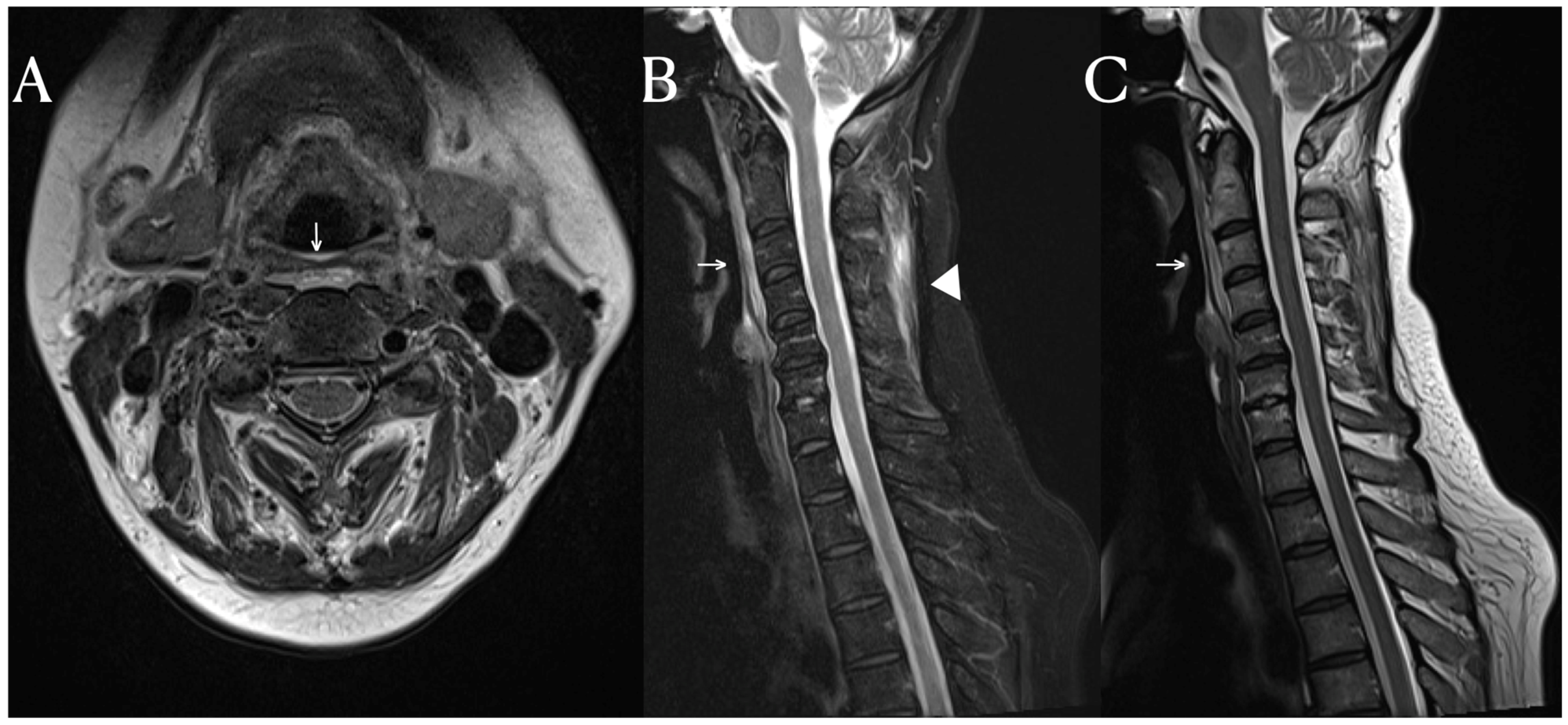
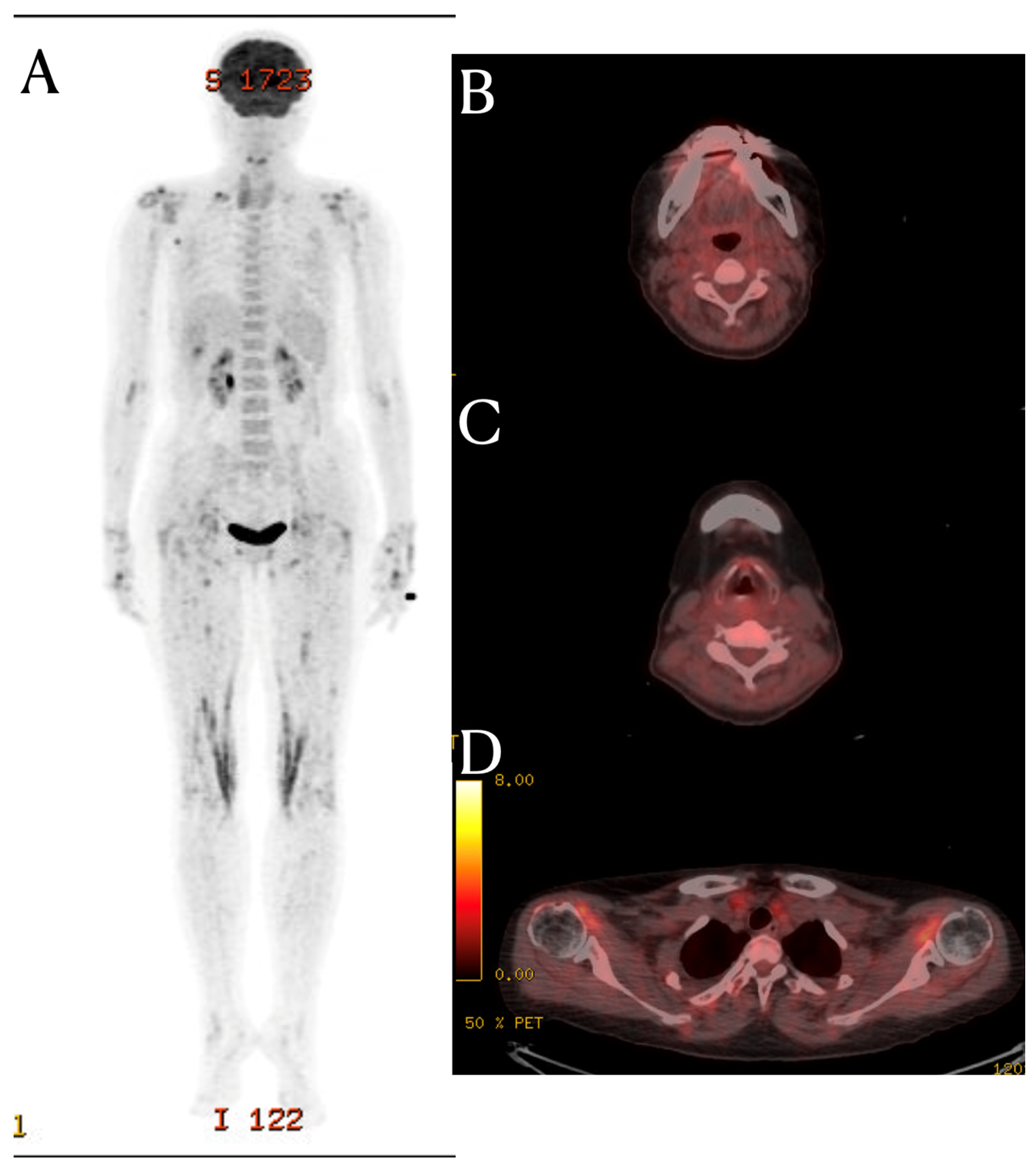
Fascial Signal Change on the Cervical MRI of a Patient with Systemic Lupus Erythematosus
Abstract
:Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.D.; Ibanez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar] [PubMed]
- Anagnostara, A.; Athanassopoulou, A.; Kailidou, E.; Markatos, A.; Eystathidis, A.; Papageorgiou, S. Traumatic retropharyngeal hematoma and prevertebral edema induced by whiplash injury. Emerg. Radiol. 2005, 11, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, T.; Demondion, X.; Stoenoiu, M.; Durez, P.; Nzeusseu Toukap, A.; Houssiau, F.; Galant, C.; Acid, S.; Lecouvet, F.; Malghem, J. Fasciae of the musculoskeletal system: Normal anatomy and MR patterns of involvement in autoimmune diseases. Insights Into Imaging 2018, 9, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; Eshed, I.; McGonagle, D. Lessons Learned from Imaging on Enthesitis in Psoriatic Arthritis. Isr. Med. Assoc. J. IMAJ 2017, 19, 708–711. [Google Scholar] [PubMed]
- Mense, S. Innervation of the thoracolumbar fascia. Eur. J. Transl. Myol. 2019, 29, 8297. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Cass, S.P. Ultrasound-guided nerve hydrodissection: What is it? A review of the literature. Curr. Sports Med. Rep. 2016, 15, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, F.; Gaudreault, N.; Venne, G. The deep fascia and its role in chronic pain and pathological conditions: A review. Clin. Anat. 2022, 35, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in myofascial neck pain: Randomized clinical trial for diagnosis and follow-up. Surg. Radiol. Anat. 2014, 36, 243–253. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Lee, D.G. Fascial Signal Change on the Cervical MRI of a Patient with Systemic Lupus Erythematosus. Diagnostics 2024, 14, 10. https://doi.org/10.3390/diagnostics14010010
Kim H-J, Lee DG. Fascial Signal Change on the Cervical MRI of a Patient with Systemic Lupus Erythematosus. Diagnostics. 2024; 14(1):10. https://doi.org/10.3390/diagnostics14010010
Chicago/Turabian StyleKim, Hyun-Je, and Dong Gyu Lee. 2024. "Fascial Signal Change on the Cervical MRI of a Patient with Systemic Lupus Erythematosus" Diagnostics 14, no. 1: 10. https://doi.org/10.3390/diagnostics14010010
APA StyleKim, H.-J., & Lee, D. G. (2024). Fascial Signal Change on the Cervical MRI of a Patient with Systemic Lupus Erythematosus. Diagnostics, 14(1), 10. https://doi.org/10.3390/diagnostics14010010





