A Modified Corona Score Using Lung Ultrasound to Identify COVID-19 Patients
Abstract
:1. Introduction
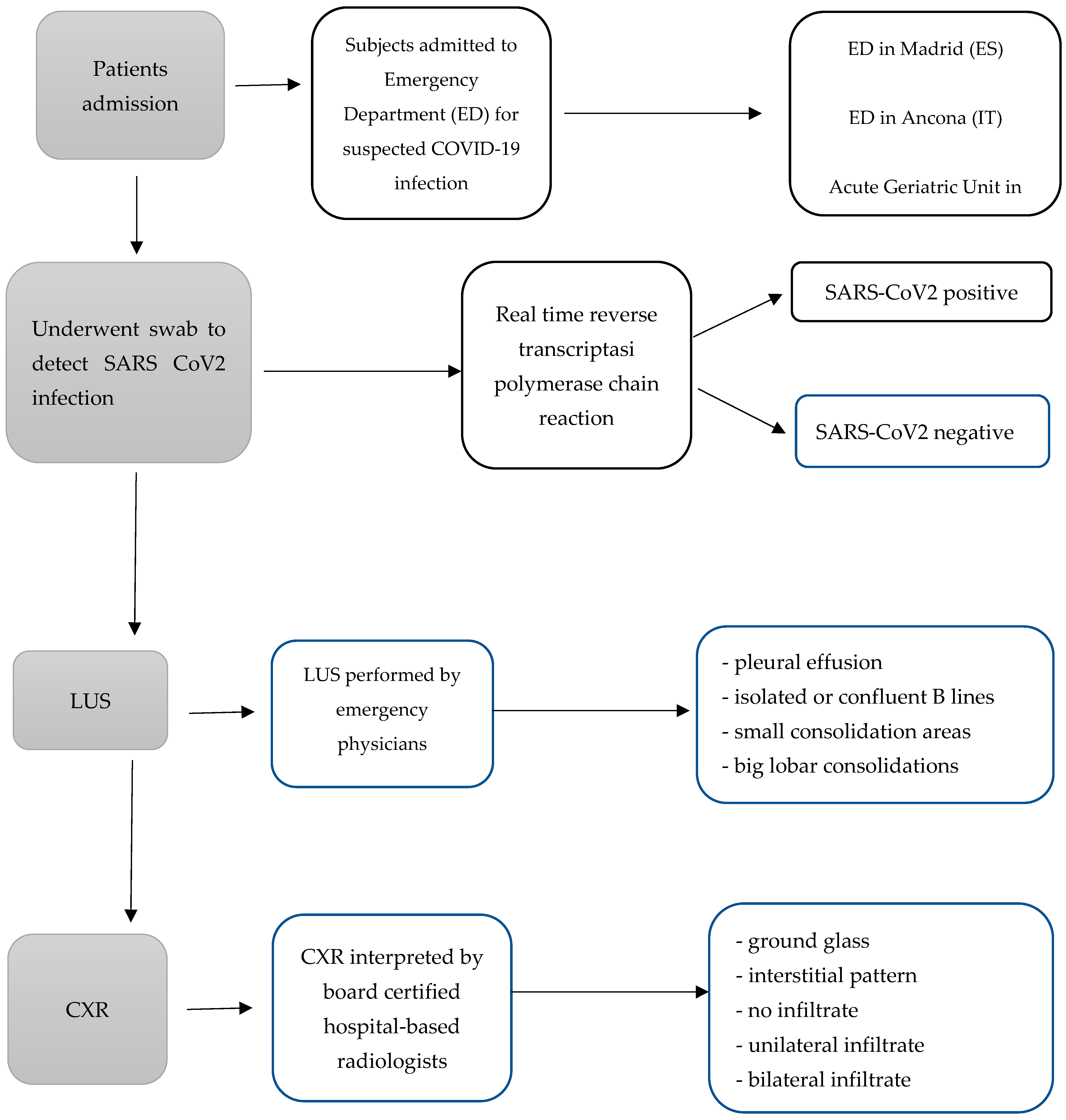
2. Materials and Methods
2.1. Study Design
2.2. Measures and Equipment
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 13 February 2022).
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; De Fazio, G.; Guglielmi, R.; Coluzzi, F.; Fiorelli, S.; Rocco, M. COVID-19 Lung Ultrasound Scores and Lessons from the Pandemic: A Narrative Review. Diagnostics 2023, 13, 1972. [Google Scholar] [CrossRef] [PubMed]
- Kurstjens, S.; van der Horst, A.; Herpers, R.; Geerits, M.W.L.; Kluiters-de Hingh, Y.C.M.; Leonne Göttgens, E.; Blaauw, M.J.T.; Thelen, M.H.M.; Elisen, M.G.L.M.; Kusters, R. Rapid identification of SARS-CoV-2-infected patients at the emergency department using routine testing. Clin. Chem. Lab. Med. 2020, 58, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Assandri, R.; Canetta, C.; Viganò, G.; Buscarini, E.; Scartabellati, A.; Montanelli, A. Laboratory markers included in the Corona Score can identify false negative results on COVID-19 RT-PCR in the emergency room. Biochem. Med. 2020, 30, 030402. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Henry, B.M.; Hoehn, J.; Benoit, S.; Benoit, J. Validation of the Corona-Score for rapid identification of SARS-CoV-2 infections in patients seeking emergency department care in the United States. Clin. Chem. Lab. Med. 2020, 58, e311–e313. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Caroselli, C.; Blaivas, M.; Marcosignori, M.; Tung Chen, Y.; Falzetti, S.; Mariz, J.; Fiorentino, R.; Silva, R.P.; Cochicho, J.G.; Sebastiani, S.; et al. Early Lung Ultrasound Findings in Patients With COVID-19 Pneumonia: A Retrospective Multicenter Study of 479 Patients. J. Ultrasound Med. 2022, 41, 2547–2556. [Google Scholar] [CrossRef]
- Tung-Chen, Y.; Martí de Gracia, M.; Díez-Tascón, A.; Alonso-González, R.; Agudo-Fernández, S.; Parra-Gordo, M.L.; Ossaba-Vélez, S.; Rodríguez-Fuertes, P.; Llamas-Fuentes, R. Correlation between Chest Computed Tomography and Lung Ultrasonography in Patients with Coronavirus Disease 2019 (COVID-19). Ultrasound Med. Biol. 2020, 46, 2918–2926. [Google Scholar] [CrossRef]
- Bashir, M.; Inzamam, W.; Banday, M.K.; Rasool, S.R.; Bhat, M.H.; Vladulescu, C.; Al-Misned, F.A.; El-Serehy, H.A. Lung Ultrasonography Is an Acceptable Imaging Modality to Diagnose COVID-19 and Effectively Correlates with HRCT Chest—A Prospective Study. Diagnostics 2023, 13, 2091. [Google Scholar] [CrossRef]
- Tung-Chen, Y.; Gil-Rodrigo, A.; Algora-Martín, A.; Llamas-Fuentes, R.; Rodríguez-Fuertes, P.; Marín-Baselga, R.; Alonso-Martínez, B.; Rodríguez, E.S.; Soriano, P.L.; Ramos-Rincón, J.-M. The lung ultrasound “Rule of 7” in the prognosis of COVID-19 patients: Results from a prospective multicentric study. Med. Clin. 2021, 59, 19–26. [Google Scholar] [CrossRef]
- de Alencar, J.C.G.; Marchini, J.F.M.; Marino, L.O.; da Costa Ribeiro, S.C.; Bueno, C.G.; da Cunha, V.P.; Neto, F.L.; Neto, R.A.B.; Souza, H.P.; the COVID U. S. P. Registry Team. Lung ultrasound score predicts outcomes in COVID-19 patients admitted to the emergency department. Ann. Intensive Care 2021, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- International Conference of Harmonization (ICH). ICH Tripartite Guideline for Good Clinical Practices E6 (R1), 10th 1996. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1Guideline.pdf (accessed on 14 September 2015).
- Regolamento Generale Sulla Protezione dei Dati (RGPD) n. 2016/679 del 27 Aprile 2016; Gazzetta Ufficiale dell’Unione Europea 4 Maggio 2016. Available online: https://europa.eu/youreurope/business/dealing-with-customers/data-protection/data-protection-gdpr/index_it.htm (accessed on 21 October 2023).
- Volpicelli, G.; Gargani, L.; Perlini, S.; Spinelli, S.; Barbieri, G.; Lanotte, A.; Casasola, G.G.; Nogué-Bou, R.; Lamorte, A.; Agricola, E.; et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: An international multicenter study. Intensive Care Med. 2021, 47, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.esaote.com/it-IT/ (accessed on 21 October 2023).
- Available online: https://www.philips.it/healthcare/solutions/ultrasound/ultrasound-general-imaging (accessed on 21 October 2023).
- Available online: https://www.gehealthcare.it/products/ultrasound (accessed on 21 October 2023).
- Available online: https://pservices.com.cy/wp-content/uploads/2021/04/Eidos_RF439_v_eng.pdf (accessed on 21 October 2023).
- Available online: https://www.molecular.abbott/int/en/products/infectious-disease/RealTime-SARS-CoV-2-Assay (accessed on 21 October 2023).
- Ord, H.L.; Griksaitis, M.J. Fifteen-minute consultation: Using point of care ultrasound to assess children with respiratory failure. Arch. Dis. Child Educ. Pract. Ed. 2019, 104, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Li, J.; Yang, J.P.; Zhan, Y.; Chen, J. Lung ultrasonography for the diagnosis of 11 patients with acute respiratory distress syndrome due to bird flu H7N9 infection. Virol. J. 2015, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Stefanini, F.; Cantisani, V.; Sidhu, P.S.; Barr, R.; Berzigotti, A.; Chammas, M.C.; Correas, J.-M.; Dietrich, C.F.; Feinstein, S.; et al. Benefits, Open questions and Challenges of the use of Ultrasound in the COVID-19 pandemic era. The views of a panel of worldwide international experts. Ultraschall Med. Eur. J. Ultrasound 2020, 41, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; Soldati, G.; Copetti, R.; Giannuzzi, R.; Portale, G.; Gentiloni-Silveri, N. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit. Care 2012, 16, R30. [Google Scholar] [CrossRef] [PubMed]
- Caroselli, C.; Cherubini, A. Should lung ultrasound be always performed in older patients with possible COVID-19 disease? Eur. Geriatr. Med. 2021, 12, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Caroselli, C.; Blaivas, M.; Falzetti, S. Diagnostic Imaging in Newborns, Children and Adolescents Infected with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Is There a Realistic Alternative to Lung High-Resolution Computed Tomography (HRCT) and Chest X-Rays? A Systematic Review of the Literature. Ultrasound Med. Biol. 2021, 47, 3034–3040. [Google Scholar]
- Lugarà, M.; Tamburrini, S.; Coppola, M.G.; Oliva, G.; Fiorini, V.; Catalano, M.; Carbone, R.; Saturnino, P.P.; Rosano, N.; Pesce, A.; et al. The Role of Lung Ultrasound in SARS-CoV-19 Pneumonia Management. Diagnostics 2022, 12, 1856. [Google Scholar] [CrossRef]
- British Society of Gastrointestinal and Abdominal Radiology/Society and College of Radiographers. Joint Guidance on Performing CT Colonography in the Early Recovery Phase of the COVID-19 Pandemic 2020. Available online: https://www.bsgar.org/media/forum/Joint_guidance_on_performing_CTcolonography_in_the_earlyrecoveryphases_of_Covid.pdf (accessed on 21 October 2023).
- Arntfield, R.; VanBerlo, B.; Alaifan, T.; Phelps, N.; White, M.; Chaudhary, R.; Ho, J.; Wu, D. Development of a convolutional neural network to differentiate among the etiology of similar appearing pathological B lines on lung ultrasound: A deep learning study. BMJ Open 2021, 11, e045120. [Google Scholar] [CrossRef]
- Bass, C.M.; Sajed, D.R.; Adedipe, A.A.; West, T.E. Pulmonary ultrasound and pulse oximetry versus chest radiography and arterial blood gas analysis for the diagnosis of acute respiratory distress syndrome: A pilot study. Crit. Care 2015, 19, 282. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, A.; Paneroni, M.; Carotenuto, M.; Bertella, E.; Cirio, S.; Gandolfo, A.; Simonelli, C.; Vigna, M.; Lastoria, C.; Malovini, A.; et al. Prevalence of exercise-induced oxygen desaturation after recovery from SARS-CoV-2 pneumonia and use of lung ultrasound to predict need for pulmonary rehabilitation. Pulmonology 2021, 29, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Blazic, I.; Cogliati, C.; Flor, N.; Frija, G.; Kawooya, M.; Umbrello, M.; Ali, S.; Baranne, M.L.; Cho, Y.J.; Pitcher, R.; et al. The use of lung ultrasound in COVID-19. ERJ Open Res. 2023, 9, 00196–02022. [Google Scholar] [CrossRef] [PubMed]
| Negative on the Molecular Test (n = 49) | Positive on the Molecular Test (n = 216) | p | |
|---|---|---|---|
| Age (years) | 65.5 ± 2.7 | 71.6 ± 1.2 | 0.03 |
| Female | 53.1% | 44.00% | ns |
| C-reactive protein (mg/dL) | 50.6 ± 11.1 | 53.7 ± 5.8 | ns |
| Ferritin (ug/L) | 492.5 ± 85 | 975.9 ± 68.2 | <0.01 |
| Lactate dehydrogenase (U/L) | 329.8 ± 41.5 | 415.1 ± 19.1 | ns |
| Absolute lymphocyte count (109/L) | 1.2 ± 0.2 | 1.2 ± 0.1 | ns |
| Absolute neutrophil count (109/L) | 6.2 ± 0.5 | 5.1 ± 0.4 | ns |
| Fever | 46.7% | 75.0% | <0.01 |
| Dyspnoea/Cough | 69.4% | 70.4% | ns |
| Asthenia | 38.8% | 26.9% | ns |
| Gastroenterological symptoms | 10.2% | 15.3% | ns |
| Hemoptysis | 0.0% | 2.8% | ns |
| Arthromyalgia | 0.0% | 4.6% | ns |
| Chest pain | 14.3% | 16.7% | ns |
| Ageusia/Anosmia | 2.0% | 3.7% | ns |
| Bradycardia | 0.0% | 1.9% | ns |
| Faringodinia | 0.0% | 2.4% | ns |
| Cefalea | 0.0% | 1.9% | ns |
| Systolic blood pressure (mmHg) | 130.6 ± 3.4 | 122.8 ± 1.7 | ns |
| Diastolic blood pressure (mmHg) | 76.1 ± 2.1 | 70.7 ± 1.0 | 0.02 |
| Heart rate (bpm) | 94.0 ± 2.4 | 90.2 ± 1.3 | ns |
| Oxygen saturation | 93.1 ± 0.9 | 90.9 ± 0.4 | 0.02 |
| Hemodynamic instability | 4.1% | 14.2% | ns |
| History of dementia | 12.2% | 45.8% | <0.01 |
| History of diabetes | 34.7% | 35.6% | ns |
| History of lung disease | 28.6% | 25.9% | ns |
| History of neoplastic disease | 28.6% | 10.2% | <0.01 |
| History of heart disease | 28.0% | 22.7% | ns |
| Positive at Swab | Negative at Swab | p | |
|---|---|---|---|
| Corona score | 9.8 ± 0.3 | 6.7 ± 0.8 | <0.001 |
| Modified 1 corona score: M1CS | 10.5 ± 0.3 | 6.4 ± 0.9 | <0.001 |
| Modified 2 corona score: M2CS | 6.4 ± 0.2 | 3.5 ± 0.5 | <0.001 |
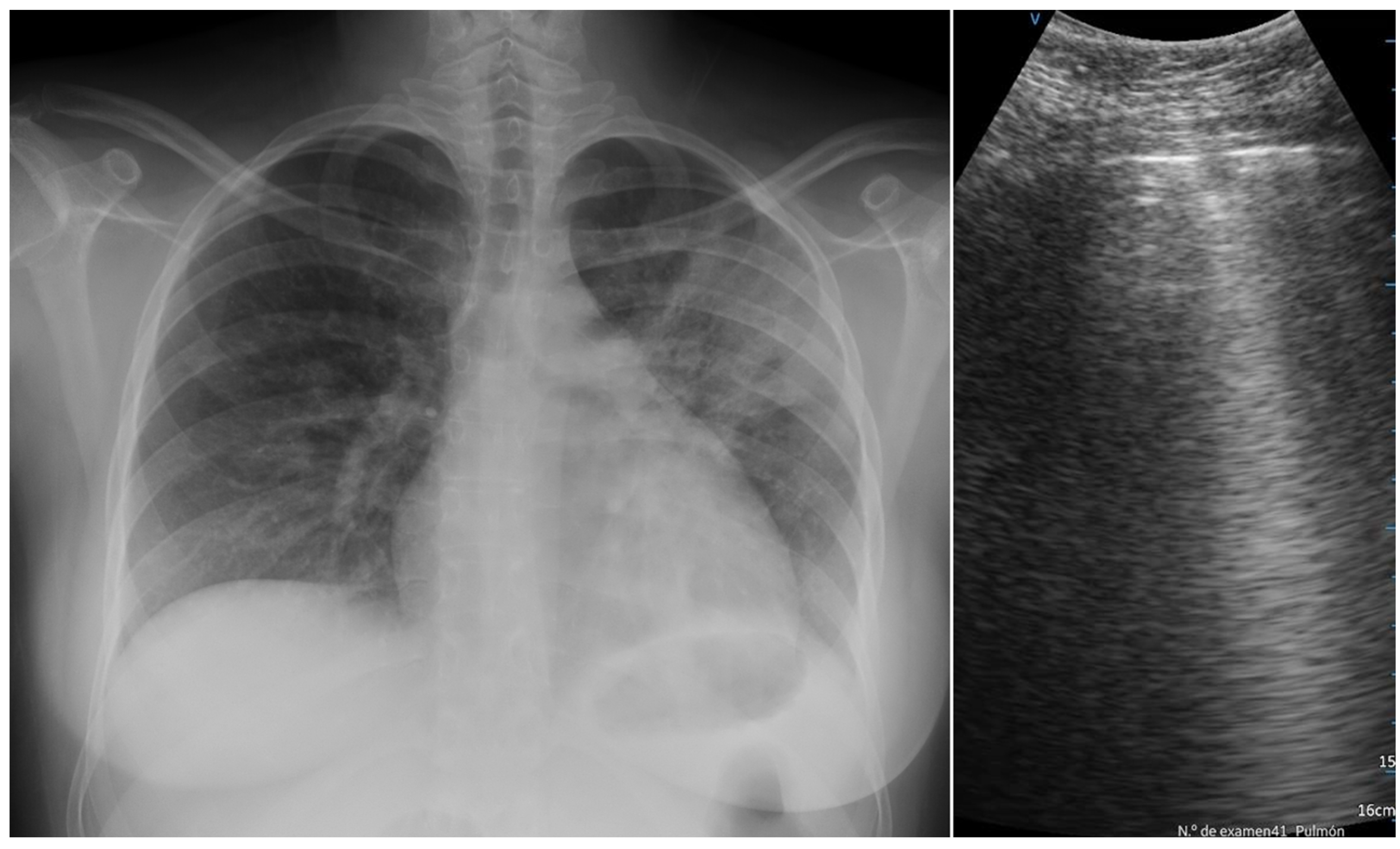
| LUS pleural effusion | 13.9% | 20.4% | ns |
| LUS isolated B lines | 81.0% | 81.6% | ns |
| LUS confluent B lines | 44.0% | 55.1% | ns |
| LUS irregular pleural line | 67.6% | 91.8% | 0.025 |
| LUS small consolidation areas | 64.8% | 57.1% | ns |
| LUS big/lobar consolidation | 13.0% | 10.2% | ns |
| X-ray normal findings | 17.0% | 26.5% | ns |
| X-ray ground glass | 35.6% | 35.5% | ns |
| X-ray interstitial pattern | 61.9% | 29.4% | <0.001 |
| X-ray no infiltrate | 27.2% | 32.3% | ns |
| X-ray unilateral infiltrate | 23.3% | 11.8% | ns |
| X-ray bilateral infiltrate | 49.5% | 55.9% | ns |
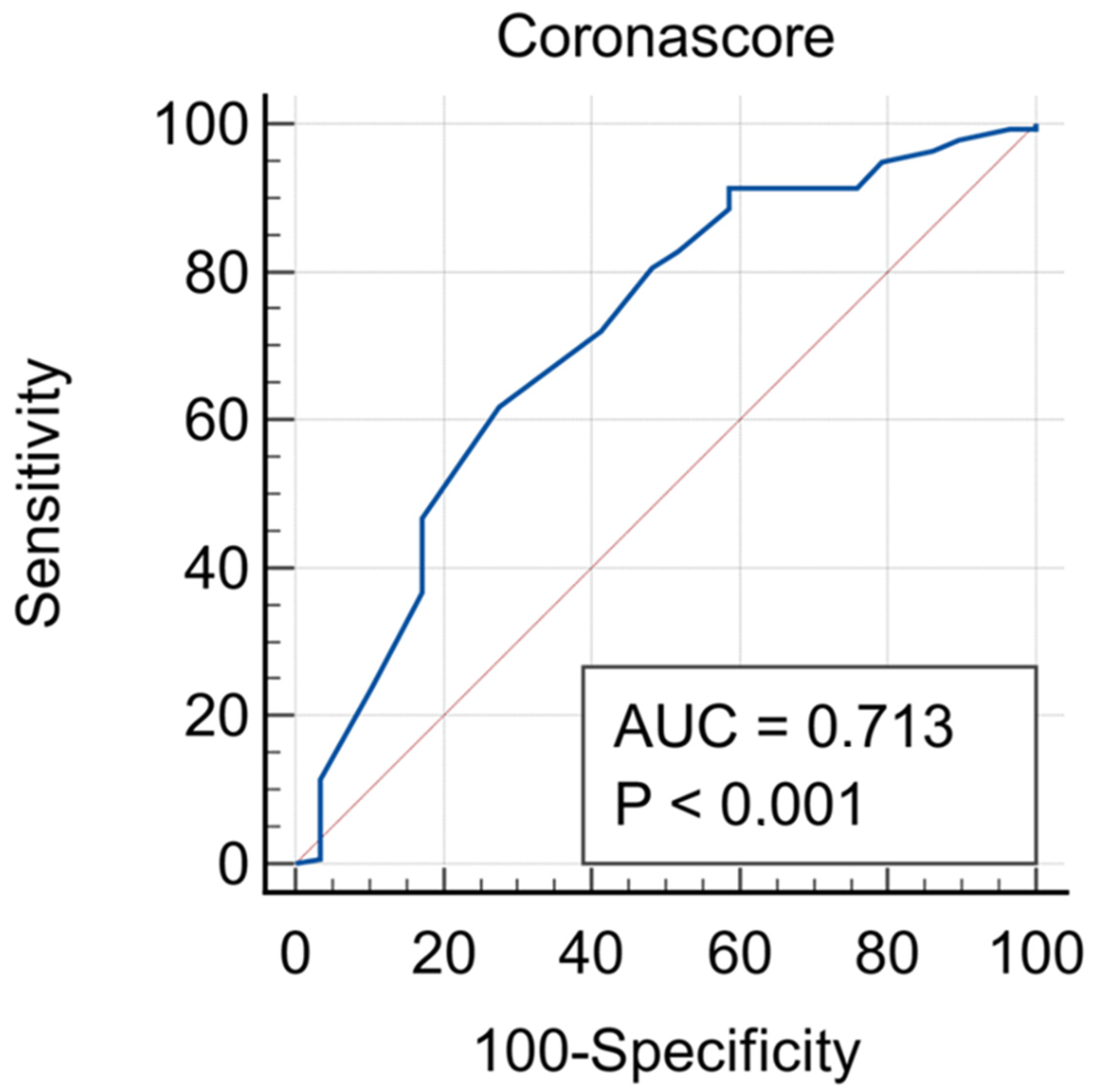
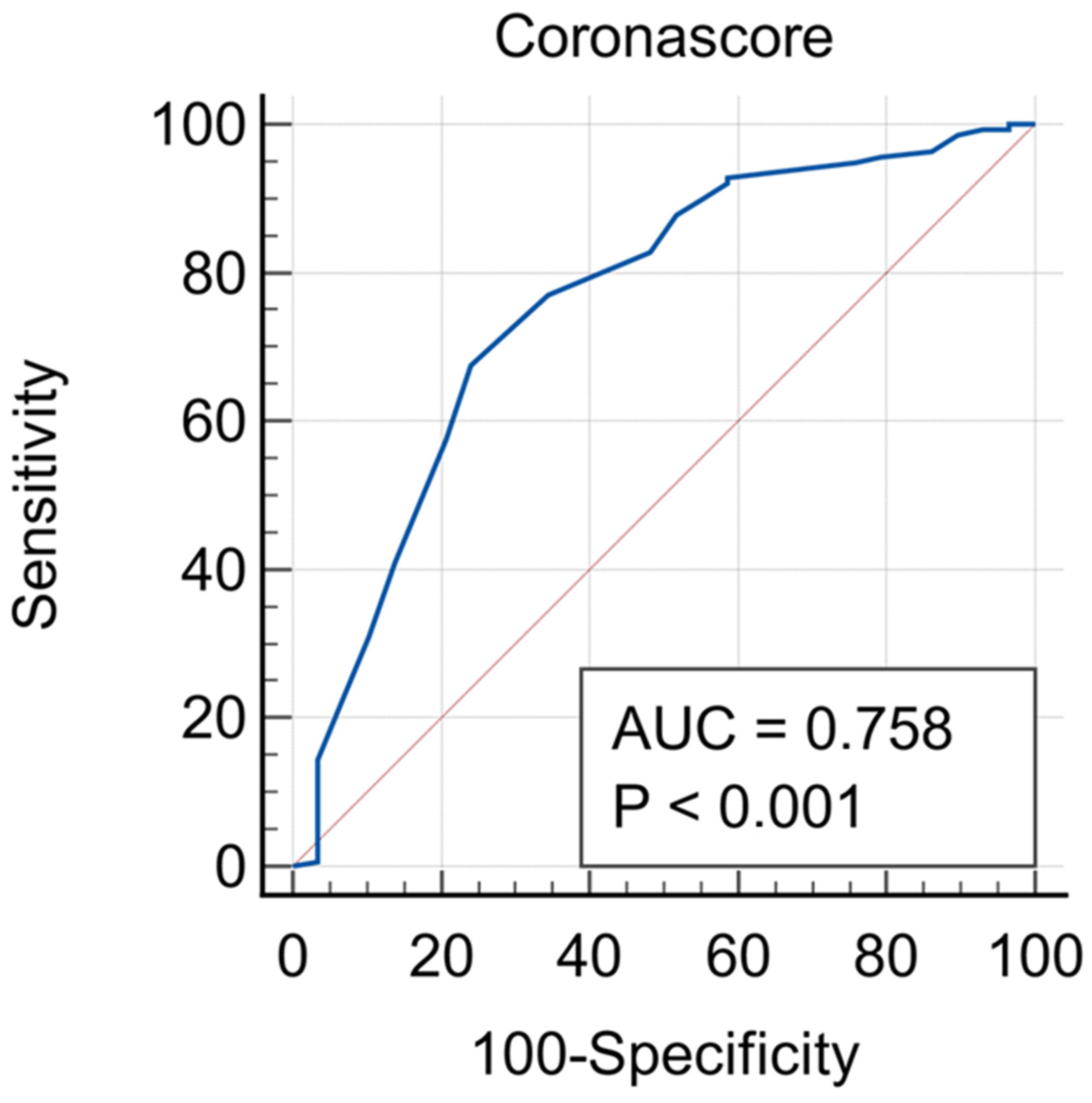
| Corona Score Cut-Off Value | M1CS | M2CS | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| 2 | 98.4% (0.95–0.99) | 8.2% (0.03–0.20) | 92.6% (0.89–0.96) | 20.4% (0.11–0.35) |
| 3 | 96.3% (0.93–0.98) | 12.4% (0.05–0.25) | 91.1% (0.83–0.97) | 30.6% (0.19–0.45) |
| 4 | 95.8% (0.93–0.98) | 24.5% (0.14–0.39) | 85.7% (0.80–0.91) | 49.0% (0.34–0.64) |
| 5 | 87.7% (0.82–0.92) | 28.6% (0.16–0.44) | 73.7% (0.67–0.80) | 59.2% (0.44–0.73) |
| 6 | 85.7% (0.79–0.90) | 32.7% (0.20–0.48) | 62.6% (0.55–0.69) | 69.4% (0.55–0.82) |
| 7 | 80.9% (0.73–0.85) | 36.8% (0.23–0.52) | 49.3% (0.42–0.55) | 75.5% (0.61–0.84) |
| 8 | 71.1% (0.64–0.77) | 49.9% (0.35–0.64) | 36.3% (0.30–0.44) | 85.7% (0.73–0.94) |
| 9 | 62.1% (0.55–0.69) | 57.8% (0.43–0.72) | 17.2% (0.12–0.23) | 96.0% (0.86–0.99) |
| 10 | 52.9% (0.46–0.60) | 70.8% (0.55–0.82) | 8.9% (0.05–0.14) | 98.0% (0.90–0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caroselli, C.; Blaivas, M.; Tung Chen, Y.; Marcosignori, M.; Cherubini, A.; Longo, D. A Modified Corona Score Using Lung Ultrasound to Identify COVID-19 Patients. Diagnostics 2024, 14, 51. https://doi.org/10.3390/diagnostics14010051
Caroselli C, Blaivas M, Tung Chen Y, Marcosignori M, Cherubini A, Longo D. A Modified Corona Score Using Lung Ultrasound to Identify COVID-19 Patients. Diagnostics. 2024; 14(1):51. https://doi.org/10.3390/diagnostics14010051
Chicago/Turabian StyleCaroselli, Costantino, Michael Blaivas, Yale Tung Chen, Matteo Marcosignori, Antonio Cherubini, and Daniele Longo. 2024. "A Modified Corona Score Using Lung Ultrasound to Identify COVID-19 Patients" Diagnostics 14, no. 1: 51. https://doi.org/10.3390/diagnostics14010051
APA StyleCaroselli, C., Blaivas, M., Tung Chen, Y., Marcosignori, M., Cherubini, A., & Longo, D. (2024). A Modified Corona Score Using Lung Ultrasound to Identify COVID-19 Patients. Diagnostics, 14(1), 51. https://doi.org/10.3390/diagnostics14010051







