Spheroids Generated from Malignant Pleural Effusion as a Tool to Predict the Response of Non-Small Cell Lung Cancer to Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Reagents
2.2. Patients
2.3. Generation of Multicellular Spheroids (Colony Formation Assay)
2.4. Immunohistochemistry Staining of TTF-1
2.5. Modified MTT Assay
2.6. Immunofluorescent Staining of Cleaved Caspase 3
3. Results
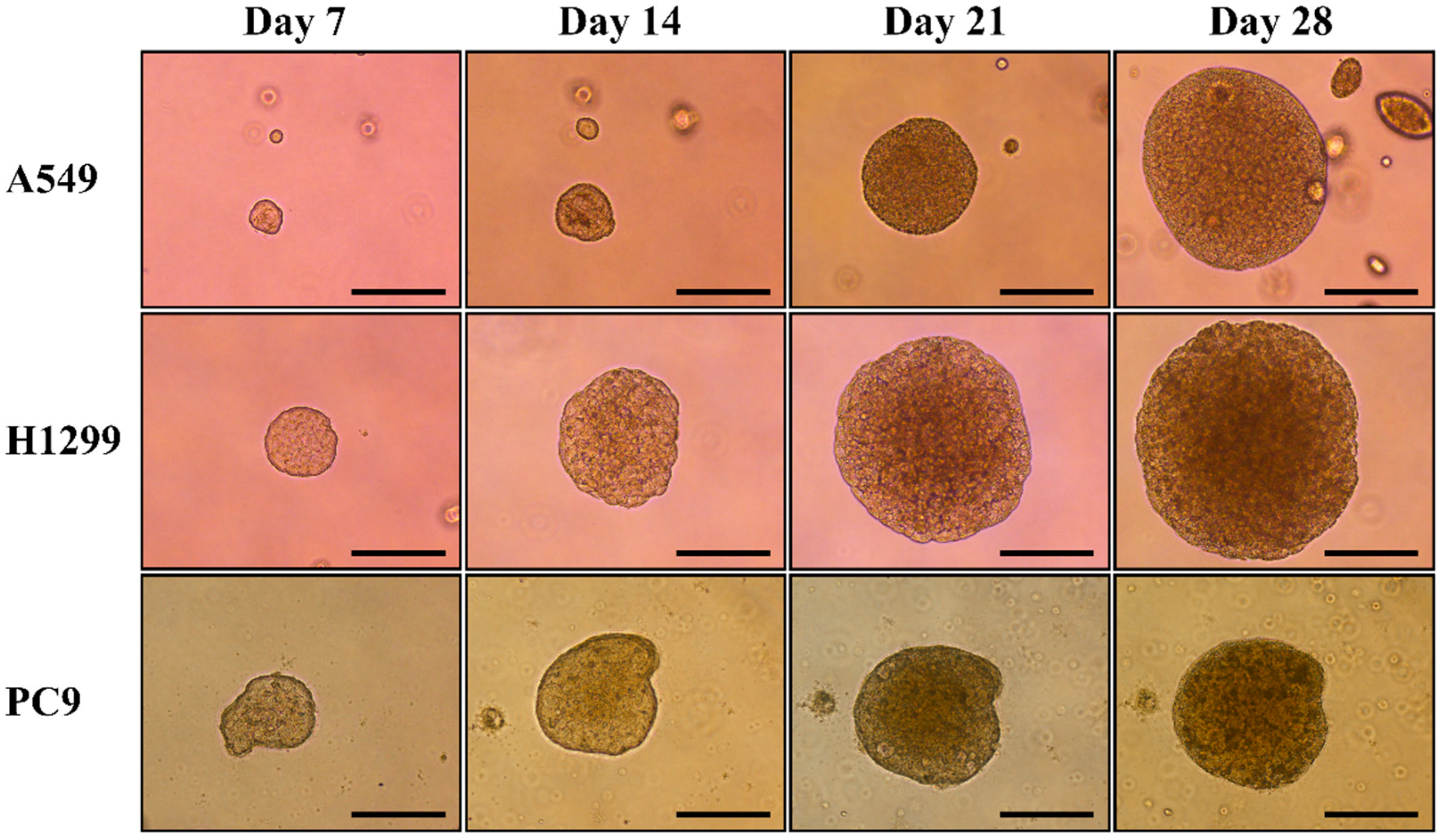
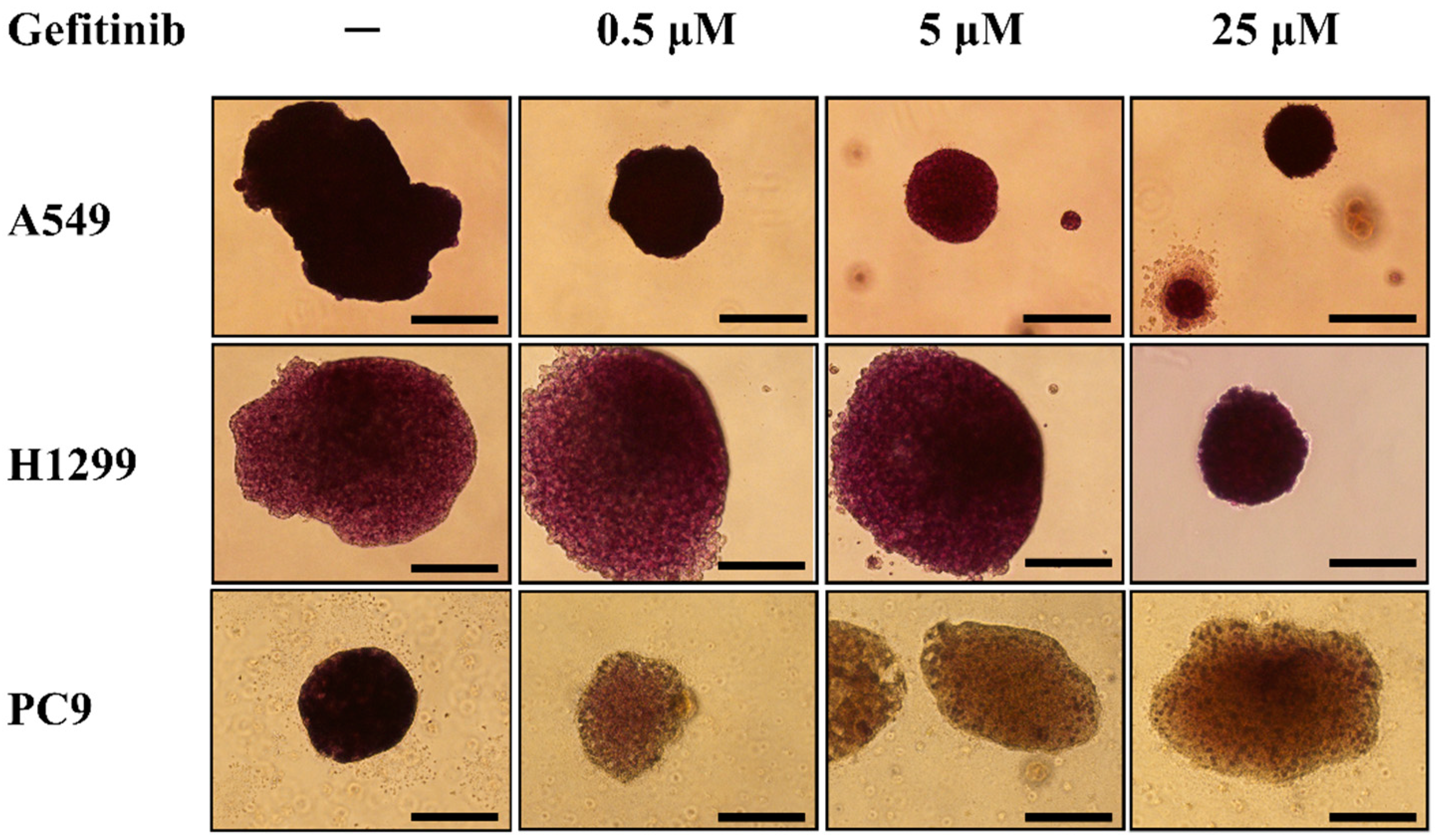
3.1. Generation of Multicellular Spheroids by NSCLC Cell Lines and Evaluation of Their Response to Gefitinib
3.2. Patient Characteristics
3.3. Modified MTT Assay of Spheroids General from NSCLC-Related Malignant Pleural Effusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Zhao, J.; Han, Y.; Zhou, J.; Li, X.; Zhang, T.; Li, W.; Xia, Y. Management of locally advanced non-small cell lung cancer: State of the art and future directions. Cancer Commun. 2024, 44, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of non-small cell lung cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (nsclc). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Tan, D.S.W. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, P.; Wistuba, I.I. Lung cancer biomarkers. Hematol. Oncol. Clin. N. Am. 2017, 31, 13–29. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in untreated egfr-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in non-small cell lung cancer: Facts and hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Loessner, D.; Rizzi, S.; Kaplan, D.L.; Mooney, D.J.; Clements, J.A. Can tissue engineering concepts advance tumor biology research? Trends Biotechnol. 2010, 28, 125–133. [Google Scholar] [CrossRef]
- Osswald, A.; Hedrich, V.; Sommergruber, W. 3d-3 tumor models in drug discovery for analysis of immune cell infiltration. Methods Mol. Biol. 2019, 1953, 151–162. [Google Scholar] [PubMed]
- Atat, O.E.; Farzaneh, Z.; Pourhamzeh, M.; Taki, F.; Abi-Habib, R.; Vosough, M.; El-Sibai, M. 3d modeling in cancer studies. Human Cell 2022, 35, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.K.; Veena, M.S.; Oh, S.; Huang, G.; Srivatsan, E.; Huang, M.; Sharma, S.; Batra, R.K. The malignant pleural effusion as a model to investigate intratumoral heterogeneity in lung cancer. PLoS ONE 2009, 4, e5884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.K.; Liu, Z.; Sa, J.K.; Shin, S.; Wang, J.; Bordyuh, M.; Cho, H.J.; Elliott, O.; Chu, T.; Choi, S.W.; et al. Pharmacogenomic landscape of patient-derived tumor cells informs precision oncology therapy. Nat. Genet. 2018, 50, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Kuraguchi, M.; Xu, M.; Portell, A.J.; Taus, L.; Diala, I.; Lalani, A.S.; Choi, J.; Chambers, E.S.; Li, S.; et al. Use of ex vivo patient-derived tumor organotypic spheroids to identify combination therapies for her2 mutant non-small cell lung cancer. Clin. Cancer Res. 2020, 26, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Jin, X.; Wang, Y.; Wang, K. Role of epidermal growth factor receptor in lung cancer and targeted therapies. Am. J. Cancer Res. 2017, 7, 187–202. [Google Scholar] [PubMed]
- Ziogas, D.C.; Tsiara, A.; Tsironis, G.; Lykka, M.; Liontos, M.; Bamias, A.; Dimopoulos, M.A. Treating alk-positive non-small cell lung cancer. Ann. Transl. Med. 2018, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- VanderLaan, P.A.; Rangachari, D.; Majid, A.; Parikh, M.S.; Gangadharan, S.P.; Kent, M.S.; McDonald, D.C.; Huberman, M.S.; Kobayashi, S.S.; Costa, D.B. Tumor biomarker testing in non-small-cell lung cancer: A decade of change. Lung Cancer 2018, 116, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Hirsh, V.; Boyer, M.; Yang, J.C.; Mok, T.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib in patients with egfr mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase iib lux-lung 7 trial. Ann. Oncol. 2017, 28, 270–277. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with egfr mutation-positive non-small-cell lung cancer (lux-lung 7): A phase 2b, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Ahn, M.J.; Tsai, C.M.; Shepherd, F.A.; Bazhenova, L.; Sequist, L.V.; Hida, T.; Yang, J.C.H.; Ramalingam, S.S.; Mitsudomi, T.; Janne, P.A.; et al. Osimertinib in patients with t790m mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer 2019, 125, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.L.; Lin, J.J.; Shaw, A.T. Alk-positive lung cancer: A moving target. Nat. Cancer 2023, 4, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in alk-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Tan, D.S.W.; Chiari, R.; Wu, Y.L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced alk-rearranged non-small-cell lung cancer (ascend-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Perol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus crizotinib in untreated alk-positive non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.; Han, J.Y.; Lee, J.S.; Hochmair, M.J.; Li, J.Y.; Chang, G.C.; Lee, K.H.; et al. Brigatinib versus crizotinib in alk-positive non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.W.; Mok, T.; Polli, A.; et al. First-line lorlatinib or crizotinib in advanced alk-positive lung cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- So, W.V.; Dejardin, D.; Rossmann, E.; Charo, J. Predictive biomarkers for pd-1/pd-l1 checkpoint inhibitor response in nsclc: An analysis of clinical trial and real-world data. J. Immunother. Cancer 2023, 11, e006464. [Google Scholar] [CrossRef] [PubMed]
- Gompelmann, D.; Sarova, P.; Mosleh, B.; Papaporfyriou, A.; Oberndorfer, F.; Idzko, M.; Hoda, M.A. Pd-l1 assessment in lung cancer biopsies-pitfalls and limitations. Int. J. Biol. Markers 2024, 39, 3–8. [Google Scholar] [CrossRef]
- Addeo, A.; Friedlaender, A.; Banna, G.L.; Weiss, G.J. Tmb or not tmb as a biomarker: That is the question. Crit. Rev. Oncol. Hematol. 2021, 163, 103374. [Google Scholar] [CrossRef]
- Di Liello, R.; Ciaramella, V.; Barra, G.; Venditti, M.; Della Corte, C.M.; Papaccio, F.; Sparano, F.; Viscardi, G.; Iacovino, M.L.; Minucci, S.; et al. Ex vivo lung cancer spheroids resemble treatment response of a patient with nsclc to chemotherapy and immunotherapy: Case report and translational study. ESMO Open 2019, 4, e000536. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Okami, J.; Okuyama, H.; Kumagai, T.; Uchida, J.; Kondo, J.; Takehara, T.; Nishizawa, Y.; Imamura, F.; Higashiyama, M.; et al. Spheroid culture of primary lung cancer cells with neuregulin 1/her3 pathway activation. J. Thorac. Oncol. 2013, 8, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Taverna, J.A.; Hung, C.N.; Williams, M.; Williams, R.; Chen, M.; Kamali, S.; Sambandam, V.; Chiu, C.H.-L.; Osmulski, P.A.; Gaczynska, M.E.; et al. Ex vivo drug testing of patient-derived lung organoids to predict treatment responses for personalized medicine. Lung Cancer 2024, 190, 107533. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Renna, M.; Park, S.J.; Menzies, F.M.; Ricketts, T.; Fullgrabe, J.; Ashkenazi, A.; Frake, R.A.; Lombarte, A.C.; Bento, C.F.; et al. Contact inhibition controls cell survival and proliferation via yap/taz-autophagy axis. Nat. Commun. 2018, 9, 2961. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Rathinam, M.K.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The soft agar colony formation assay. J. Vis. Exp. 2014, 92, e51998. [Google Scholar]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2h-tetrazolium bromide (mtt) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Huyck, L.; Ampe, C.; Van Troys, M. The xtt cell proliferation assay applied to cell layers embedded in three-dimensional matrix. Assay Drug Dev. Technol. 2012, 10, 382–392. [Google Scholar] [CrossRef]
- Rolver, M.G.; Elingaard-Larsen, L.O.; Pedersen, S.F. Assessing cell viability and death in 3d spheroid cultures of cancer cells. J. Vis. Exp. 2019, 148, e59714. [Google Scholar]



| Case | Gender | Age | Cell Type | EGFR Mutation | TKI Treatment | Response to TKI |
|---|---|---|---|---|---|---|
| 1 | Female | 80.6 | Adenocarcinoma | Wild type | No | Not Applicable |
| 2 | Male | 82.7 | Adenocarcinoma | Wild type | No | Not Applicable |
| 3 | Male | 61.0 | Adenocarcinoma | Wild type | Erlotinib | Disease progression |
| 4 | Female | 79.0 | Adenocarcinoma | Wild type | No | Not Applicable |
| 5 | Female | 49.6 | Adenocarcinoma | Wild type | No | Not Applicable |
| 6 | Male | 63.0 | Adenocarcinoma | Wild type | No | Not Applicable |
| 7 | Female | 74.5 | Adenocarcinoma | Exon 20 insertion | No | Not Applicable |
| 8 | Male | 77.6 | Adenocarcinoma | L861Q | Afatinib | Partial response |
| 9 | Male | 53.5 | Adenocarcinoma | L858R | Erlotinib | Partial response |
| 10 | Female | 77.9 | Adenocarcinoma | L858R | Erlotinib | Partial response |
| 11 | Male | 59.2 | Adenocarcinoma | L858R | Gefitinib | Partial response |
| 12 | Male | 80.4 | Adenocarcinoma | L858R | Afatinib | Partial response |
| 13 | Female | 73.6 | Adenocarcinoma | Exon 19 deletion | Erlotinib | Disease progression |
| 14 | Female | 82.4 | Adenocarcinoma | Exon 19 deletion | Gefitinib | Disease progression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-M.; Fang, Y.-H.; Lin, C.-M.; Chen, M.-F.; Lin, C.-L. Spheroids Generated from Malignant Pleural Effusion as a Tool to Predict the Response of Non-Small Cell Lung Cancer to Treatment. Diagnostics 2024, 14, 998. https://doi.org/10.3390/diagnostics14100998
Yang T-M, Fang Y-H, Lin C-M, Chen M-F, Lin C-L. Spheroids Generated from Malignant Pleural Effusion as a Tool to Predict the Response of Non-Small Cell Lung Cancer to Treatment. Diagnostics. 2024; 14(10):998. https://doi.org/10.3390/diagnostics14100998
Chicago/Turabian StyleYang, Tsung-Ming, Yu-Hung Fang, Chieh-Mo Lin, Miao-Fen Chen, and Chun-Liang Lin. 2024. "Spheroids Generated from Malignant Pleural Effusion as a Tool to Predict the Response of Non-Small Cell Lung Cancer to Treatment" Diagnostics 14, no. 10: 998. https://doi.org/10.3390/diagnostics14100998
APA StyleYang, T.-M., Fang, Y.-H., Lin, C.-M., Chen, M.-F., & Lin, C.-L. (2024). Spheroids Generated from Malignant Pleural Effusion as a Tool to Predict the Response of Non-Small Cell Lung Cancer to Treatment. Diagnostics, 14(10), 998. https://doi.org/10.3390/diagnostics14100998






