Coronary Tortuosity Index vs. Angle Measurement Method for the Quantification of the Tortuosity of Coronary Arteries in Non-Obstructive Coronary Disease
Abstract
:1. Introduction
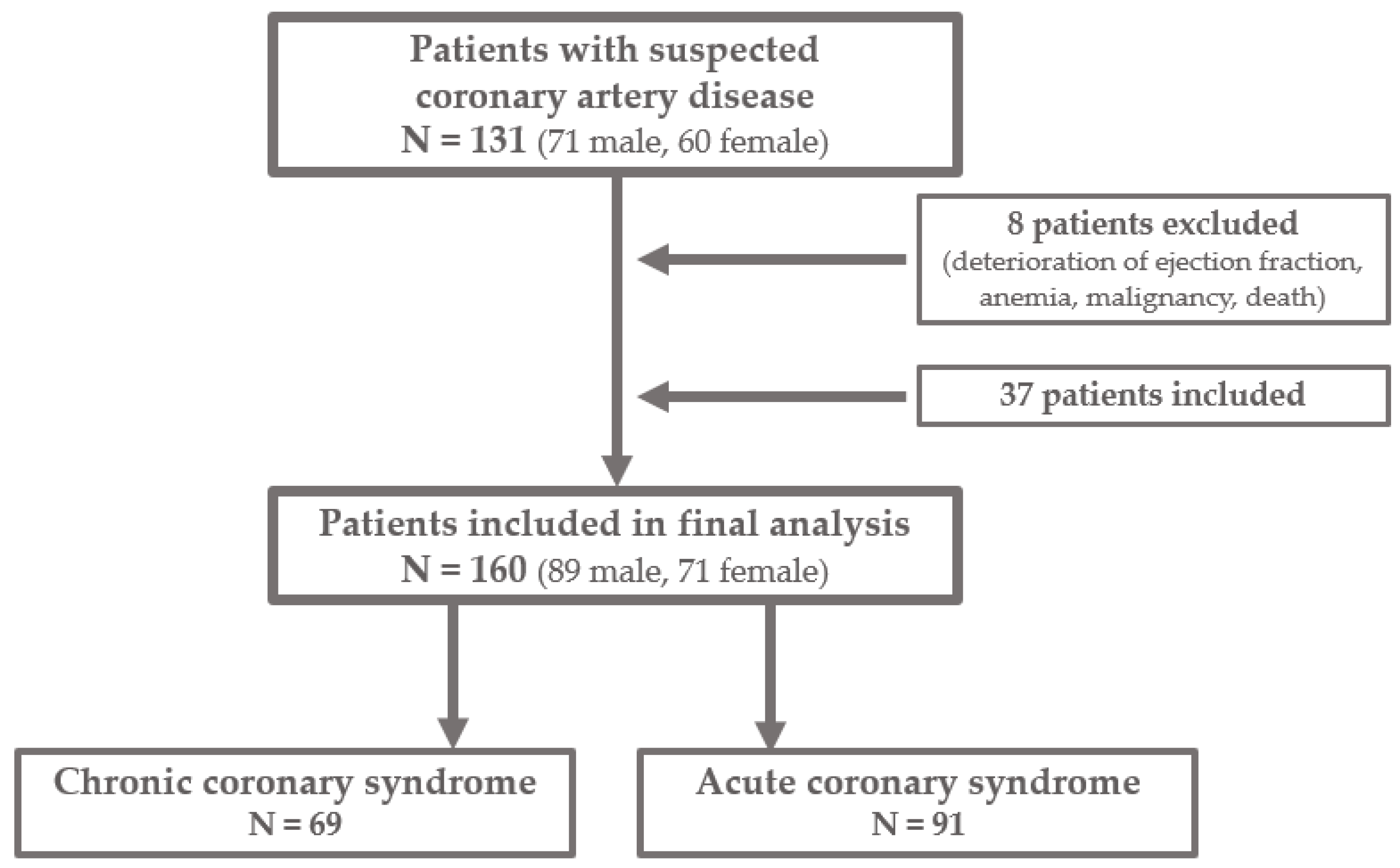
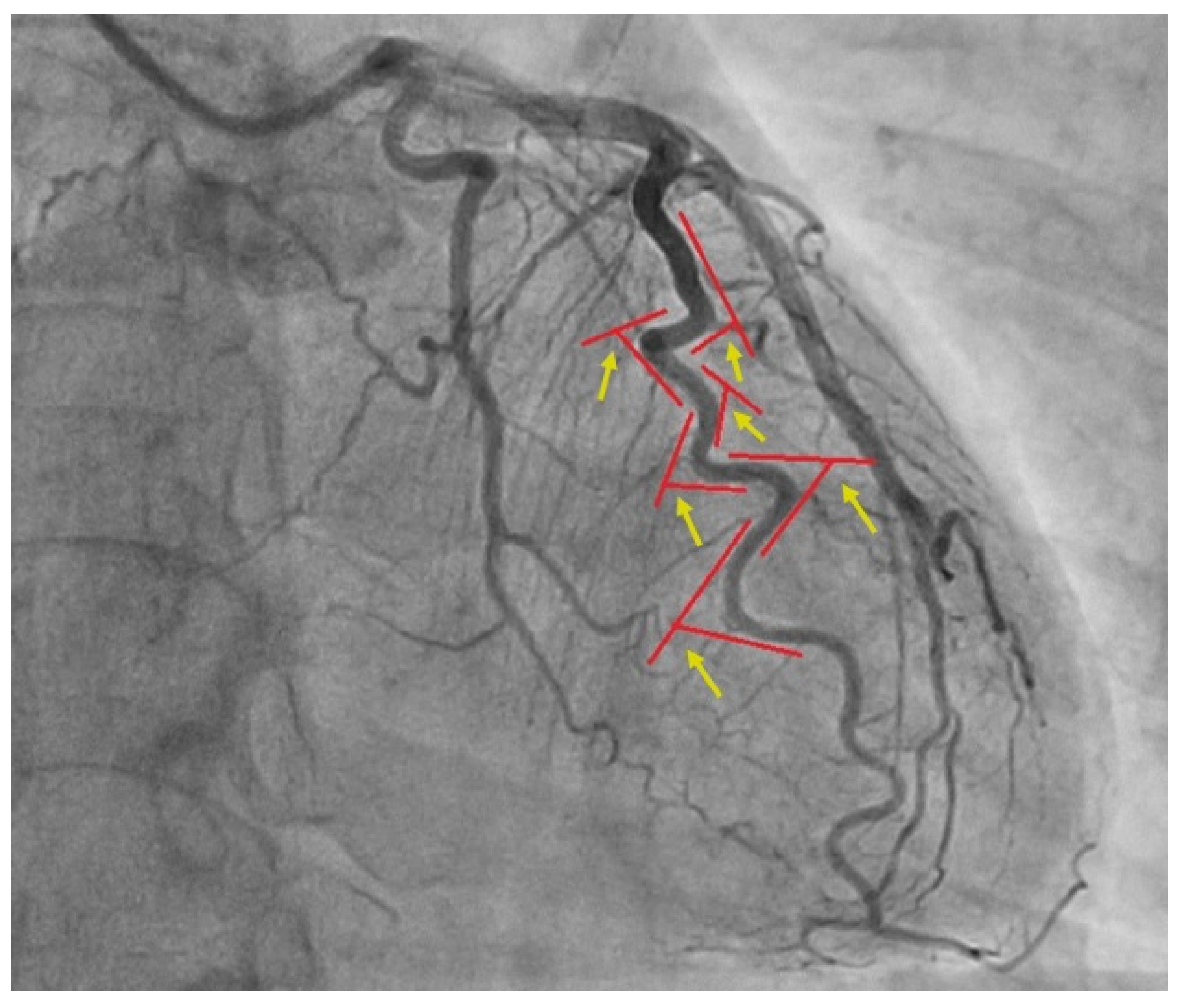
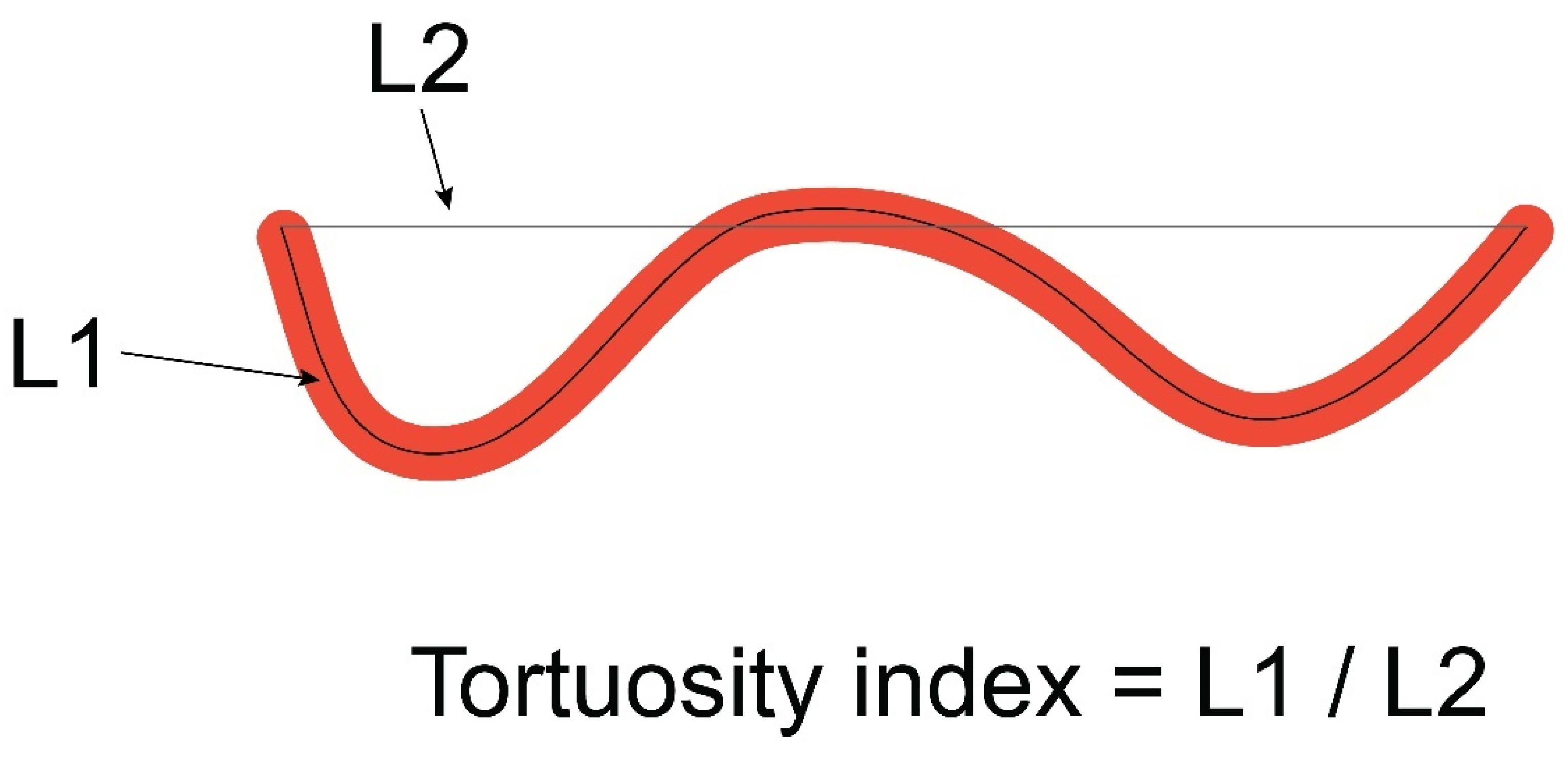
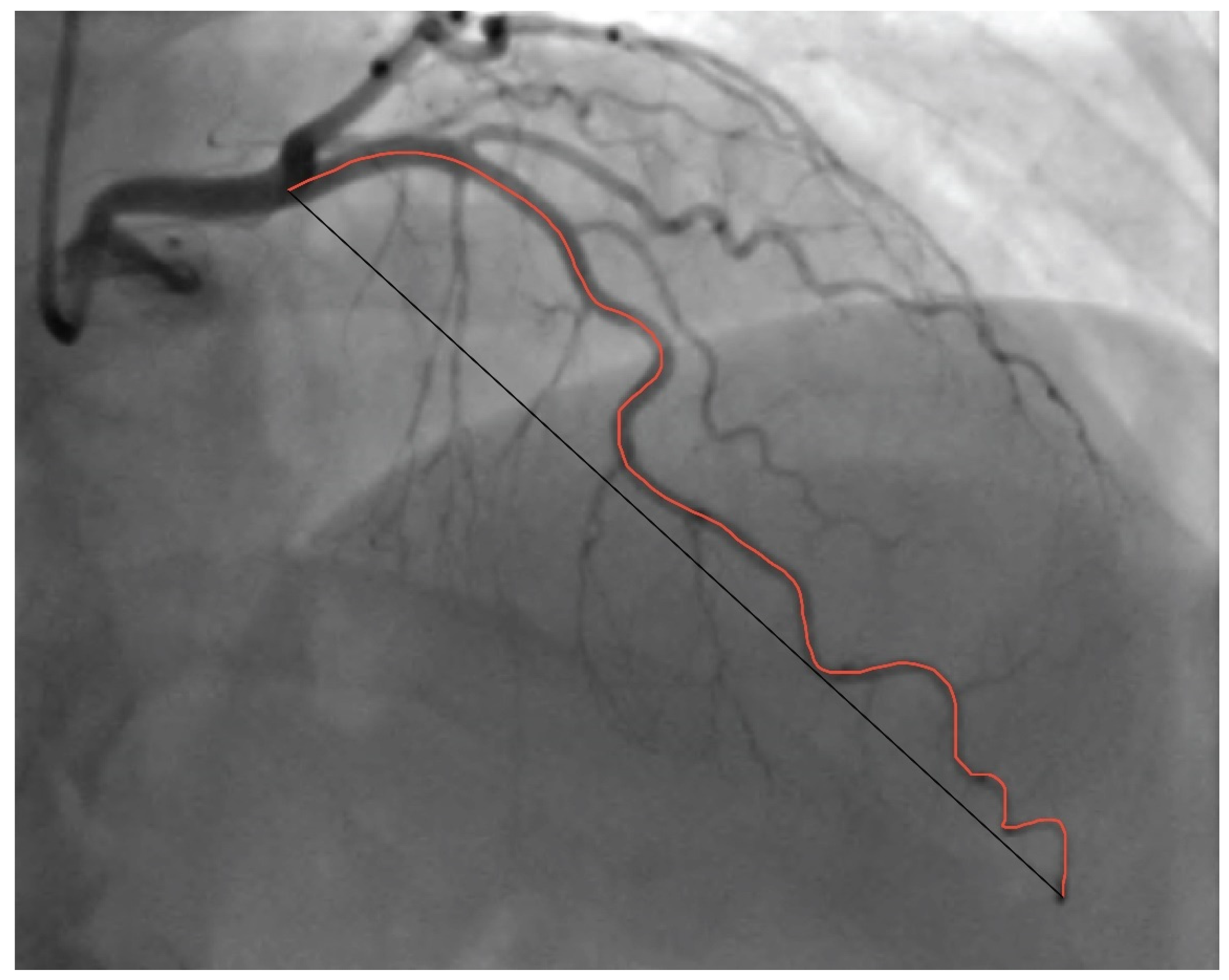
2. Materials and Methods
2.1. Patients and Procedures
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Kenkre, T.S.; Malhotra, P.; Johnson, B.D.; Handberg, E.M.; Thompson, D.V.; Marroquin, O.C.; Rogers, W.J.; Pepine, C.J.; Bairey Merz, C.N.; Kelsey, S.F. Ten-year mortality in the WISE study (Women’s Ischemia Syndrome Evaluation). Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003863. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch. Intern. Med. 2009, 169, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Schumann, C.L.; Mathew, R.C.; Dean, J.L.; Yang, Y.; Balfour, P.C., Jr.; Shaw, P.W.; Robinson, A.A.; Salerno, M.; Kramer, C.M.; Bourque, J.M. Functional and Economic Impact of INOCA and Influence of Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2021, 14, 1369–1379. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Estrada, A.; Sousa, A.S.; Mesquita, C.T.; Villacorta, H. Coronary Tortuosity as a New Phenotype for Ischemia without Coronary Artery Disease. Arq. Bras. Cardiol. 2022, 119, 883–890. [Google Scholar] [CrossRef]
- Zegers, E.S.; Meursing, B.T.; Zegers, E.B.; Oude Ophuis, A.J. Coronary tortuosity: A long and winding road. Neth. Heart J. 2007, 15, 191–195. [Google Scholar] [CrossRef]
- Ciurică, S.; Lopez-Sublet, M.; Loeys, B.L.; Radhouani, I.; Natarajan, N.; Vikkula, M.; Maas, A.H.E.M.; Adlam, D.; Persu, A. Arterial Tortuosity. Hypertension 2019, 73, 951–960. [Google Scholar] [CrossRef]
- Jakob, M.; Spasojevic, D.; Krogmann, O.N.; Wiher, H.; Hug, R.; Hess, O.M. Tortuosity of coronary arteries in chronic pressure and volume overload. Catheter. Cardiovasc. Diagn. 1996, 38, 25–31. [Google Scholar] [CrossRef]
- Turgut, O.; Yilmaz, A.; Yalta, K.; Yilmaz, B.M.; Ozyol, A.; Kendirlioglu, O.; Karadas, F.; Tandogan, I. Tortuosity of coronary arteries: An indicator for impaired left ventricular relaxation? Int. J. Cardiovasc. Imaging 2007, 23, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, S.; Spadafora, L.; Bernardi, M.; Galli, M.; Betti, M.; Perone, F.; Nicolaio, G.; Marzetti, E.; Martone, A.M.; Landi, F.; et al. Management of Coronary Artery Disease in Older Adults: Recent Advances and Gaps in Evidence. J. Clin. Med. 2023, 12, 5233. [Google Scholar] [CrossRef] [PubMed]
- Zebic Mihic, P.; Saric, S.; Bilic Curcic, I.; Mihaljevic, I.; Juric, I. The Association of Severe Coronary Tortuosity and Non-Obstructive Coronary Artery Disease. Medicina 2023, 59, 1619. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Guddeti, R.R.; Tweet, M.S.; Lerman, A.; Singh, M.; Best, P.J.; Vrtiska, T.J.; Prasad, M.; Rihal, C.S.; Hayes, S.N.; et al. Coronary artery tortuosity in spontaneous coronary artery dissection: Angiographic characteristics and clinical implications. Circ. Cardiovasc. Interv. 2014, 7, 656–662. [Google Scholar] [CrossRef]
- Zaacks, S.M.; Allen, J.E.; Calvin, J.E.; Schaer, G.L.; Palvas, B.W.; Parrillo, J.E.; Klein, L.W. Value of the American College of Cardiology/American Heart Association stenosis morphology classification for coronary interventions in the late 1990s. Am. J. Cardiol. 1998, 82, 43–49. [Google Scholar] [CrossRef]
- Hassan, A.K.M.; Abd-El Rahman, H.; Hassan, S.G.; Ahmed, T.A.N.; Youssef, A.A.A. Validity of tortuosity severity index in chest pain patients with abnormal exercise test and normal coronary angiography. Egypt. Heart J. 2018, 70, 381–387. [Google Scholar] [CrossRef]
- Li, Y.; Shen, C.; Ji, Y.; Feng, Y.; Ma, G.; Liu, N. Clinical implication of coronary tortuosity in patients with coronary artery disease. PLoS ONE 2011, 6, e24232. [Google Scholar] [CrossRef]
- Chiha, J.; Mitchell, P.; Gopinath, B.; Burlutsky, G.; Kovoor, P.; Thiagalingam, A. Gender differences in the prevalence of coronary artery tortuosity and its association with coronary artery disease. Int. J. Cardiol. Heart Vasc. 2016, 14, 23–27. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Rigo, F.; Reverberi, C. Severe coronary tortuosity or myocardial bridging in patients with chest pain, normal coronary arteries, and reversible myocardial perfusion defects. Am. J. Cardiol. 2011, 108, 973–978. [Google Scholar] [CrossRef]
- Oehler, A.C.; Minnier, J.; Lindner, J.R. Increased Coronary Tortuosity Is Associated with Increased Left Ventricular Longitudinal Myocardial Shortening. J. Am. Soc. Echocardiogr. 2017, 30, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef] [PubMed]
- Herring, N.; Paterson, D.J. ECG diagnosis of acute ischaemia and infarction: Past, present and future. QJM 2006, 99, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Fathala, A. Myocardial perfusion scintigraphy: Techniques, interpretation, indications and reporting. Ann. Saudi Med. 2011, 31, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Kusunose, K.; Abe, T.; Haga, A.; Fukuda, D.; Yamada, H.; Harada, M.; Sata, M. A Deep Learning Approach for Assessment of Regional Wall Motion Abnormality From Echocardiographic Images. JACC Cardiovasc. Imaging 2020, 13 Pt 1, 374–381. [Google Scholar] [CrossRef]
- Mihai, D.E.; Lupasteanu, I.; Dan, G.A. Impact of coronary artery tortuosity in ischemic and non-ischemic cardiovascular pathology. Rom. J. Intern. Med. 2021, 59, 119–126. [Google Scholar] [CrossRef]
- Hassanin Hanboly, N.; Ghany Abdel, M.M.; El-Kaffas Helmy, S.M.; Ahmed Udden, T. Prevalence, risk factors, and coronary angiographic profile in patients with tortuous coronary artery. Cor Vasa 2021, 63, 547–554. [Google Scholar] [CrossRef]
- Dvir, D.; Kornowski, R.; Gurevich, J.; Orlov, B.; Aravot, D. Degrees of severe stenoses in sigma-shaped versus C-shaped right coronary arteries. Am. J. Cardiol. 2003, 92, 294–298. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Ma, G.; Shen, C.; Liu, N. Coronary tortuosity is negatively correlated with coronary atherosclerosis. J. Int. Med. Res. 2018, 46, 5205–5209. [Google Scholar] [CrossRef] [PubMed]
- Khosravani-Rudpishi, M.; Joharimoghadam, A.; Rayzan, E. The significant coronary tortuosity and atherosclerotic coronary artery disease; What is the relation? J. Cardiovasc. Thorac. Res. 2018, 10, 209–213. [Google Scholar] [CrossRef]
- Vorobtsova, N.; Chiastra, C.; Stremler, M.A.; Sane, D.C.; Migliavacca, F.; Vlachos, P. Effects of Vessel Tortuosity on Coronary Hemodynamics: An Idealized and Patient-Specific Computational Study. Ann. Biomed. Eng. 2016, 44, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- El Tahlawi, M.; Sakrana, A.; Elmurr, A.; Gouda, M.; Tharwat, M. The Relation between coronary tortuosity and Calcium Score in Patients with Chronic Stable Angina and Normal Coronaries by CT angiography. Atherosclerosis 2016, 246, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wingert, A.; Wang, J.; Zhang, J.; Wang, X.; Sun, J.; Chen, F.; Khalid, S.G.; Jiang, J.; Zheng, D. Extraction of Coronary Atherosclerotic Plaques From Computed Tomography Imaging: A Review of Recent Methods. Front. Cardiovasc. Med. 2021, 8, 597568. [Google Scholar] [CrossRef]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbæk, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef]
| Group | |||
|---|---|---|---|
| Variable | NOCAD (N = 85) | OCAD (N = 75) | Statistics |
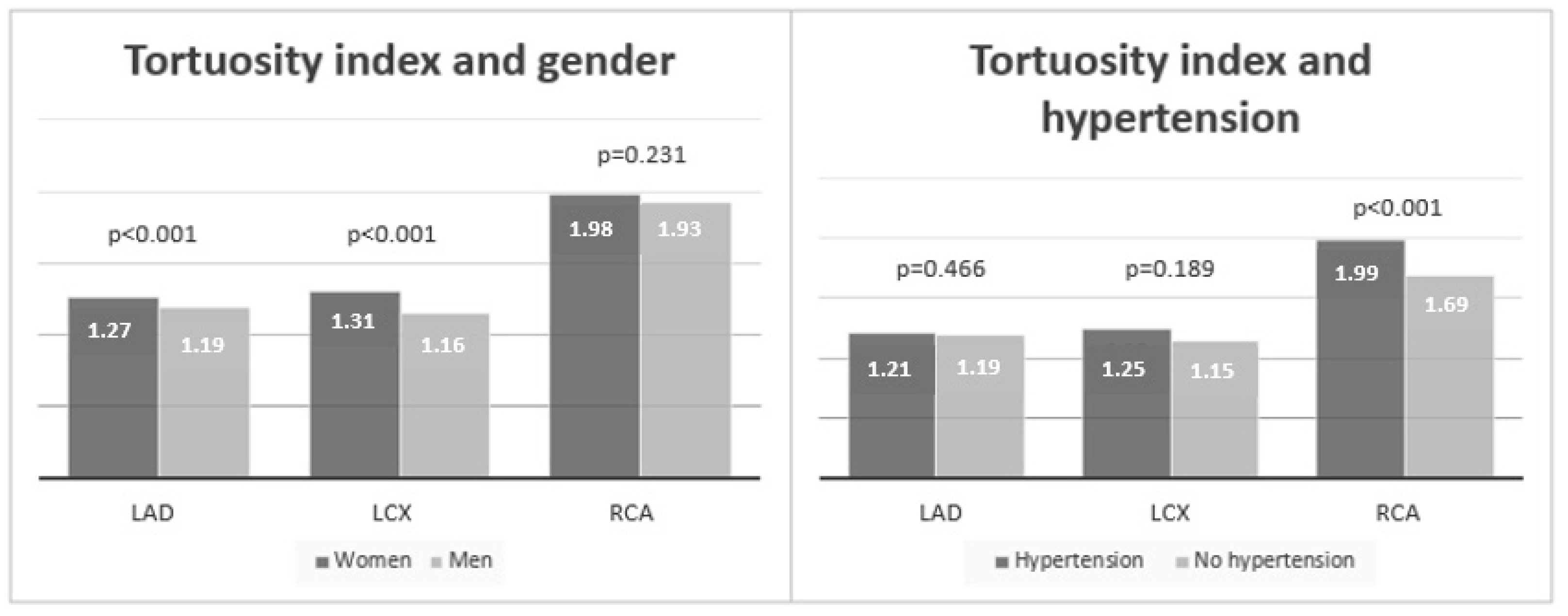
| Female; N (%) | 51 (60.0) | 20 (26.7) | χ2 = 17.8, p < 0.001 |
| Age, yrs; mean (SD) | 61.0 (10.7) | 62.1 (9.5) | 0.490 * |
| BMI, kgm−2; M (IQR) | 27.75 (25.92–31.36) | 28.40 (26.24–31.33) | 0.285 ** |
| Smoking; N (%) | χ2 = 6.6, p = 0.010 | ||
| Non smokers | 64 (75.3) | 42 (56.0) | |
| Smokers | 21 (24.7) | 33 (44.0) | |
| Medical history; N (%) | |||
| DM | 17 (20.0) | 26 (34.7) | χ2 = 4.3, p = 0.037 |
| HTA | 68 (80.0) | 56 (74.7) | χ2 = 0.6, p = 0.422 |
| HLP | 38 (44.7) | 42 (56.0) | χ2 = 2.0, p = 0.155 |
| Diagnosis of referral; N (%) | χ2 = 13.1, p < 0.001 | ||
| CCS | 48 (56.5) | 21 (28.0) | |
| ACS | 37 (43.5) | 54 (72.0) | |
| Group | |||
|---|---|---|---|
| Variable | NOCAD (N = 85) | OCAD (N = 75) | Statistics |
| Tortuous coronary artery; N (%) | |||
| LCX | 34 (40.0) | 11 (14.7) | χ2 = 12.6, p < 0.001 |
| LAD | 28 (32.9) | 8 (10.7) | χ2 = 11.3, p < 0.001 |
| RCA | 8 (9.4) | 0 (0.0) | χ2 = 7.38, p = 0.007 |
| Tortuosity index; M (IQR) | |||
| LCX | 1.30 (1.13–1.47) | 1.15 (1.10–1.30) | p < 0.001 * |
| LAD | 1.26 (1.18–1.45) | 1.18 (1.14–1.26) | p < 0.001 * |
| RCA | 2.00 (1.79–2.27) | 1.87 (1.65–2.17) | p = 0.014 * |
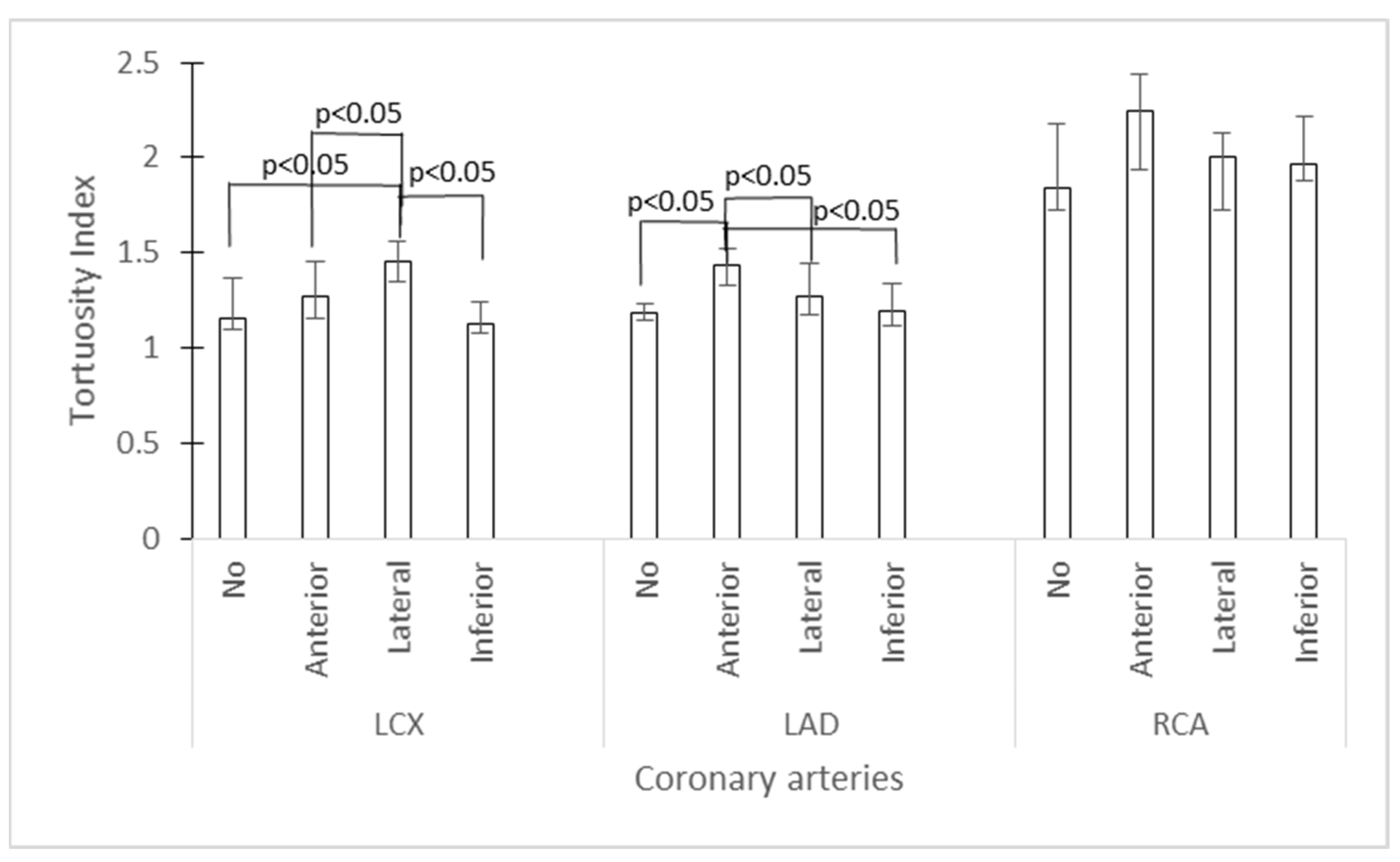
| Myocardial ischemia; N (%) | |||
| Anterior | 20 (23.5) | ||
| Lateral | 35 (41.2) | ||
| Inferior | 16 (18.8) | ||
| None | 14 (16.5) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zebic Mihic, P.; Arambasic, J.; Mlinarevic, D.; Saric, S.; Labor, M.; Bosnjak, I.; Mihaljevic, I.; Bilic Curcic, I.; Juric, I. Coronary Tortuosity Index vs. Angle Measurement Method for the Quantification of the Tortuosity of Coronary Arteries in Non-Obstructive Coronary Disease. Diagnostics 2024, 14, 35. https://doi.org/10.3390/diagnostics14010035
Zebic Mihic P, Arambasic J, Mlinarevic D, Saric S, Labor M, Bosnjak I, Mihaljevic I, Bilic Curcic I, Juric I. Coronary Tortuosity Index vs. Angle Measurement Method for the Quantification of the Tortuosity of Coronary Arteries in Non-Obstructive Coronary Disease. Diagnostics. 2024; 14(1):35. https://doi.org/10.3390/diagnostics14010035
Chicago/Turabian StyleZebic Mihic, Petra, Jerko Arambasic, Drazen Mlinarevic, Sandra Saric, Marina Labor, Ivica Bosnjak, Ivica Mihaljevic, Ines Bilic Curcic, and Iva Juric. 2024. "Coronary Tortuosity Index vs. Angle Measurement Method for the Quantification of the Tortuosity of Coronary Arteries in Non-Obstructive Coronary Disease" Diagnostics 14, no. 1: 35. https://doi.org/10.3390/diagnostics14010035
APA StyleZebic Mihic, P., Arambasic, J., Mlinarevic, D., Saric, S., Labor, M., Bosnjak, I., Mihaljevic, I., Bilic Curcic, I., & Juric, I. (2024). Coronary Tortuosity Index vs. Angle Measurement Method for the Quantification of the Tortuosity of Coronary Arteries in Non-Obstructive Coronary Disease. Diagnostics, 14(1), 35. https://doi.org/10.3390/diagnostics14010035






