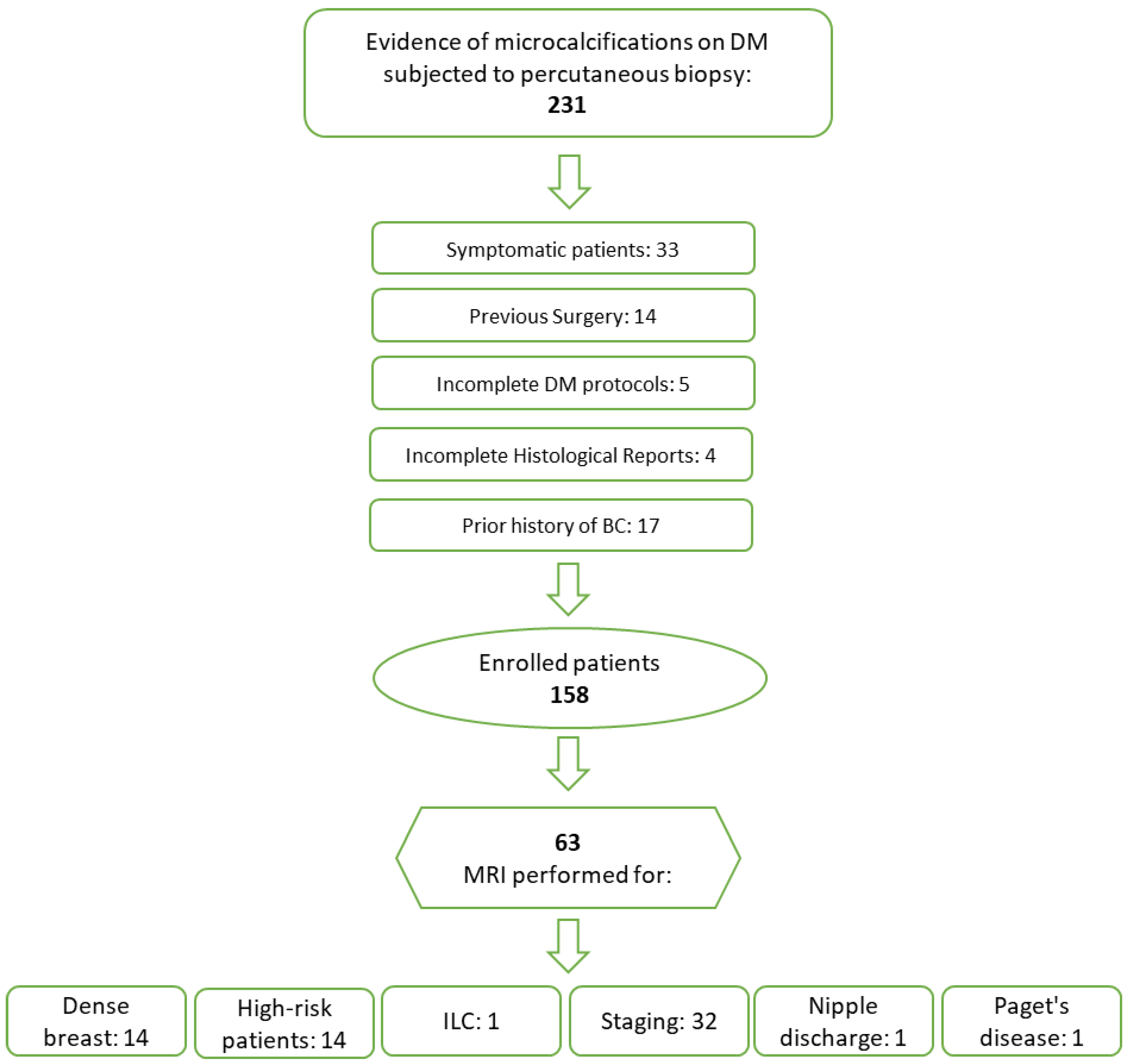
2.1. Study Design and Patient Population
2.2. Digital Mammography and Microcalcifications
2.3. Breast DCE-MRI
- -
- Axial two-dimensional (2D) fast spin-echo (FSE) T2-weighted fat-suppressed (FS) sequence based on a three-point Dixon technique (IDEAL);
- -
- Axial dynamic dual-echo 3D spoiled gradient-recalled (DISCO) T1-weighted fat-suppressed sequence, acquired once before and nine times after the injection of contrast media (Gadoteridol-Prohance 279.3 mg/mL; Bracco Imaging Italia S.r.l., Milano, Italy).
2.4. Percutaneous Biopsy and Histopathological Findings
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Digital Mammography
3.3. Breast DCE-MRI
3.4. DM vs. Breast DCE-MRI
3.5. Univariate and Multivariate Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Winters, S.; Martin, C.; Murphy, D.; Shokar, N.K. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mol. Biol. Transl. Sci. 2017, 151, 1–32. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi: Acr BI-rAdS® Atlas—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=ACR%20BI-RADS%20Atlas%2C%20Breast%20Imaging%20Reporting%20and%20Data%20System&author=C.J.%20D%27Orsi&publication_year=2013 (accessed on 29 November 2023).
- Zhang, L.; Hao, C.; Wu, Y.; Zhu, Y.; Ren, Y.; Tong, Z. Microcalcification and BMP-2 in breast cancer: Correlation with clinicopathological features and outcomes. OncoTargets Ther. 2019, 12, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.E.; Turner, R.M.; Ciatto, S.; Marinovich, M.L.; French, J.R.; Macaskill, P.; Houssami, N. Ductal carcinoma in situ at core-needle biopsy: Meta-analysis of underestimation and predictors of invasive breast cancer. Radiology 2011, 260, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ian, T.W.M.; Tan, E.Y.; Chotai, N. Role of mammogram and ultrasound imaging in predicting breast cancer subtypes in screening and symptomatic patients. World J. Clin. Oncol. 2021, 12, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.S.; Denley, H.E.; Pinder, S.E.; Ellis, I.O.; Elston, C.W.; Vujovic, P.; Macmillan, R.D.; Evans, A.J.; Nottingham Breast Team. Excision biopsy findings of patients with breast needle core biopsies reported as suspicious of malignancy (B4) or lesion of uncertain malignant potential (B3). Histopathology 2003, 42, 331–336. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.E.; Rakha, E.A.; Reed, J.; Lee, A.H.S.; Evans, A.J.; Ellis, I.O. Predictive value of needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Histopathology 2008, 53, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Ciatto, S.; Bilous, M.; Vezzosi, V.; Bianchi, S. Borderline breast core needle histology: Predictive values for malignancy in lesions of uncertain malignant potential (B3). Br. J. Cancer 2007, 96, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Richter-Ehrenstein, C.; Maak, K.; Röger, S.; Ehrenstein, T. Lesions of “uncertain malignant potential” in the breast (B3) identified with mammography screening. BMC Cancer 2018, 18, 829. [Google Scholar] [CrossRef]
- Lieske, B.; Ravichandran, D.; Alvi, A.; Lawrence, D.A.S.; Wright, D.J. Screen-detected breast lesions with an indeterminate (B3) core needle biopsy should be excised. Eur. J. Surg. Oncol. 2008, 34, 1293–1298. [Google Scholar] [CrossRef]
- Hayes, B.D.; O’Doherty, A.; Quinn, C.M. Correlation of needle core biopsy with excision histology in screen-detected B3 lesions: The Merrion Breast Screening Unit experience. J. Clin. Pathol. 2009, 62, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Chadashvili, T.; Ghosh, E.; Fein-Zachary, V.; Mehta, T.S.; Venkataraman, S.; Dialani, V.; Slanetz, P.J. Nonmass Enhancement on Breast MRI: Review of Patterns with Radiologic-Pathologic Correlation and Discussion of Management. Am. J. Roentgenol. 2015, 204, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Panzironi, G.; Moffa, G.; Galati, F.; Marzocca, F.; Rizzo, V.; Pediconi, F. Peritumoral edema as a biomarker of the aggressiveness of breast cancer: Results of a retrospective study on a 3 T scanner. Breast Cancer Res. Treat. 2020, 181, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Needle Core Biopsy Result Code for Breast. Available online: https://www.datadictionary.nhs.uk/attributes/needle_core_biopsy_result_code_for_breast.html (accessed on 29 November 2023).
- Perry, N.; Broeders, M.; de Wolf, C.; Törnberg, S.; Holland, R.; von Karsa, L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—Summary document. Ann. Oncol. 2008, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Tran, T.X.M.; Song, H.; Park, B. Microcalcifications, mammographic breast density, and risk of breast cancer: A cohort study. Breast Cancer Res. 2022, 24, 96. [Google Scholar] [CrossRef] [PubMed]
- Ferranti, C.; Yoldi, G.; Biganzoli, E.; Bergonzi, S.; Mariani, L.; Scaperrotta, G.; Marchesini, M. Relationships between age, mammographic features and pathological tumour characteristics in non-palpable breast cancer. Br. J. Radiol. 2000, 73, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, R.; Scimeca, M.; Toschi, N.; Pistolese, C.A.; Giannini, E.; Antonacci, C.; Ciuffa, S.; Tancredi, V.; Tarantino, U.; Albonici, L.; et al. Radiological, Histological and Chemical Analysis of Breast Microcalcifications: Diagnostic Value and Biological Significance. J. Mammary Gland. Biol. Neoplasia 2018, 23, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Laya, M.B.; Gallagher, J.C.; Schreiman, J.S.; Larson, E.B.; Watson, P.; Weinstein, L. Effect of postmenopausal hormonal replacement therapy on mammographic density and parenchymal pattern. Radiology 1995, 196, 433–437. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Lerda, D.; Quinn, C.; Follmann, M.; Alonso-Coello, P.; Giorgi Rossi, P.; Lebeau, A.; Nyström, L.; Broeders, M.; Ioannidou-Mouzaka, L.; et al. Breast Cancer Screening and Diagnosis: A Synopsis of the European Breast Guidelines. Ann. Intern. Med. 2020, 172, 46–56. [Google Scholar] [CrossRef]
- Coleman, C. Early Detection and Screening for Breast Cancer. Semin. Oncol. Nurs. 2017, 33, 141–155. [Google Scholar] [CrossRef]
- Frykberg, E.R.; Bland, K.I. Management of in situ and minimally invasive breast carcinoma. World J. Surg. 1994, 18, 45–57. [Google Scholar] [CrossRef]
- Naseem, M.; Murray, J.; Hilton, J.F.; Karamchandani, J.; Muradali, D.; Faragalla, H.; Polenz, C.; Han, D.; Bell, D.C.; Brezden-Masley, C. Mammographic microcalcifications and breast cancer tumorigenesis: A radiologic-pathologic analysis. BMC Cancer 2015, 15, 307. [Google Scholar] [CrossRef]
- Galati, F.; Rizzo, V.; Moffa, G.; Caramanico, C.; Kripa, E.; Cerbelli, B.; D’Amati, G.; Pediconi, F. Radiologic-pathologic correlation in breast cancer: Do MRI biomarkers correlate with pathologic features and molecular subtypes? Eur. Radiol. Exp. 2022, 6, 39. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.Y.; Kim, E.K.; Kim, M.J.; Moon, H.J.; Yoon, J.H. Evaluation of malignancy risk stratification of microcalcifications detected on mammography: A study based on the 5th edition of BI-RADS. Ann. Surg. Oncol. 2015, 22, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Luiten, J.D.; Voogd, A.C.; Luiten, E.J.T.; Broeders, M.J.; Roes, K.C.; Tjan-Heijnen, V.C.; Duijm, L.E. Recall and Outcome of Screen-detected Microcalcifications during 2 Decades of Mammography Screening in the Netherlands National Breast Screening Program. Radiology 2020, 294, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Tabár, L.; Chen, H.H.; Duffy, S.W.; Yen, M.F.; Chiang, C.F.; Dean, P.B.; Smith, R.A. A novel method for prediction of long-term outcome of women with T1a, T1b, and 10-14 mm invasive breast cancers: A prospective study. Lancet 2000, 355, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Rominger, M.B.; Steinmetz, C.; Westerman, R.; Ramaswamy, A.; Albert, U.S. Microcalcification-Associated Breast Cancer: Presentation, Successful First Excision, Long-Term Recurrence and Survival Rate. Breast Care 2015, 10, 380–385. [Google Scholar] [CrossRef]
- Rageth, C.J.; O’Flynn, E.A.M.; Pinker, K.; Kubik-Huch, R.A.; Mundinger, A.; Decker, T.; Tausch, C.; Dammann, F.; Baltzer, P.A.; Fallenberg, E.M.; et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res. Treat. 2019, 174, 279–296. [Google Scholar] [CrossRef]
- McCroskey, Z.; Sneige, N.; Herman, C.R.; Miller, R.A.; Venta, L.A.; Ro, J.Y.; Schwartz, M.R.; Ayala, A.G. Flat epithelial atypia in directional vacuum-assisted biopsy of breast microcalcifications: Surgical excision may not be necessary. Mod. Pathol. 2018, 31, 1097–1106. [Google Scholar] [CrossRef]
- Forgeard, C.; Benchaib, M.; Guerin, N.; Thiesse, P.; Mignotte, H.; Faure, C.; Clement-Chassagne, C.; Treilleux, I. Is surgical biopsy mandatory in case of atypical ductal hyperplasia on 11-gauge core needle biopsy? A retrospective study of 300 patients. Am. J. Surg. 2008, 196, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Rauch, G.M.; Hobbs, B.P.; Kuerer, H.M.; Scoggins, M.E.; Benveniste, A.P.; Park, Y.M.; Caudle, A.S.; Fox, P.S.; Smith, B.D.; Adrada, B.E.; et al. Microcalcifications in 1657 Patients with Pure Ductal Carcinoma in Situ of the Breast: Correlation with Clinical, Histopathologic, Biologic Features, and Local Recurrence. Ann. Surg. Oncol. 2016, 23, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Mariscotti, G.; Durando, M.; Ruggirello, I.; Belli, P.; Caumo, F.; Nori, J.; Zuiani, C.; Tagliafico, A.; Bicchierai, G.; Romanucci, G.; et al. Lesions of uncertain malignant potential of the breast (B3) on vacuum-assisted biopsy for microcalcifications: Predictors of malignancy. Eur. J. Radiol. 2020, 130, 109194. [Google Scholar] [CrossRef]
- Avdan Aslan, A.; Gültekin, S.; Esendağli Yilmaz, G.; Kurukahvecioğlu, O. Is There Any Association Between Mammographic Features of Microcalcifications and Breast Cancer Subtypes in Ductal Carcinoma In Situ? Acad. Radiol. 2021, 28, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, H.S.; Choi, N.; Yang, J.H.; Yoo, Y.B.; Park, K.S. Screening mammography-detected ductal carcinoma in situ: Mammographic features based on breast cancer subtypes. Clin. Imaging 2015, 39, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Moon, W.K.; Chang, J.M.; Cho, N.; Park, S.Y.; Won, J.K.; Jeon, Y.K.; Moon, H.G.; Han, W.; Park, I.A. Mammographic features of calcifications in DCIS: Correlation with oestrogen receptor and human epidermal growth factor receptor 2 status. Eur. Radiol. 2013, 23, 2072–2078. [Google Scholar] [CrossRef]
- Brnic, D.; Brnic, D.; Simundic, I.; Vanjaka Rogosic, L.; Tadic, T. MRI and comparison mammography: A worthy diagnostic alliance for breast microcalcifications? Acta Radiol. 2016, 57, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Pöschke, P.; Wenkel, E.; Hack, C.C.; Beckmann, M.W.; Uder, M.; Ohlmeyer, S. Low-Risk Women with Suspicious Microcalcifications in Mammography—Can an Additional Breast MRI Reduce the Biopsy Rate? Diagnostics 2023, 13, 1197. [Google Scholar] [CrossRef]
- Fueger, B.J.; Clauser, P.; Kapetas, P.; Pötsch, N.; Helbich, T.H.; Baltzer, P.A.T. Can supplementary contrast-enhanced MRI of the breast avoid needle biopsies in suspicious microcalcifications seen on mammography? A systematic review and meta-analysis. Breast 2021, 56, 53–60. [Google Scholar] [CrossRef]
- Bennani-Baiti, B.; Baltzer, P.A. MR Imaging for Diagnosis of Malignancy in Mammographic Microcalcifications: A Systematic Review and Meta-Analysis. Radiology 2017, 283, 692–701. [Google Scholar] [CrossRef]
- Pustahija, A.H.; Ivanac, G.; Brkljacic, B. US and MRI in the evaluation of mammographic BI-RADS 4 and 5 microcalcifications. Diagn. Interv. Radiol. 2018, 24, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Cilotti, A.; Iacconi, C.; Marini, C.; Moretti, M.; Mazzotta, D.; Traino, C.; Naccarato, A.G.; Piagneri, V.; Giaconi, C.; Bevilacqua, G.; et al. Contrast-enhanced MR imaging in patients with BI-RADS 3-5 microcalcifications. Radiol. Med. 2007, 112, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Dietzel, M.; Kaiser, C.G.; Wenkel, E.; Clauser, P.; Uder, M.; Schulz-Wendtland, R.; Baltzer, P.A. Differentiation of ductal carcinoma in situ versus fibrocystic changes by magnetic resonance imaging: Are there pathognomonic imaging features? Acta Radiol. 2017, 58, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Moradi, B.; Gity, M.; Etesam, F.; Borhani, A.; Ahmadinejad, N.; Kazemi, M.A. Correlation of apparent diffusion coefficient values and peritumoral edema with pathologic biomarkers in patients with breast cancer. Clin. Imaging 2020, 68, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, P.A.T.; Yang, F.; Dietzel, M.; Herzog, A.; Simon, A.; Vag, T.; Gajda, M.; Camara, O.; Kaiser, W.A. Sensitivity and specificity of unilateral edema on T2w-TSE sequences in MR-Mammography considering 974 histologically verified lesions. Breast J. 2010, 16, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Belli, P.; Distefano, D.; Bufi, E.; Di Matteo, M.; Rinaldi, P.; Giuliani, M.; Petrone, G.; Magno, S.; Bonomo, L. Magnetic resonance imaging features in triple-negative breast cancer: Comparison with luminal and HER2-overexpressing tumors. Clin. Breast Cancer 2012, 12, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Kim, H.J.; Kim, T.H.; Ryeom, H.K.; Lee, J.; Kim, G.C.; Yuk, J.S.; Kim, W.H. Invasive Breast Cancer: Prognostic Value of Peritumoral Edema Identified at Preoperative MR Imaging. Radiology 2018, 287, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Song, S.E.; Shin, S.U.; Moon, H.G.; Ryu, H.S.; Kim, K.; Moon, W.K. MR imaging features associated with distant metastasis-free survival of patients with invasive breast cancer: A case-control study. Breast Cancer Res. Treat. 2017, 162, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Shin, S.U.; Ryu, H.S.; Han, W.; Im, S.A.; Park, I.A.; Noh, D.Y.; Moon, W.K. Pretreatment MR Imaging Features of Triple-Negative Breast Cancer: Association with Response to Neoadjuvant Chemotherapy and Recurrence-Free Survival. Radiology 2016, 281, 392–400. [Google Scholar] [CrossRef]
- Rizzo, V.; Cicciarelli, F.; Galati, F.; Moffa, G.; Maroncelli, R.; Pasculli, M.; Pediconi, F. Could breast multiparametric MRI discriminate between pure ductal carcinoma in situ and microinvasive carcinoma? Acta Radiol. 2024, 9, 2841851231225807. [Google Scholar] [CrossRef]
- Baltzer, P.A.T.; Bennani-Baiti, B.; Stöttinger, A.; Bumberger, A.; Kapetas, P.; Clauser, P. Is breast MRI a helpful additional diagnostic test in suspicious mammographic microcalcifications? Magn. Reson. Imaging 2018, 46, 70–74. [Google Scholar] [CrossRef]
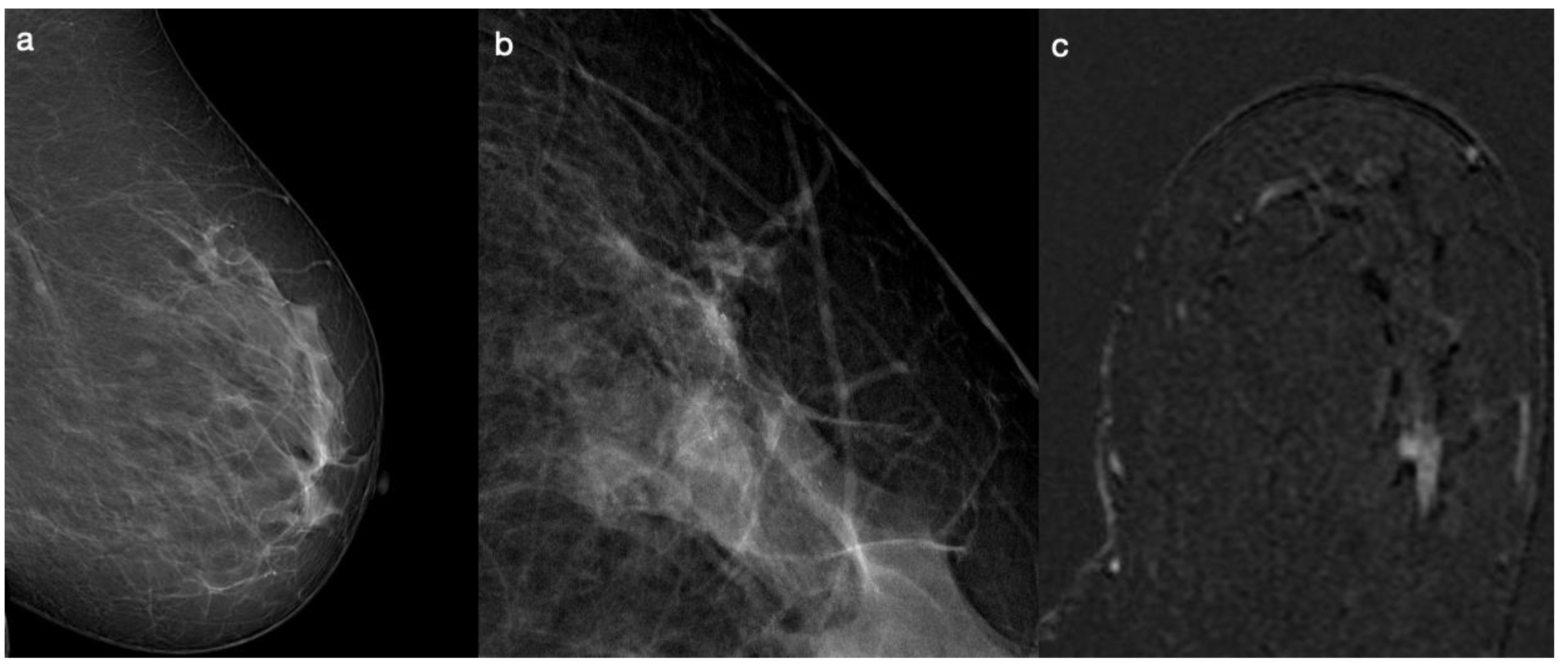

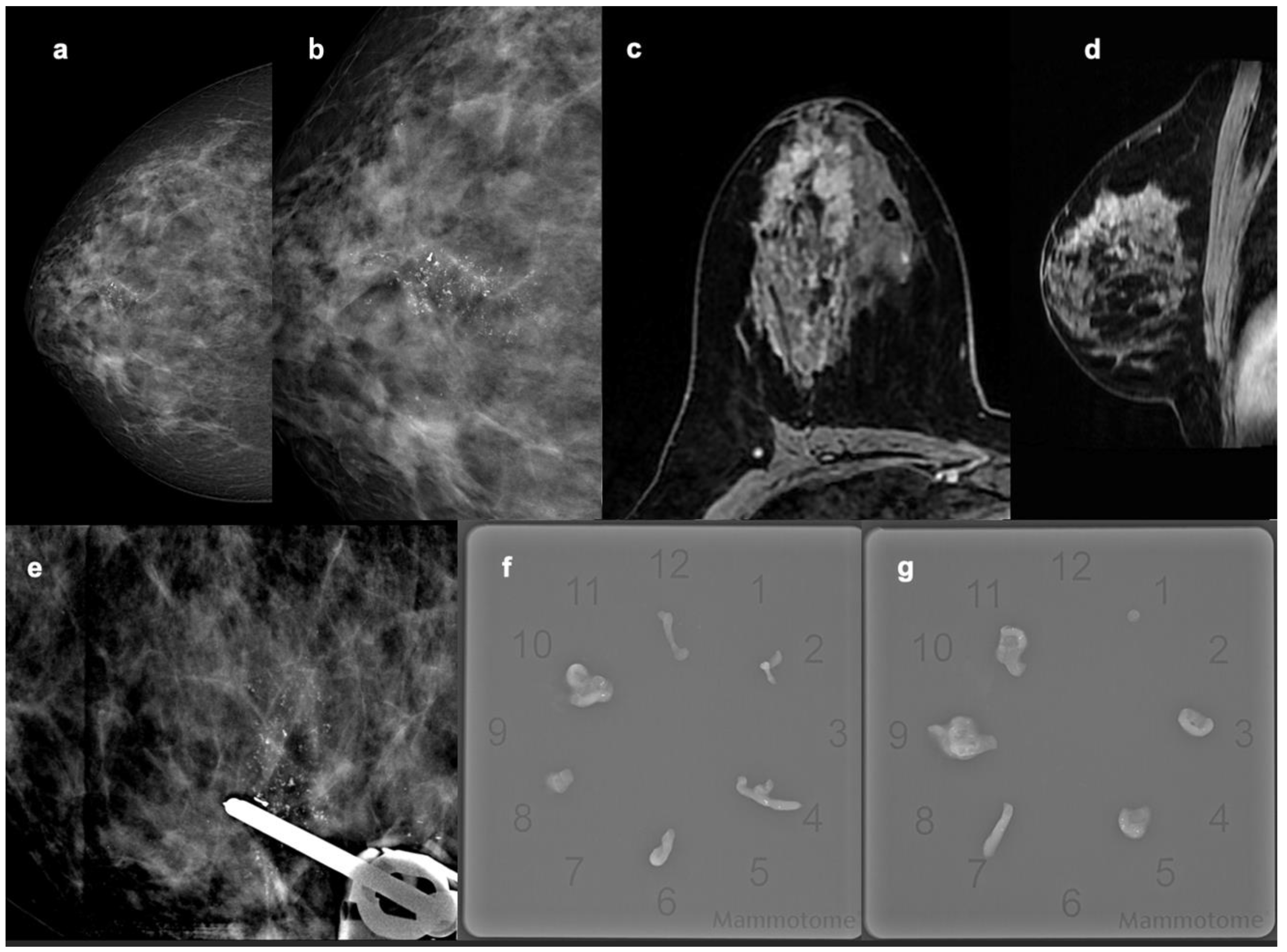
| Patient Cohort Features | N | Median | % |
|---|---|---|---|
| Patients | 158 | ||
| Age | 29–89 years (min.–max.) | 50 years | |
| Menopausal stage | 81 | 51.3% | |
| Pre-menopausal stage | 77 | 48.7% | |
| Tumor size on DM | 3–109 cm (min.–max.) | 15 cm |
| B2 Lesions | B3 Lesions | Malignant Lesions | |||
|---|---|---|---|---|---|
| Fibrocystic mastopathy | 25 | FEA | 9 | DCIS | 46 |
| Fibroadenoma | 6 | Radial scar | 1 | IDC | 34 |
| Micropapillary apocrine metaplasia | 4 | ALH | 4 | ILC | 3 |
| Steatonecrosis | 1 | ADH | 5 | Paget disease | 1 |
| Sclerosing adenosis | 7 | ||||
| Fibroadipose involution | 4 | ||||
| Columnar cell alteration | 5 | ||||
| Typical ductal hyperplasia | 1 | ||||
| Stromal fibrosis | 1 | ||||
| PASH | 1 | ||||
| Total | 55 | Total | 19 | Total | 84 |
| Histological Subtype | Total | |||||
|---|---|---|---|---|---|---|
| DCIS | IDC | ILC | Paget Disease | |||
| Molecular subtype | Luminal A | 19 | 8 | 3 | 0 | 30 |
| Luminal B Her− | 12 | 12 | 0 | 1 | 25 | |
| Luminal B Her+ | 5 | 10 | 0 | 0 | 15 | |
| Her2+ | 8 | 4 | 0 | 0 | 12 | |
| TN | 2 | 0 | 0 | 0 | 2 | |
| Total | 46 | 34 | 3 | 1 | 84 | |
| DM Microcalcifications | N | % |
|---|---|---|
| Distribution | ||
| Grouped | 83 | 52.5% |
| Diffuse | 5 | 3.2% |
| Regional | 45 | 28.5% |
| Linear | 11 | 6.9% |
| Segmental | 14 | 8.9% |
| Morphology | ||
| Amorphous | 48 | 30.4% |
| Coarse heterogeneous | 39 | 24.7% |
| Fine pleomorphic | 52 | 32.9% |
| Fine linear or fine linear branching | 19 | 12.0% |
| Peritumoral Edema | ||
|---|---|---|
| Histological Subtype | Presence | Absence |
| DCIS | 0 | 25 |
| IDC | 4 | 10 |
| ILC | 0 | 1 |
| Paget disease | 0 | 1 |
| Non-malignant lesion | 0 | 22 |
| Total | 4 | 59 |
| Molecular Subtype | |||||
|---|---|---|---|---|---|
| Luminal A | Luminal B Her− | Luminal B Her+ | Her2+ | TN | |
| Mass | 0 | 5 | 2 | 1 | 0 |
| Non-mass | 15 | 7 | 9 | 2 | 0 |
| Univariate Analysis | Multivariate Analysis (Stepwise Method) | |||
|---|---|---|---|---|
| OR (CI 95%) | p-Value | OR (CI 95%) | p-Value | |
| Age | 1.040 (1.012–1.069) | 0.005 | 1.052 (1.021–1.083) | <0.001 |
| Menopause | 0.356 (0.187–0.679) | 0.002 | Eliminated * | |
| Microcalcifications distribution | 1.534 (1.191–1.975) | <0.001 | 1.693 (1.285–2.231) | <0.001 |
| Microcalcifications morphology | 1.301 (0.953–1.776) | 0.098 | ||
| Associate opacity | 1.293 (0.684–2.446) | 0.429 | ||
| Enhancement lesion | Out of scale | 0.999 | ||
| Non-mass distribution | 1.952 (0.826–4.614) | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicciarelli, F.; Guiducci, E.; Galati, F.; Moffa, G.; Ricci, P.; Pediconi, F.; Rizzo, V. Digital Mammography (DM) vs. Dynamic Contrast Enhancement-Magnetic Resonance Imaging (DCE-MRI) in Microcalcifications Assessment: A Radiological–Pathological Comparison. Diagnostics 2024, 14, 1063. https://doi.org/10.3390/diagnostics14111063
Cicciarelli F, Guiducci E, Galati F, Moffa G, Ricci P, Pediconi F, Rizzo V. Digital Mammography (DM) vs. Dynamic Contrast Enhancement-Magnetic Resonance Imaging (DCE-MRI) in Microcalcifications Assessment: A Radiological–Pathological Comparison. Diagnostics. 2024; 14(11):1063. https://doi.org/10.3390/diagnostics14111063
Chicago/Turabian StyleCicciarelli, Federica, Elisa Guiducci, Francesca Galati, Giuliana Moffa, Paolo Ricci, Federica Pediconi, and Veronica Rizzo. 2024. "Digital Mammography (DM) vs. Dynamic Contrast Enhancement-Magnetic Resonance Imaging (DCE-MRI) in Microcalcifications Assessment: A Radiological–Pathological Comparison" Diagnostics 14, no. 11: 1063. https://doi.org/10.3390/diagnostics14111063
APA StyleCicciarelli, F., Guiducci, E., Galati, F., Moffa, G., Ricci, P., Pediconi, F., & Rizzo, V. (2024). Digital Mammography (DM) vs. Dynamic Contrast Enhancement-Magnetic Resonance Imaging (DCE-MRI) in Microcalcifications Assessment: A Radiological–Pathological Comparison. Diagnostics, 14(11), 1063. https://doi.org/10.3390/diagnostics14111063






