Rare Driver Mutations in Advanced, Oncogene-Addicted Non-Small Cell Lung Cancer: A North Italian, Real-World, Registry Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Immunoscores Definition
2.3. Statistical Analysis
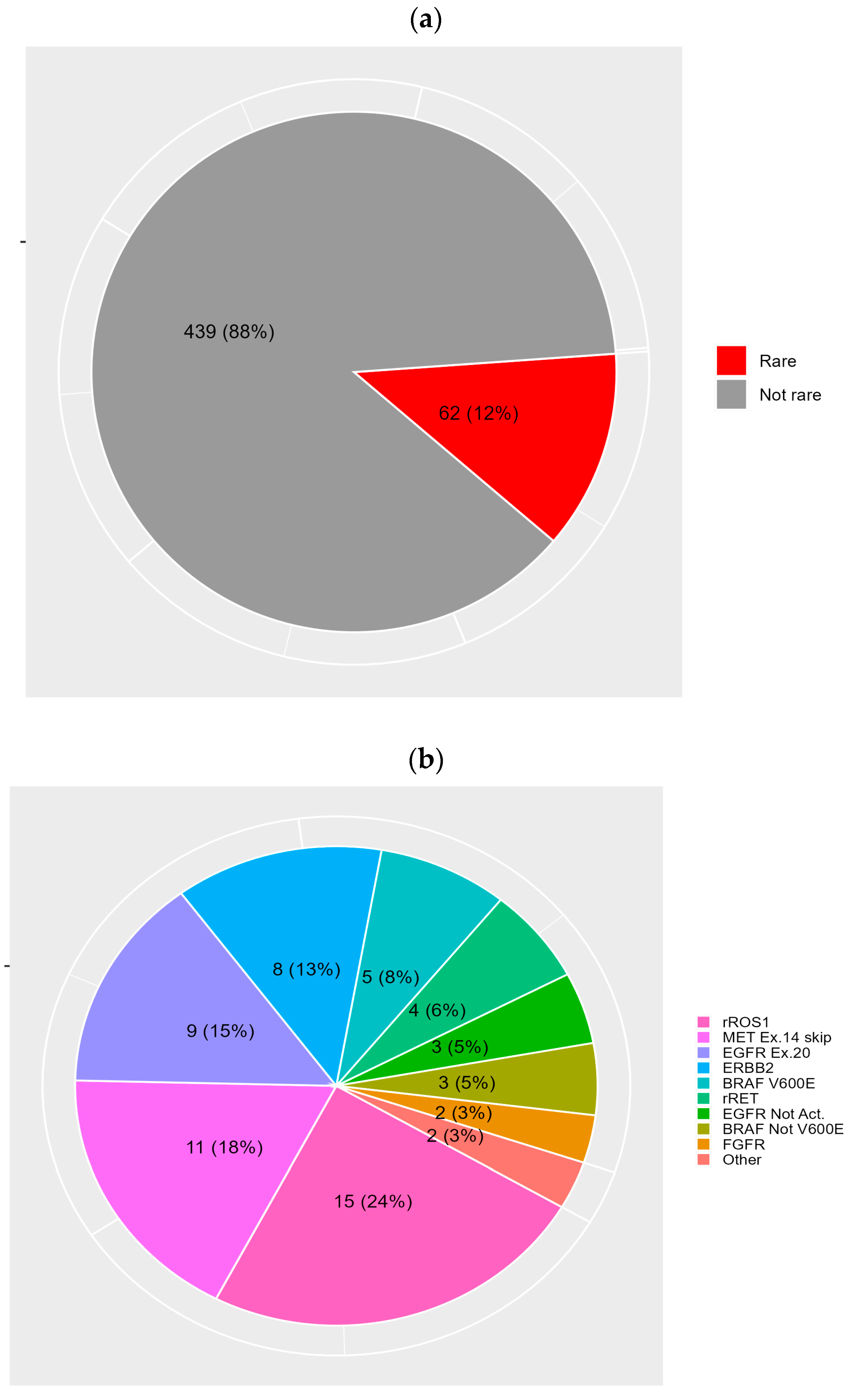
3. Results
3.1. Molecular and Clinical Features
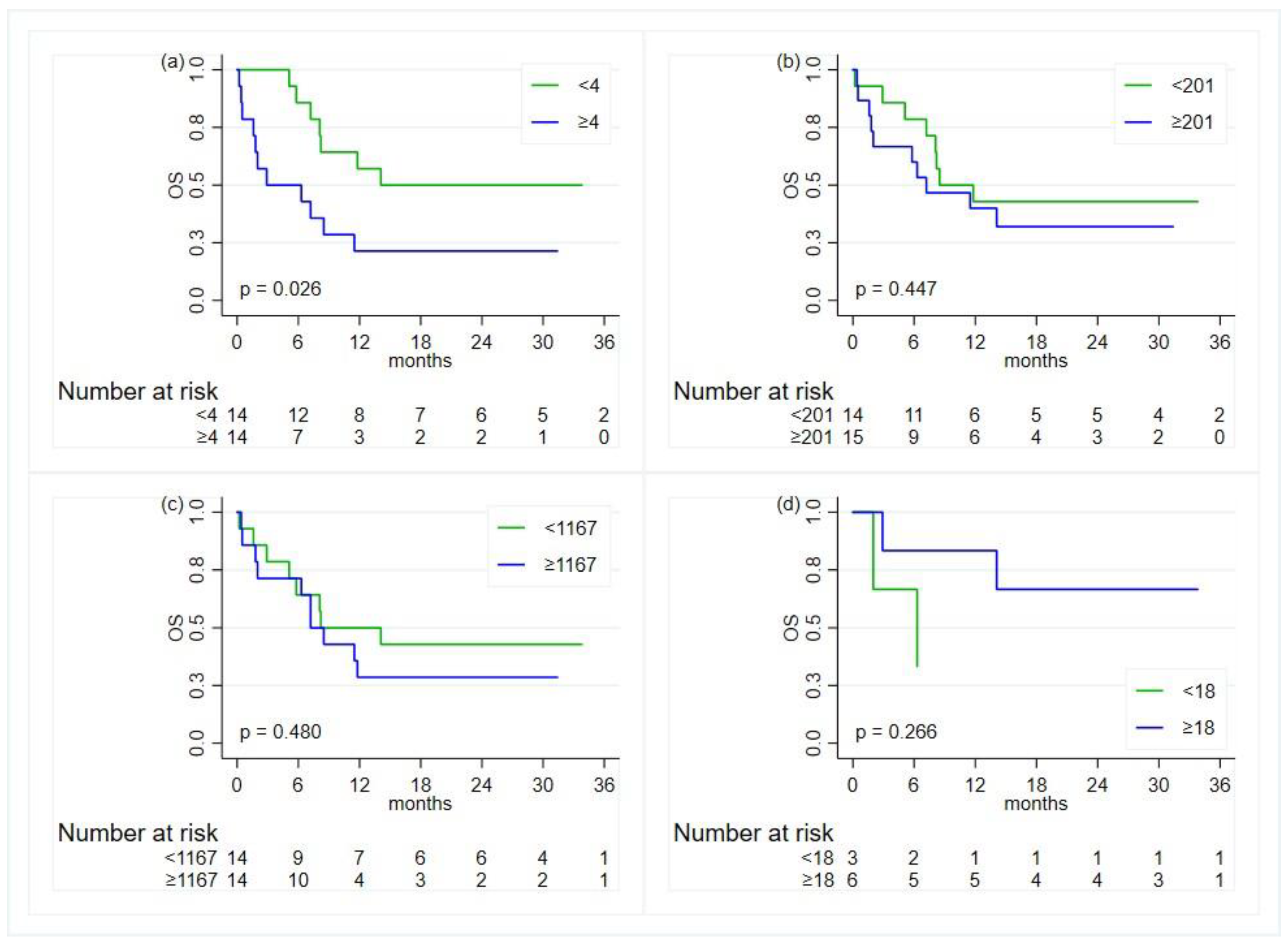
3.2. Treatments and Clinical Outcomes
3.3. Patients with Co-Mutations
3.4. Association with Inflammatory Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart (IARC, 2015); Schabath, M.B.; Cote, M.L. Cancer progress and priorities: Lung cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefi tinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.F.; et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014, 311, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Mazieres, J.; Merlio, J.P.; Debieuvre, D.; Mosser, J.; Lena, H.; Ouafik, L.; Besse, B.; Rouquette, I.; Westeel, V.; et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016, 387, 1415–1426. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Johnson, A.; Sklar, J.; Lindeman, N.I.; Moore, K.; Ganesan, S.; Lovly, C.M.; Perlmutter, J.; Gray, S.W.; Hwang, J.; et al. Somatic Genomic Testing in Patients with Metastatic or Advanced Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2022, 40, 1231–1258. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.Y.; Kim, S.W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results from the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhu, J.; Zhang, W.; Yu, A.; Zhou, W.; Xu, K. Efficacy and toxicity of drugs targeting KRASG12C mutation in non-small cell lung cancer: A meta- analysis. Expert Rev. Anticancer. Ther. 2023, 23, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Sforza, V.; Palumbo, G.; Cascetta, P.; Carillio, G.; Manzo, A.; Montanino, A.; Sandomenico, C.; Costanzo, R.; Esposito, G.; Laudato, F.; et al. BRAF Inhibitors in Non-Small Cell Lung Cancer. Cancers 2022, 14, 4863. [Google Scholar] [CrossRef] [PubMed]
- Chagas, G.C.L.; Rangel, A.R.; El Osta, B. MET alterations in advanced non-small cell lung cancer. Curr. Probl. Cancer 2024, 12, 101075. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.F.; Felip, E.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Paz-Ares Rodríguez, L.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Redman, M.W.; Lara, P.N., Jr.; Borghaei, H.; Hoffman, P.; Bradley, J.D.; Newman, A.J., III; Feldman, M.J.; Minichiello, K.; Miao, J.; et al. SWOG S1400D (NCT02965378), a Phase II Study of the Fibroblast Growth Factor Receptor Inhibitor AZD4547 in Previously Treated Patients with Fibroblast Growth Factor Pathway-Activated Stage IV Squamous Cell Lung Cancer (Lung-MAP Substudy). J. Thorac. Oncol. 2019, 14, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Delmonte, A.; Burgio, M.A.; Verlicchi, A.; Bronte, G.; Cravero, P.; Ulivi, P.; Martinelli, G.; Crinò, L. New generation anaplastic lymphoma kinase inhibitors. Transl. Lung Cancer Res. 2019, 8 (Suppl. S3), S280–S289. [Google Scholar] [CrossRef] [PubMed]
- Gálffy, G.; Morócz, É.; Korompay, R.; Hécz, R.; Bujdosó, R.; Puskás, R.; Lovas, T.; Gáspár, E.; Yahya, K.; Király, P.; et al. Targeted therapeutic options in early and metastatic NSCLC-overview. Pathol. Oncol. Res. 2024, 30, 1611715. [Google Scholar] [CrossRef]
- Zhou, C.; Solomon, B.; Loong, H.H.; Park, K.; Pérol, M.; Arriola, E.; Novello, S.; Han, B.; Zhou, J.; Ardizzoni, A.; et al. First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RET Fusion-Positive NSCLC. N. Engl. J. Med. 2023, 389, 1839–1850. [Google Scholar] [CrossRef]
- Chen, J.; Xu, C.; Lv, J.; Lu, W.; Zhang, Y.; Wang, D.; Song, Y. Clinical characteristics and targeted therapy of different gene fusions in non-small cell lung cancer: A narrative review. Transl. Lung Cancer Res. 2023, 12, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Doebele, R.C.; Kerr, K.M. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat. Rev. Clin. Oncol. 2019, 16, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.; Ogata, Y.; Ihara, S.; Yamamoto, S.; Komuta, K. Neutrophil-to-Lymphocyte Ratio Predicts Overall Survival of Advanced Non-Small Cell Lung Cancer Harboring Mutant Epidermal Growth Factor Receptor. World J. Oncol. 2017, 8, 180–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chan, S.W.S.; Smith, E.; Aggarwal, R.; Balaratnam, K.; Chen, R.; Hueniken, K.; Fazelzad, R.; Weiss, J.; Jiang, S.; Shepherd, F.A.; et al. Systemic Inflammatory Markers of Survival in Epidermal Growth Factor-Mutated Non-Small-Cell Lung Cancer: Single-Institution Analysis, Systematic Review, and Meta-analysis. Clin. Lung Cancer 2021, 22, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Ozyurek, B.A.; Ozdemirel, T.S.; Ozden, S.B.; Erdoğan, Y.; Ozmen, O.; Kaplan, B.; Kaplan, T. Does advanced lung inflammation index (ALI) have prognostic significance in metastatic non-small cell lung cancer? Clin. Respir. J. 2018, 12, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Luo, J.; Wen, J.; Jiang, M. The Relationship Between Systemic Immune Inflammatory Index and Prognosis of Patients with Non-Small Cell Lung Cancer: A Meta-Analysis and Systematic Review. Front. Surg. 2022, 9, 898304. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Alberti, G.; Petracci, E.; Bettelli, S.; Ulivi, P.; Cravero, P.; Guaitoli, G.; Zanelli, F.; Burgio, M.A.; Andrikou, K.; et al. Genomic profiling in advanced NSCLC in Italy: First results of NERoNE (NSCLC Emilia Romagna Next Generation Sequencing Evaluation) project. J. Clin. Oncol. 2023, 41, e21077. [Google Scholar] [CrossRef]
- Oxnard, G.R.; Lo, P.C.; Nishino, M.; Dahlberg, S.E.; Lindeman, N.I.; Butaney, M.; Jackman, D.M.; Johnson, B.E.; Jänne, P.A. Natural history and molecular characteristics of lung cancers harbouring EGFR exon 20 insertions. J. Thorac. Oncol. 2013, 8, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Arcila, M.E.; Fara, M.; Sima, C.S.; Miller, V.A.; Kris, M.G.; Ladanyi, M.; Riely, G.J. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J. Clin. Oncol. 2011, 29, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Hu, Z.I.; Lai, G.G.Y.; Tan, D.S.W. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat. Rev. Clin. Oncol. 2018, 15, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Schrock, A.B.; Frampton, G.M.; Suh, J.; Chalmers, Z.R.; Rosenzweig, M.; Erlich, R.L.; Halmos, B.; Goldman, J.; Forde, P.; Leuenberger, K.; et al. Characterization of 298 Patients with Lung Cancer Harboring MET Exon 14 Skipping Alterations. J. Thorac. Oncol. 2016, 11, 1493–1502. [Google Scholar] [CrossRef]
- Pacini, L.; Jenks, A.D.; Lima, N.C.; Huang, P.H. Targeting the Fibroblast Growth Factor Receptor (FGFR) Family in Lung Cancer. Cells 2021, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.D.; Doebele, R.C. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. 2013, 19, 4040–4045. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, R.; Cortot, A.B.; Gautschi, O.; Mazieres, J.; Camidge, D.R. PD-1/PD-L1 inhibitor activity in patients with gene-rearrangement positive non-small cell lung cancer-an IMMUNOTARGET case series. Transl. Lung Cancer Res. 2022, 11, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, P.; Urbini, M.; Petracci, E.; Canale, M.; Dubini, A.; Bartolini, D.; Calistri, D.; Cravero, P.; Fonzi, E.; Martinelli, G.; et al. Wide Next-Generation Sequencing Characterization of Young Adults Non-Small-Cell Lung Cancer Patients. Cancers 2022, 14, 2352. [Google Scholar] [CrossRef]




| n | % | |
|---|---|---|
| Sex | ||
| M | 27 | (43.6) |
| F | 35 | (56.5) |
| Age at diagnosis (y) | ||
| Median (IQ–IIIQ) | 69.2 (65.0–76.2) | |
| Min–max | 35.2–87.0 | |
| <65 | 15 | (24.2) |
| ≥65 | 47 | (75.8) |
| Smoking habit | ||
| Never-smoker | 23 | (37.1) |
| Ex-smoker | 28 | (45.2) |
| Current smoker | 11 | (17.7) |
| ECOG PS | ||
| 0 | 19 | (30.7) |
| 1 | 30 | (48.4) |
| 2 | 10 | (16.1) |
| 3 | 2 | (3.2) |
| 4 | 1 | (1.6) |
| BMI | ||
| Median (IQ–IIIQ) | 24 (22.0–28.0) | |
| Min–max | 16–48 | |
| <18.50 | 6 | (9.7) |
| 18.5–24.99 | 29 | (46.8) |
| 25.00–29.99 | 17 | (27.4) |
| ≥30.00 | 10 | (16.1) |
| Histotype | ||
| Adenocarcinoma | 60 | (96.8) |
| Squamous cell carcinoma | 2 | (3.2) |
| Type of treatment | ||
| Target therapy | 20 | (32.3) |
| Chemo-immunotherapy | 20 | (32.3) |
| Immunotherapy | 10 | (16.1) |
| Chemotherapy | 8 | (12.9) |
| Clinical trial | 4 | (6.5) |
| PD-L1 | ||
| <1% | 8 | (13.1) |
| 1–49% | 35 | (57.4) |
| ≥50% | 18 | (29.5) |
| missing | 1 | |
| NLR | ||
| Median (IQ–IIIQ) | 4.0 (2.3–4.8) | |
| Min–max | 0.6–25.2 | |
| missing | 13 | |
| PLR | ||
| Median (IQ–IIIQ) | 194.4 (138.7–257.5) | |
| Min–max | 23.1–779.6 | |
| missing | 12 | |
| ALI | ||
| Median (IQ–IIIQ) | 24.6 (15.1–50.8) | |
| Min–max | 3.5–56.7 | |
| missing | 41 | |
| ALI | ||
| <18 | 7 | 33.3 |
| ≥18 | 14 | 66.7 |
| missing | 41 | |
| SII | ||
| Median (IQ–IIIQ) | 1095.2 (641.1–1842.1) | |
| Min–max | 143.9–10,337.7 | |
| missing | 13 | |
| rROS1 (n = 15) | MET Ex.14 Skip (n = 11) | EGFR Ex.20 (n = 9) | ERBB2 (n = 8) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| M | 7 | (46.7) | 6 | (54.6) | 1 | (11.1) | 2 | (25.0) |
| F | 8 | (53.3) | 5 | (45.5) | 8 | (88.9) | 6 | (75.0) |
| Age at diagnosis (y) | ||||||||
| Median (IQ–IIIQ) | 69.6 (55.3–76.2) | 74.5 (69.0–78.5) | 72.4 (63.2–76.3) | 68.5 (63.3–69.2) | ||||
| Min–max | 35.2–82.4 | 65.0–83.7 | 57.1–80.1 | 53.3–83.7 | ||||
| <65 | 6 | (40.0) | - | 3 | (33.3) | 2 | (25.0) | |
| ≥65 | 9 | (60.0) | 11 | (100.0) | 6 | (66.7) | 6 | (75.0) |
| Smoking habit | ||||||||
| Never smoker | 6 | (40.0) | 2 | (18.2) | 6 | (66.7) | 5 | (62.5) |
| Ex-smoker | 7 | (46.7) | 7 | (63.6) | 1 | (11.1) | 1 | (12.5) |
| Current smoker | 2 | (13.3) | 2 | (18.2) | 2 | (22.2) | 2 | (25.0) |
| ECOG PS | ||||||||
| 0 | 9 | (60.0) | 1 | (9.1) | 4 | (44.4) | - | |
| 1 | 4 | (26.7) | 8 | (72.7) | 4 | (44.4) | 5 | (62.5) |
| 2 | 1 | (6.7) | 2 | (18.2) | 1 | (11.1) | 2 | (25.0) |
| 3 | - | - | - | 1 | (12.5) | |||
| 4 | 1 | (6.7) | - | - | - | |||
| Histotype | ||||||||
| Adenocarcinoma | 14 | (93.3) | 11 | (100.0) | 9 | (100.0) | 8 | (100.0) |
| Squamous cell carcinoma | 1 | (6.7) | - | - | - | |||
| Type of treatment | ||||||||
| Target therapy | 13 | (86.7) | 1 | (9.1) | - | - | ||
| Chemo-immunotherapy | 1 | (6.7) | 3 | (27.3) | 3 | (33.3) | 5 | (52.5) |
| Immunotherapy | 1 | (6.7) | 5 | (45.5) | - | 1 | (12.5) | |
| Chemotherapy | - | 1 | (9.1) | 3 | (33.3) | 2 | (25.0) | |
| Clinical trial | - | 1 | (9.1) | 3 | (33.3) | - | ||
| Presence of co-mutations | ||||||||
| No | 10 | (66.7) | 10 | (90.9) | 9 | (100.0) | 6 | (75.0) |
| Yes | 5 | (33.3) | 1 | (9.1) | - | 2 | (25.0) | |
| PD-L1 | ||||||||
| <1% | - | - | 2 | (22.2) | 3 | (37.5) | ||
| 1–49% | 10 | (71.4) | 5 | (45.5) | 4 | (44.4) | 5 | (62.5) |
| ≥50% | 4 | (28.6) | 6 | (54.6) | 3 | (33.3) | - | |
| Missing | 1 | - | - | - | ||||
| NLR | ||||||||
| Median (IQ–IIIQ) | 2.4 (2.1–4.6) | 4.2 (3.8–8.5) | 2.2 (1.7–4.4) | 4.4 (3.8–5.6) | ||||
| Min–max | 0.6–15.4 | 2.1–12.7 | 0.8–6.7 | 1.9–9.4 | ||||
| Missing | 4 | 2 | 1 | - | ||||
| PLR | ||||||||
| Median (IQ–IIIQ) | 159.2 (108.6–234.2) | 186.1 (151.7–240.9) | 191.4 (130.3–235.4) | 271.8 (170.4–321.7) | ||||
| Min–max | 26.7–779.6 | 110.2–437.8 | 23.1–346.3 | 114.6–365.0 | ||||
| Missing | 4 | 2 | 1 | |||||
| ALI | ||||||||
| Median (IQ–IIIQ) | 43.5 (4.4–57.6) | 14.9 (14.8–14.9) | 55.4 (25.2–57.4) | 17.3 (12.7–22.0) | ||||
| Min–max | 4.2–58.0 | 14.8–14.9 | 25.2–57.4 | 12.7–22.0 | ||||
| Missing | 8 | 9 | 6 | 6 | ||||
| ALI | ||||||||
| <18 | 2 | 28.6 | 2 | (100.0) | - | 1 | (50.0) | |
| ≥18 | 5 | 71.4 | - | 3 | (100.0) | 1 | (50.0) | |
| Missing | 8 | 9 | 6 | 6 | ||||
| SII | ||||||||
| Median (IQ–IIIQ) | 656.3 (519.4–1598.5) | 1360.9 (1109.6–2724.2) | 707.9 (483.3–1045.5) | 1490.4 (1127.1–2056.7) | ||||
| Min–max | 143.9–10,337.7 | 282.1–5130.7 | 226.1–1842.1 | 555.9–3004.6 | ||||
| Missing | 4 | 2 | 1 | - | ||||
| OS | PFS | |||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | |
| NLR | ||||||
| <4.0 | 1.0 | 1.0 | ||||
| ≥4.0 | 2.16 | (0.94–4.93) | 0.069 | 1.42 | (0.71–2.84) | 0.327 |
| Log-transformed NLR | 1.37 | (0.73–2.56) | 0.324 | 1.17 | (0.71–1.94) | 0.539 |
| PLR | ||||||
| <194.4 | 1.0 | 1.0 | ||||
| ≥194.4 | 1.45 | (0.65–3.25) | 0.364 | 1.18 | (0.59–2.37) | 0.635 |
| Log-transformed PLR | 0.96 | (0.52–1.76) | 0.890 | 1.01 | (0.62–1.64) | 0.968 |
| SII | ||||||
| <1095.2 | 1.0 | 1.0 | ||||
| ≥1095.2 | 1.92 | (0.84–4.38) | 0.123 | 1.28 | (0.63–2.57) | 0.483 |
| Log-transformed SII | 1.17 | (0.72–1.90) | 0.532 | 1.06 | (0.72–1.58) | 0. 774 |
| ALI | ||||||
| <18 | 1.0 | 1.0 | ||||
| ≥18 | 0.75 | (0.17–3.35) | 0.705 | 0.46 | (0.15–1.45) | 0.188 |
| Log-transformed ALI | 0.89 | (0.37–2.13) | 0.797 | 0.83 | (0.45–1.57) | 0.581 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrikou, K.; Ulivi, P.; Petracci, E.; Azzali, I.; Bertolini, F.; Alberti, G.; Bettelli, S.; Calistri, D.; Chiadini, E.; Capelli, L.; et al. Rare Driver Mutations in Advanced, Oncogene-Addicted Non-Small Cell Lung Cancer: A North Italian, Real-World, Registry Experience. Diagnostics 2024, 14, 1024. https://doi.org/10.3390/diagnostics14101024
Andrikou K, Ulivi P, Petracci E, Azzali I, Bertolini F, Alberti G, Bettelli S, Calistri D, Chiadini E, Capelli L, et al. Rare Driver Mutations in Advanced, Oncogene-Addicted Non-Small Cell Lung Cancer: A North Italian, Real-World, Registry Experience. Diagnostics. 2024; 14(10):1024. https://doi.org/10.3390/diagnostics14101024
Chicago/Turabian StyleAndrikou, Kalliopi, Paola Ulivi, Elisabetta Petracci, Irene Azzali, Federica Bertolini, Giulia Alberti, Stefania Bettelli, Daniele Calistri, Elisa Chiadini, Laura Capelli, and et al. 2024. "Rare Driver Mutations in Advanced, Oncogene-Addicted Non-Small Cell Lung Cancer: A North Italian, Real-World, Registry Experience" Diagnostics 14, no. 10: 1024. https://doi.org/10.3390/diagnostics14101024
APA StyleAndrikou, K., Ulivi, P., Petracci, E., Azzali, I., Bertolini, F., Alberti, G., Bettelli, S., Calistri, D., Chiadini, E., Capelli, L., Cravero, P., Guaitoli, G., Zanelli, F., Burgio, M. A., Pagano, M., Verlicchi, A., Martinelli, E., Di Emidio, K., Dominici, M., ... Delmonte, A. (2024). Rare Driver Mutations in Advanced, Oncogene-Addicted Non-Small Cell Lung Cancer: A North Italian, Real-World, Registry Experience. Diagnostics, 14(10), 1024. https://doi.org/10.3390/diagnostics14101024







