In Vivo Classification and Characterization of Carotid Atherosclerotic Lesions with Integrated 18F-FDG PET/MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Acquisition Protocols with the Integrated PET/MRI System
2.3. Analysis of FDG PET/MR Images
2.4. Clinical Evaluation
2.5. Statistical Methods
3. Results
3.1. Patient Population
3.2. Features of Advanced Carotid Plaques on MR Vessel Wall Imaging
3.3. 18F-FDG Uptake in Advanced Carotid Plaques Measured with PET
3.4. Reclassification of Ischemic Stroke Risk
4. Discussion
4.1. Association between Morphological and Inflammation Features of Carotid Plaque
4.2. Association between Component and Inflammation Features of Advanced Carotid Plaque
4.3. Incremental Value of 18F-FDG PET in Classification Pre-Symptomatic Stage of Advanced Carotid Plaque
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, S.F.; Brown, M.M.; Simister, R.J.; Richards, T. Contemporary prevalence of carotid stenosis in patients presenting with ischaemic stroke. Br. J. Surg. 2019, 106, 872–878. [Google Scholar] [CrossRef] [PubMed]
- BD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Bonati, L.H.; Jansen, O.; de Borst, G.J.; Brown, M.M. Management of atherosclerotic extracranial carotid artery stenosis. Lancet Neurol. 2022, 21, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.P.J.; Gaziano, L.; Rothwell, P.M.; Oxford Vascular, S. Risk of stroke in relation to degree of asymptomatic carotid stenosis: A population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. 2021, 20, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Eliasziw, M.; Gutnikov, S.A.; Fox, A.J.; Taylor, D.W.; Mayberg, M.R.; Warlow, C.P.; Barnett, H.J.; for the Carotid Endarterectomy Trialists’ Collaboration. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003, 361, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Halliday, A.; Mansfield, A.; Marro, J.; Peto, C.; Peto, R.; Potter, J.; Thomas, D.; MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet 2004, 363, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Saam, T.; Jager, H.R.; Yuan, C.; Hatsukami, T.S.; Saloner, D.; Wasserman, B.A.; Bonati, L.H.; Wintermark, M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019, 18, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Bos, D.; Arshi, B.; van den Bouwhuijsen, Q.J.A.; Ikram, M.K.; Selwaness, M.; Vernooij, M.W.; Kavousi, M.; van der Lugt, A. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J. Am. Coll. Cardiol. 2021, 77, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Brunner, G.; Virani, S.S.; Sun, W.; Liu, L.; Dodge, R.C.; Nambi, V.; Coresh, J.; Mosley, T.H.; Sharrett, A.R.; Boerwinkle, E.; et al. Associations between carotid artery plaque burden, plaque characteristics, and cardiovascular events: The ARIC Carotid Magnetic Resonance Imaging Study. JAMA Cardiol. 2021, 6, 79–86. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Ji, A.; Lv, P.; Zhang, J.; Fu, C.; Lin, J. Identification of high-risk carotid plaque with MRI-based radiomics and machine learning. Eur. Radiol. 2021, 31, 3116–3126. [Google Scholar] [CrossRef]
- Senders, M.L.; Calcagno, C.; Tawakol, A.; Nahrendorf, M.; Mulder, W.J.M.; Fayad, Z.A. PET/MR imaging of inflammation in atherosclerosis. Nat. Biomed. Eng. 2023, 7, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.J.; Lau, H.C.; Naseer, R.; Sandhu, S.; Raynor, W.Y.; Werner, T.J.; Alavi, A. Atherosclerosis imaging: Positron emission tomography. PET Clin. 2023, 18, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Camps-Renom, P.; Giannotti, N.; Marti-Fabregas, J.; McNulty, J.P.; Baron, J.C.; Barry, M.; Coutts, S.B.; Cronin, S.; Delgado-Mederos, R.; et al. A Risk Score Including Carotid Plaque Inflammation and Stenosis Severity Improves Identification of Recurrent Stroke. Stroke 2020, 51, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Friera, L.; Fuster, V.; Lopez-Melgar, B.; Oliva, B.; Sanchez-Gonzalez, J.; Macias, A.; Perez-Asenjo, B.; Zamudio, D.; Alonso-Farto, J.C.; Espana, S.; et al. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J. Am. Coll. Cardiol. 2019, 73, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, H.; Jiang, H.; Balu, N.; Geleri, D.B.; Chu, B.; Watase, H.; Zhao, X.; Li, R.; Xu, J.; et al. Domain adaptive and fully automated carotid artery atherosclerotic lesion detection using an artificial intelligence approach (LATTE) on 3D MRI. Magn. Reson. Med. 2021, 86, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.M.; Hatsukami, T.S.; Ferguson, M.S.; Small, R.; Polissar, N.L.; Yuan, C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002, 106, 1368–1373. [Google Scholar] [CrossRef]
- Bucerius, J.; Hyafil, F.; Verberne, H.J.; Slart, R.H.; Lindner, O.; Sciagra, R.; Agostini, D.; Ubleis, C.; Gimelli, A.; Hacker, M.; et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 780–792. [Google Scholar] [CrossRef] [PubMed]
- McCabe, J.J.; Camps-Renom, P.; Giannotti, N.; McNulty, J.P.; Coveney, S.; Murphy, S.; Barry, M.; Harbison, J.; Cronin, S.; Williams, D.; et al. Carotid plaque inflammation imaged by PET and prediction of recurrent stroke at 5 years. Neurology 2021, 97, e2282–e2291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, B.; Yang, H.; Ma, J.; Yang, Y.; Yang, B.; Ma, Y.; Jiao, L.; Li, X.; Lu, J. Morphological feature and mapping inflammation in classified carotid plaques in symptomatic and asymptomatic patients: A hybrid (18)F-FDG PET/MR study. Front. Neurosci. 2023, 17, 1144248. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B., Sr.; D’Agostino, R.B., Jr.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172; discussion 207–112. [Google Scholar] [CrossRef]
- Lairez, O.; Hyafil, F. A Clinical Role of PET in Atherosclerosis and Vulnerable Plaques? Semin. Nucl. Med. 2020, 50, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Piri, R.; Gerke, O.; Hoilund-Carlsen, P.F. Molecular imaging of carotid artery atherosclerosis with PET: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2016–2025. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, E.S.; Park, K.Y.; Seok, J.W.; Kwon, O.S. Comparison of [(18)F]-FDG and [(18)F]-NaF Positron Emission Tomography on Culprit Carotid Atherosclerosis: A Prospective Study. JACC Cardiovasc. Imaging 2019, 12, 370–372. [Google Scholar] [CrossRef]
- Chaker, S.; Al-Dasuqi, K.; Baradaran, H.; Demetres, M.; Delgado, D.; Nehmeh, S.; Osborne, J.R.; Christos, P.J.; Kamel, H.; Gupta, A. Carotid plaque positron emission tomography imaging and cerebral ischemic disease. Stroke 2019, 50, 2072–2079. [Google Scholar] [CrossRef]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s choice—European Society for Vascular Surgery (ESVS) 2023 clinical practice guidelines on the management of atherosclerotic carotid and vertebral artery disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, J.N.; Lovett, J.K.; Gallagher, P.J.; Rothwell, P.M. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: The Oxford plaque study. Circulation 2006, 113, 2320–2328. [Google Scholar] [CrossRef]
- Figueroa, A.L.; Subramanian, S.S.; Cury, R.C.; Truong, Q.A.; Gardecki, J.A.; Tearney, G.J.; Hoffmann, U.; Brady, T.J.; Tawakol, A. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: A comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ. Cardiovasc. Imaging 2012, 5, 69–77. [Google Scholar] [CrossRef]
- Moghbel, M.; Al-Zaghal, A.; Werner, T.J.; Constantinescu, C.M.; Hoilund-Carlsen, P.F.; Alavi, A. The Role of PET in Evaluating Atherosclerosis: A Critical Review. Semin. Nucl. Med. 2018, 48, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Toutouzas, K.; Koutagiar, I.; Benetos, G.; Aggeli, C.; Georgakopoulos, A.; Athanasiadis, E.; Pianou, N.; Trachanellis, S.; Patelis, N.; Agrogiannis, G.; et al. Inflamed human carotid plaques evaluated by PET/CT exhibit increased temperature: Insights from an in vivo study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1236–1244. [Google Scholar] [CrossRef]
- Kwee, R.M.; Teule, G.J.; van Oostenbrugge, R.J.; Mess, W.H.; Prins, M.H.; van der Geest, R.J.; Ter Berg, J.W.; Franke, C.L.; Korten, A.G.; Meems, B.J.; et al. Multimodality imaging of carotid artery plaques: 18F-fluoro-2-deoxyglucose positron emission tomography, computed tomography, and magnetic resonance imaging. Stroke 2009, 40, 3718–3724. [Google Scholar] [CrossRef]
- Li, X.; Heber, D.; Rausch, I.; Beitzke, D.; Mayerhoefer, M.E.; Rasul, S.; Kreissl, M.; Mitthauser, M.; Wadsak, W.; Hartenbach, M.; et al. Quantitative assessment of atherosclerotic plaques on (18)F-FDG PET/MRI: Comparison with a PET/CT hybrid system. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, F.; Schindler, A.; Sepp, D.; Obenhuber, T.; Bayer-Karpinska, A.; Boeckh-Behrens, T.; Hohn, S.; Hacker, M.; Nekolla, S.G.; Rominger, A.; et al. High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 270–279. [Google Scholar] [CrossRef]
- Chai, J.T.; Biasiolli, L.; Li, L.; Alkhalil, M.; Galassi, F.; Darby, C.; Halliday, A.W.; Hands, L.; Magee, T.; Perkins, J.; et al. Quantification of lipid-rich core in carotid atherosclerosis using magnetic resonance T(2) mapping: Relation to clinical presentation. JACC Cardiovasc. Imaging 2017, 10, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Sadat, U.; Teng, Z.; Young, V.E.; Graves, M.J.; Gillard, J.H. Three-dimensional volumetric analysis of atherosclerotic plaques: A magnetic resonance imaging-based study of patients with moderate stenosis carotid artery disease. Int. J. Cardiovasc. Imaging 2010, 26, 897–904. [Google Scholar] [CrossRef]
- Kelly, P.J.; Camps-Renom, P.; Giannotti, N.; Marti-Fabregas, J.; Murphy, S.; McNulty, J.; Barry, M.; Barry, P.; Calvet, D.; Coutts, S.B.; et al. Carotid plaque inflammation imaged by (18)F-fluorodeoxyglucose positron emission tomography and risk of early recurrent stroke. Stroke 2019, 50, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
| n | % | |
|---|---|---|
| Vascular risk factors/comorbidities | ||
| Active smoking | 92 | 32.9 |
| Former smoking | 54 | 19.3 |
| Hypertension | 213 | 76.1 |
| Diabetes mellitus | 109 | 38.9 |
| Hyperlipidemia | 90 | 32.1 |
| Coronary artery disease | 68 | 24.3 |
| Overweight (body mass index > 25 kg/m2) | 155 | 55.4 |
| Baseline medication | ||
| Antiplatelet agent plus statin | 188 | 67.1 |
| Statin only | 13 | 4.6 |
| Antiplatelet agent only | 10 | 3.6 |
| Neither antiplatelet agent nor statin | 69 | 24.7 |
| Features | Symptomatic Plaque (n = 87) | Asymptomatic Plaque (n = 315) | p Value |
|---|---|---|---|
| MRI | |||
| Mean luminal diameter, mm, median (IQR) | 2.43 (1.69–2.95) | 3.11 (2.32–4.10) | <0.001 |
| Mean wall diameter, mm, ± SD | 8.46 ± 1.99 | 8.39 ± 1.61 | 0.938 |
| Mean wall thickness, mm, median (IQR) | 2.98 (2.40–3.55) | 2.43 (1.95–3.04) | <0.001 |
| Stenosis degree, %, median (IQR) | 61.5% (53.3–70.5%) | 50.0% (38.0–63.0%) | <0.001 |
| Wall area, mm2, median (IQR) | 55.46 (39.23–75.95) | 49.11 (38.47–63.17) | 0.069 |
| Luminal area, mm2, median (IQR) | 4.97 (2.48–7.28) | 8.09 (4.57–13.82) | <0.001 |
| Normalized Wall Index, median (IQR) | 0.93 (0.88–0.97) | 0.85 (0.75–0.93) | <0.001 |
| Total vessel area, mm2, median (IQR) | 59.05 (43.82–83.22) | 60.94 (46.31–76.10) | 0.789 |
| Remodeling index, median (IQR) | 1.44 (1.06–2.14) | 1.36 (1.00–1.77) | 0.121 |
| AHA lesion types | |||
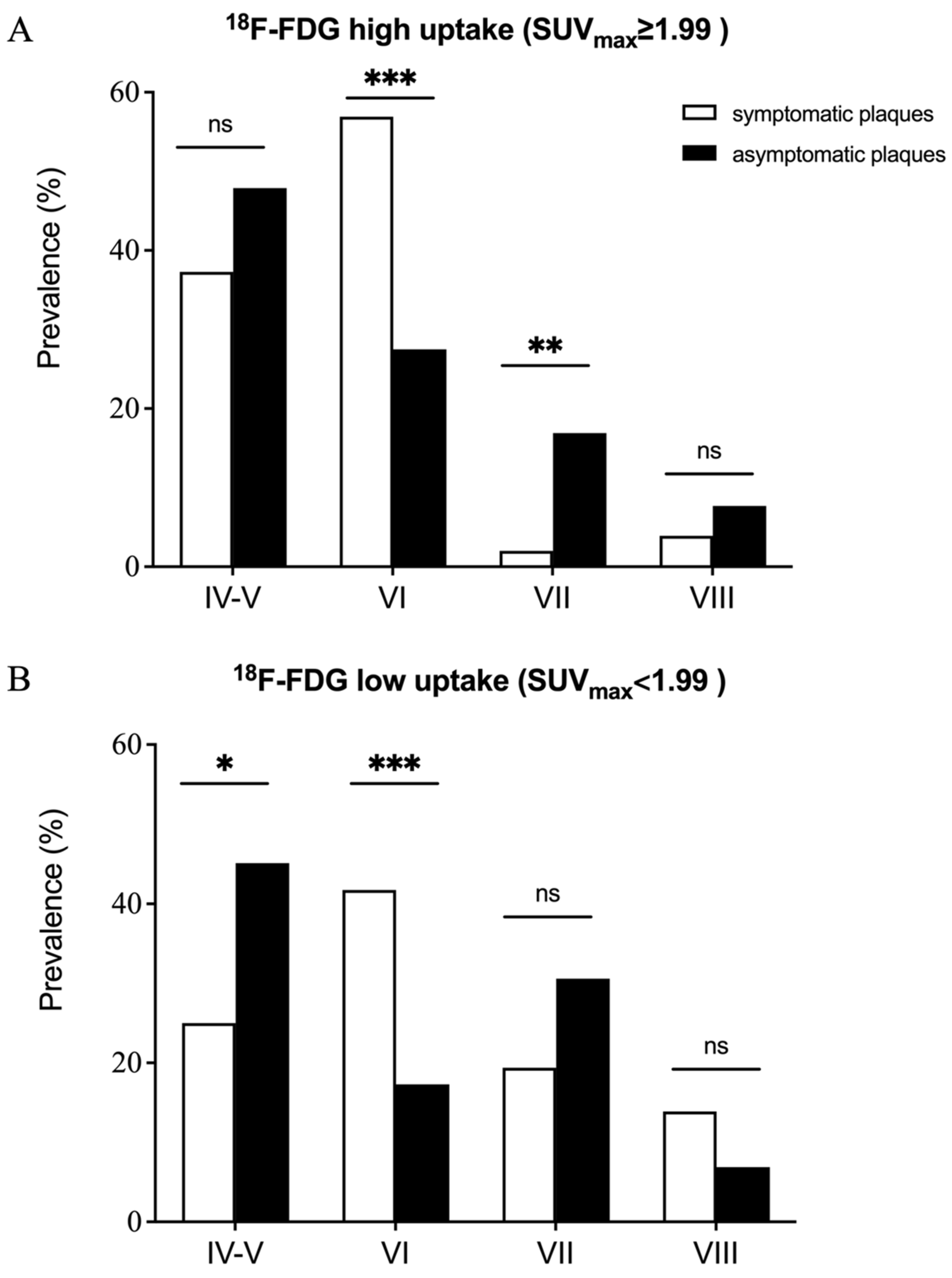
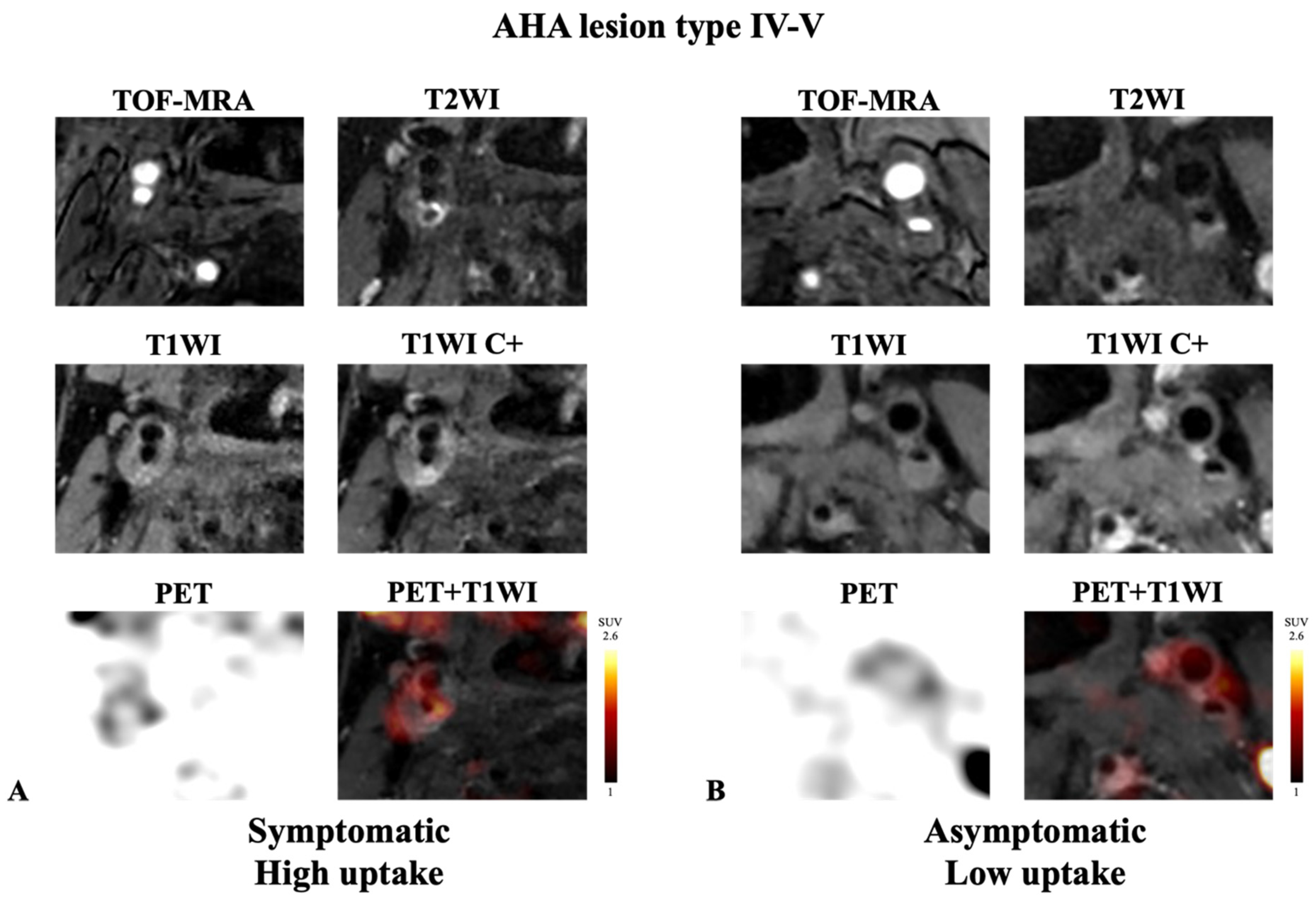
| Type IV-V, n (%) | 28 (32.2%) | 146 (46.3%) | 0.018 |
| Type VI, n (%) | 44 (50.6%) | 69 (21.9%) | <0.001 |
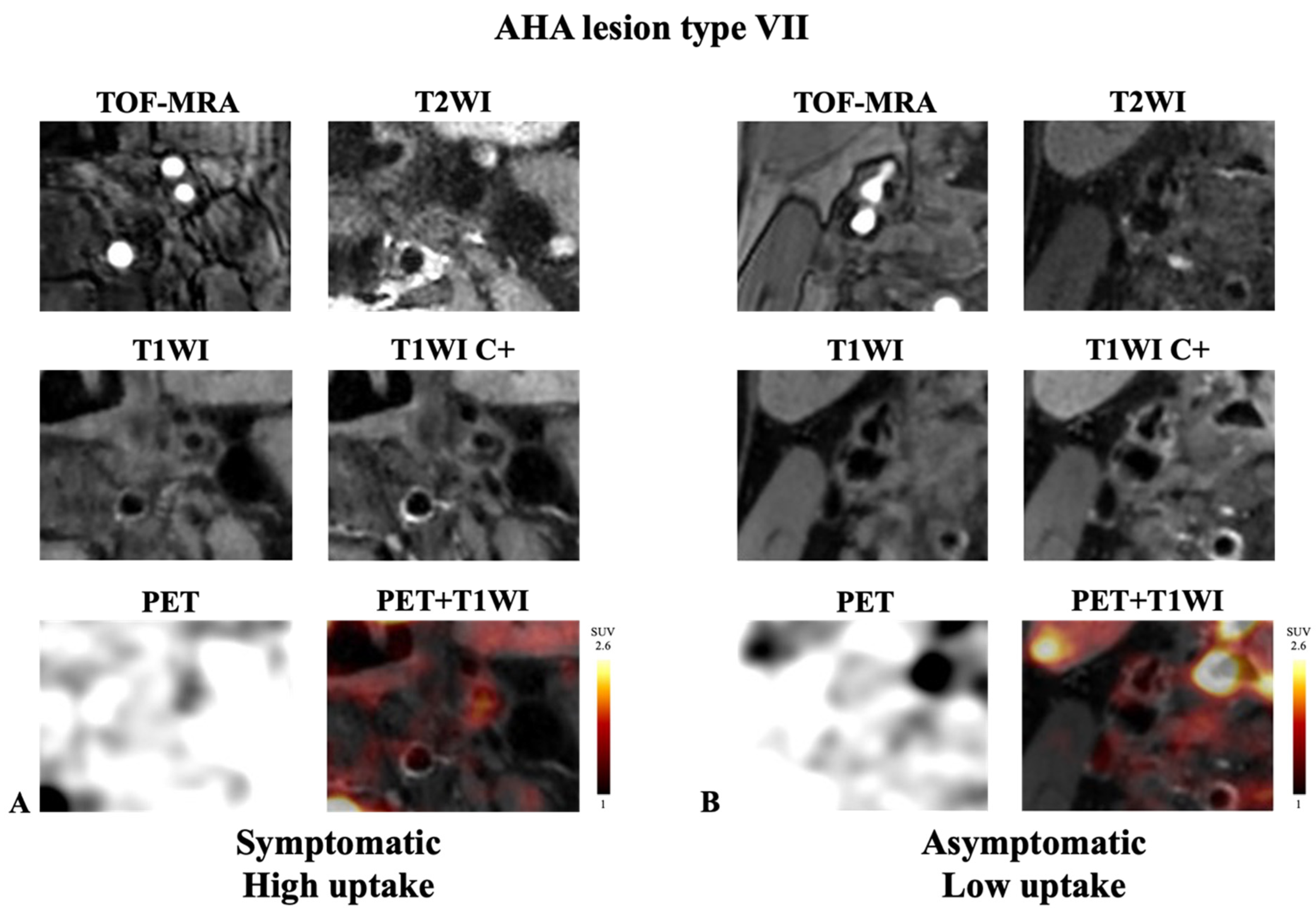
| Type VII, n (%) | 8 (9.2%) | 77 (24.4%) | 0.002 |
| Type VIII, n (%) | 7 (8.0%) | 23 (7.3%) | 0.815 |
| PET | |||
| SUVmax, median (IQR) | 2.30 (1.68–2.92) | 1.93 (1.52–2.49) | 0.007 |
| TBR, median (IQR) | 2.96 (2.35–3.80) | 2.32 (1.81–3.00) | <0.001 |
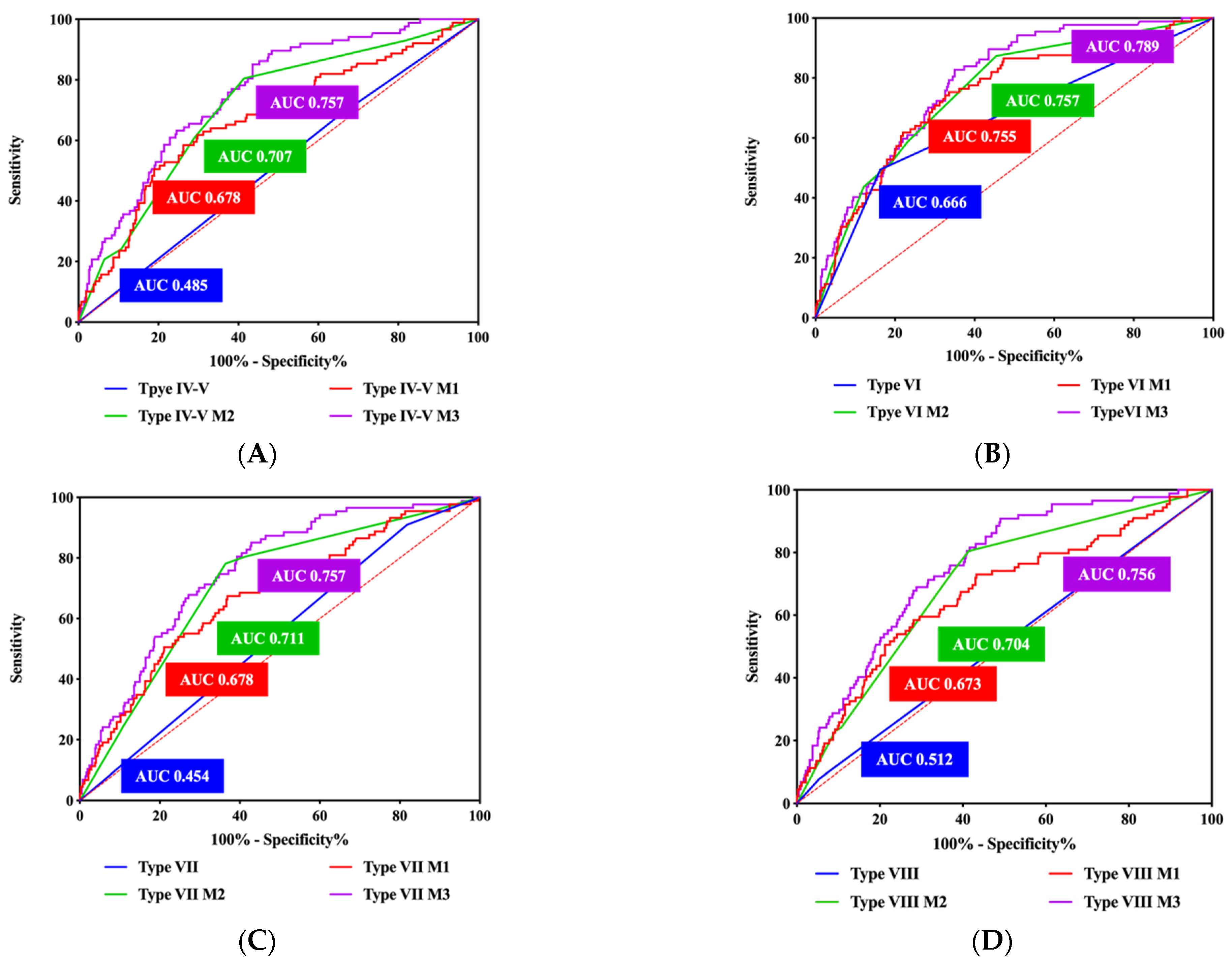
| OR | 95% CI | p Value | AUC | p Value | ||
|---|---|---|---|---|---|---|
| Stenosis degree | 1.742 | 1.191 | 2.549 | 0.004 | 0.702 | <0.001 |
| Type IV–V | 5.269 | 1.178 | 23.568 | 0.030 | 0.485 | 0.648 |
| Type VI | 14.596 | 3.218 | 66.202 | <0.001 | 0.666 | <0.001 |
| Type VII | 4.195 | 0.852 | 20.642 | 0.078 | 0.454 | 0.150 |
| Type VIII | 7.297 | 1.329 | 40.068 | 0.022 | 0.512 | 0.722 |
| SUVmax | 0.947 | 0.625 | 1.434 | 0.799 | - | - |
| TBR | 1.456 | 1.077 | 1.968 | 0.014 | 0.673 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Zhang, Y.; Sun, H.; Li, X.; Shan, Y.; Zheng, C.; Cui, B.; Li, J.; Yang, Y.; Yang, B.; et al. In Vivo Classification and Characterization of Carotid Atherosclerotic Lesions with Integrated 18F-FDG PET/MRI. Diagnostics 2024, 14, 1006. https://doi.org/10.3390/diagnostics14101006
Yu F, Zhang Y, Sun H, Li X, Shan Y, Zheng C, Cui B, Li J, Yang Y, Yang B, et al. In Vivo Classification and Characterization of Carotid Atherosclerotic Lesions with Integrated 18F-FDG PET/MRI. Diagnostics. 2024; 14(10):1006. https://doi.org/10.3390/diagnostics14101006
Chicago/Turabian StyleYu, Fan, Yue Zhang, Heyu Sun, Xiaoran Li, Yi Shan, Chong Zheng, Bixiao Cui, Jing Li, Yang Yang, Bin Yang, and et al. 2024. "In Vivo Classification and Characterization of Carotid Atherosclerotic Lesions with Integrated 18F-FDG PET/MRI" Diagnostics 14, no. 10: 1006. https://doi.org/10.3390/diagnostics14101006
APA StyleYu, F., Zhang, Y., Sun, H., Li, X., Shan, Y., Zheng, C., Cui, B., Li, J., Yang, Y., Yang, B., Ma, Y., Wang, Y., Jiao, L., Li, X., & Lu, J. (2024). In Vivo Classification and Characterization of Carotid Atherosclerotic Lesions with Integrated 18F-FDG PET/MRI. Diagnostics, 14(10), 1006. https://doi.org/10.3390/diagnostics14101006







