Trends in the Use of Second-Generation Androgen Receptor Axis Inhibitors for Metastatic Hormone-Sensitive Prostate Cancer and Clinical Factors Predicting Biological Recurrence
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Statistical Analysis
3. Results
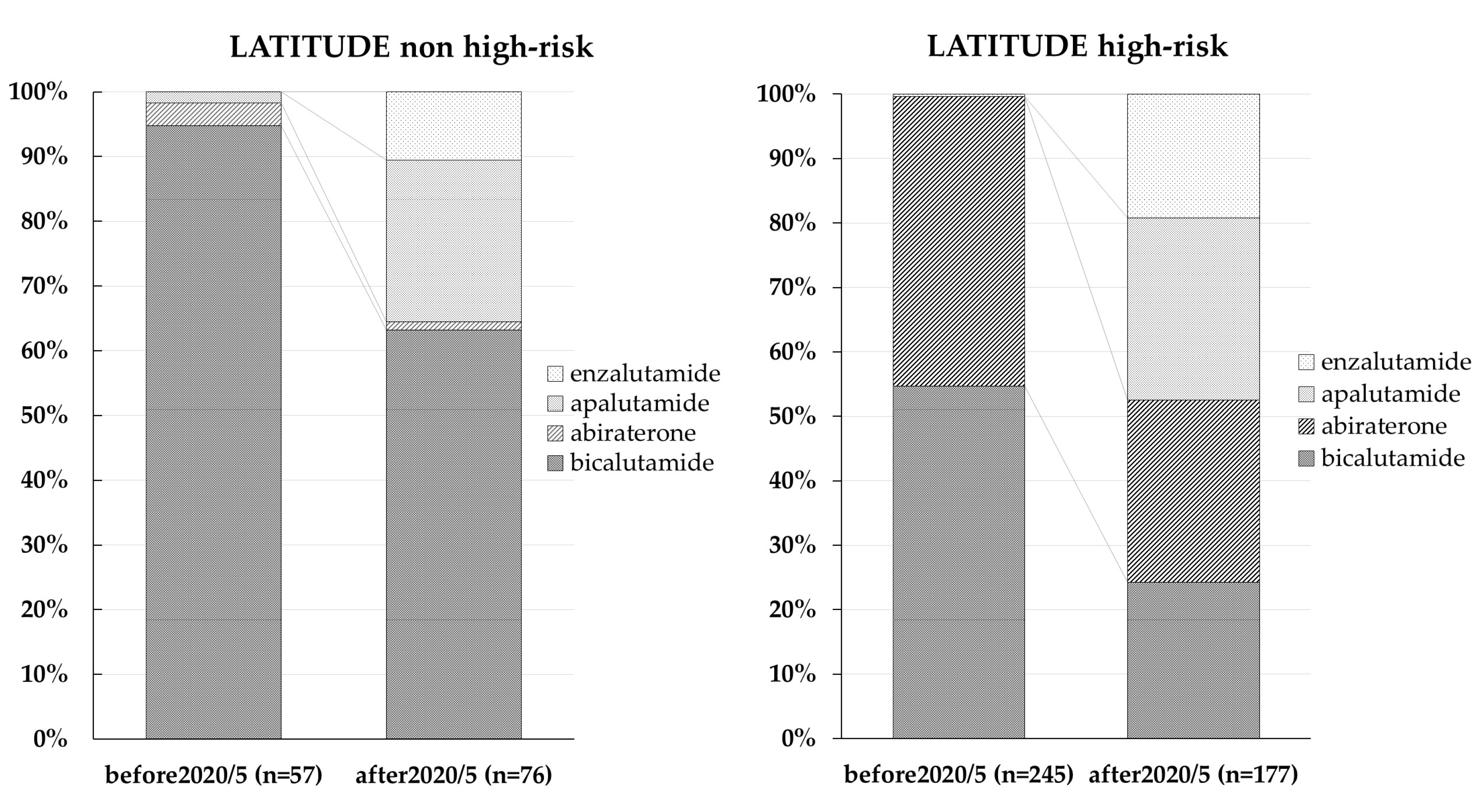
3.1. Trends in ARAT Agent Use
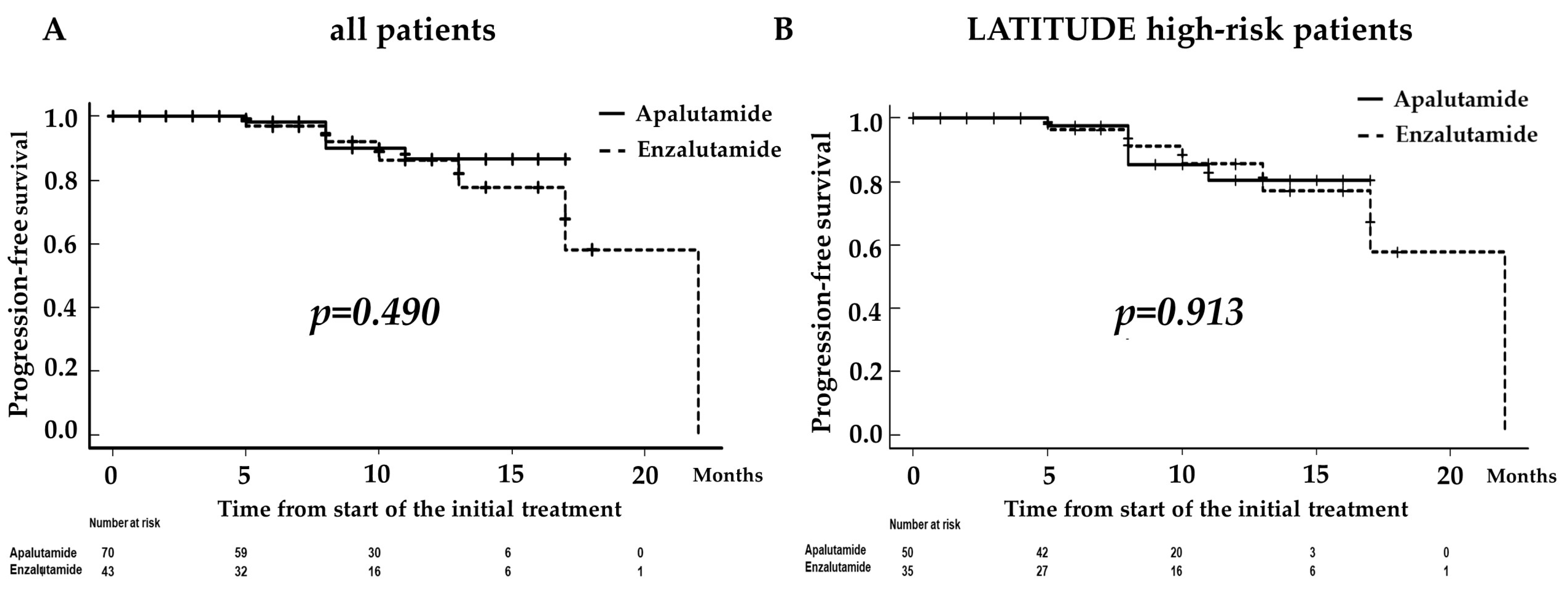
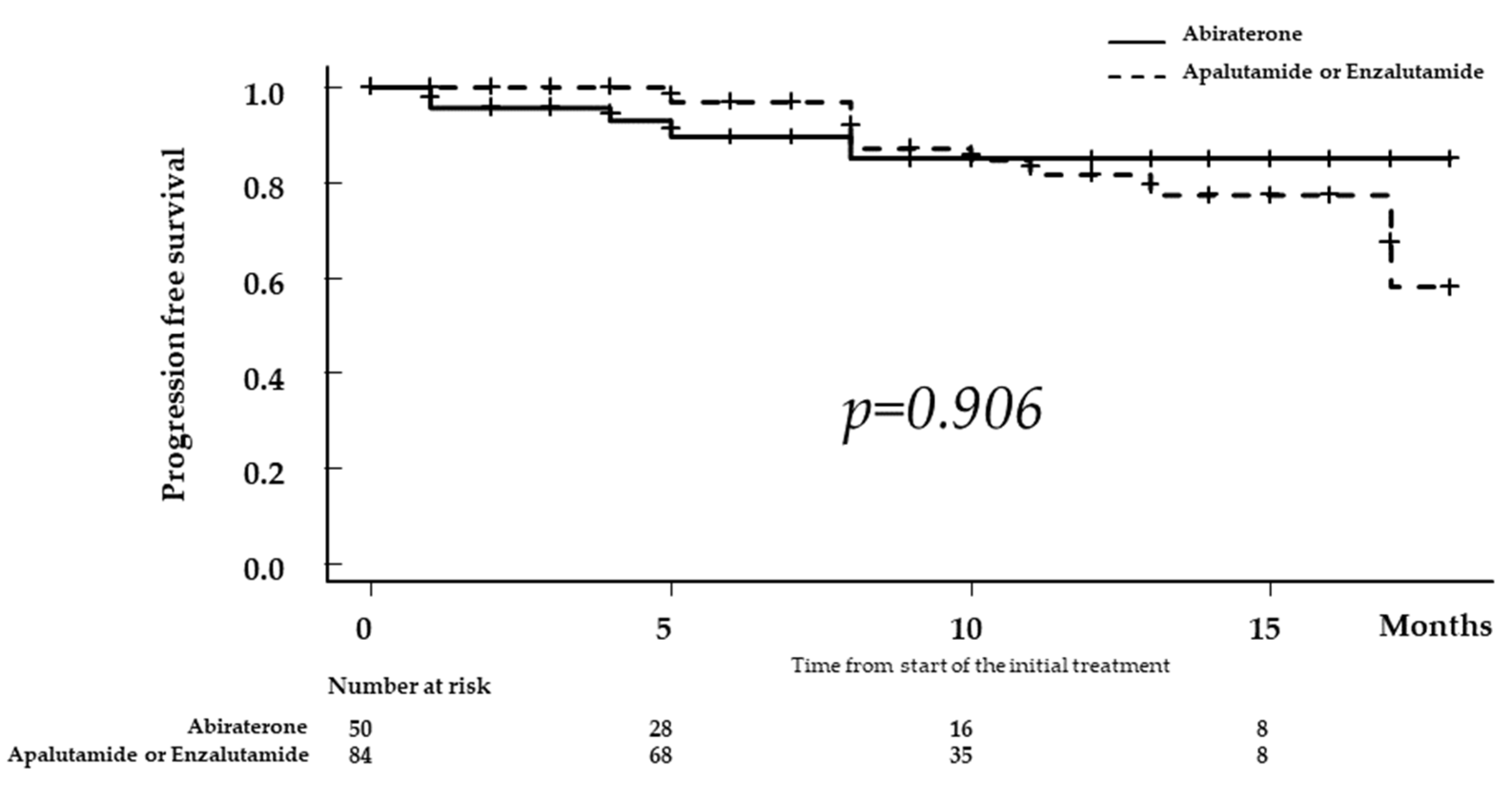
3.2. Comparison of Progression-Free Survival according to ARAT
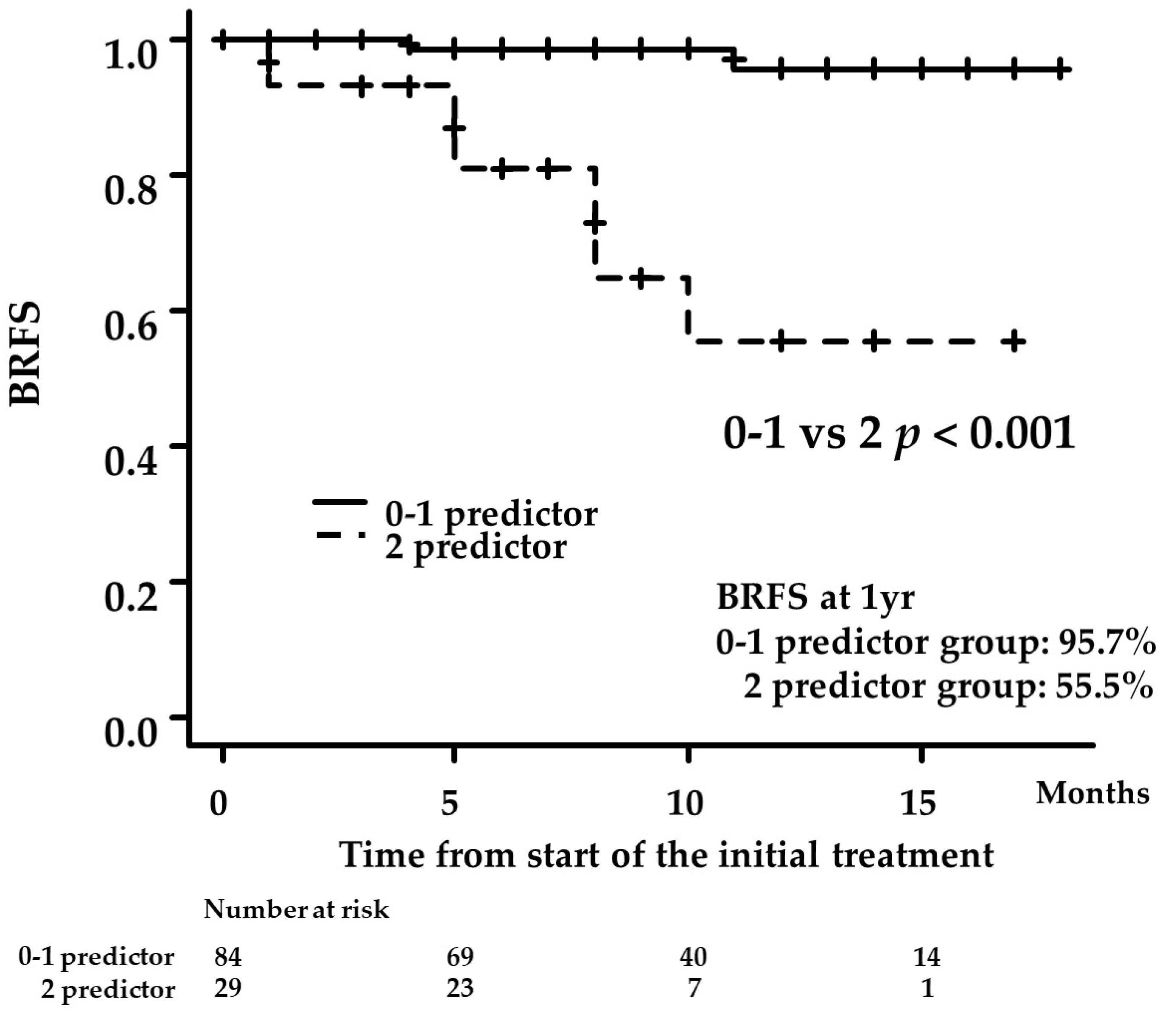
3.3. Uni- and Multivariate Analyses for Factors Predicting BCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akaza, H.; Hinotsu, S.; Usami, M.; Arai, Y.; Kanetake, H.; Naito, S.; Hirao, Y. Combined androgen blockade with bicalutamide for advanced prostate cancer: Long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 2009, 115, 3437–3445. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Jenkins, C.; Tannock, I.F. Should docetaxel be standard of care for patients with metastatic hormone-sensitive prostate cancer? Pro and contra. Ann. Oncol. 2015, 26, 16601667. [Google Scholar] [CrossRef] [PubMed]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Motoyama, D.; Ito, T.; Sugiyama, T.; Otsuka, A. Prognostic Significance of Time to Castration Resistance in Patients with Metastatic Castration-sensitive Prostate Cancer. Anticancer Res. 2019, 39, 1391–1396. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1187. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Takahara, K.; Naiki, T.; Ito, T.; Nakane, K.; Koie, T.; Yasui, T.; Miyake, H.; Shiroki, R. Useful predictors of progression-free survival for Japanese patients with LATITUDE-high-risk metastatic castration-sensitive prostate cancer who received upfront abiraterone acetate. Int. J. Urol. 2022, 29, 229–234. [Google Scholar] [CrossRef]
- Naiki, T.; Takahara, K.; Ito, T.; Nakane, K.; Sugiyama, Y.; Koie, T.; Shiroki, R.; Miyake, H.; Yasui, T. Comparison of clinical outcomes between androgen deprivation therapy with up-front abiraterone and bicalutamide for Japanese patients with LATITUDE high-risk prostate cancer in a real-world retrospective analysis. Int. J. Clin. Oncol. 2022, 27, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Leith, A.; Ribbands, A.; Kim, J.; Clayton, E.; Gillespie-Akar, L.; Yang, L.; Ghate, S.R. Impact of next-generation hormonal agents on treatment patterns among patients with metastatic hormone-sensitive prostate cancer: A real-world study from the United States, five European countries and Japan. BMC Urol. 2022, 22, 33. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients with Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Wang, B.; Luo, G.; Gu, L.; Chen, L.; Liu, K.; Gao, Y.; Zhang, X. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Localized and Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0153981. [Google Scholar] [CrossRef]
- Tomioka-Inagawa, R.; Nakane, K.; Enomoto, T.; Tomioka, M.; Taniguchi, T.; Ishida, T.; Ozawa, K.; Takagi, K.; Ito, H.; Takeuchi, S.; et al. The Impact of Neutrophil-to-Lymphocyte Ratio after Two Courses of Pembrolizumab for Oncological Outcomes in Patients with Metastatic Urothelial Carcinoma. Biomedicines 2022, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, K.; Enomoto, T.; Kawada, K.; Fujimoto, S.; Ishida, T.; Takagi, K.; Nagai, S.; Ito, H.; Kawase, M.; Nakai, C.; et al. Utility of Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Systemic Immune Inflammation Index as Prognostic, Predictive Biomarkers in Patients with Metastatic Renal Cell Carcinoma Treated with Nivolumab and Ipilimumab. J. Clin. Med. 2021, 10, 5325. [Google Scholar] [CrossRef] [PubMed]
- Loubersac, T.; Nguile-Makao, M.; Pouliot, F.; Fradet, V.; Toren, P. Neutrophil-to-lymphocyte Ratio as a Predictive Marker of Response to Abiraterone Acetate: A Retrospective Analysis of the COU302 Study. Eur. Urol. Oncol. 2020, 3, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.D.; Shayegan, B.; Morgan, S.C.; Hamilton, R.J.; Cagiannos, I.; Basappa, N.S.; Ferrario, C.; Gotto, G.T.; Fernandes, R.; Roy, S.; et al. Prognostic Association between Common Laboratory Tests and Overall Survival in Elderly Men with De Novo Metastatic Castration Sensitive Prostate Cancer: A Population-Based Study in Canada. Cancers 2021, 13, 2844. [Google Scholar] [CrossRef]
- Salciccia, S.; Frisenda, M.; Bevilacqua, G.; Viscuso, P.; Casale, P.; De Berardinis, E.; Di Pierro, G.B.; Cattarino, S.; Giorgino, G.; Rosati, D.; et al. Comparative Prospective and Longitudinal Analysis on the Platelet-to-Lymphocyte, Neutrophil-to-Lymphocyte, and Albumin-to-Globulin Ratio in Patients with Non-Metastatic and Metastatic Prostate Cancer. Curr. Oncol. 2022, 29, 9474–9500. [Google Scholar] [CrossRef]
- Matsubara, N.; Chi, K.N.; Özgüroğlu, M.; Rodriguez-Antolin, A.; Feyerabend, S.; Fein, L.; Alekseev, B.Y.; Sulur, G.; Protheroe, A.; Li, S.; et al. Correlation of Prostate-specific Antigen Kinetics with Overall Survival and Radiological Progression-free Survival in Metastatic Castration-sensitive Prostate Cancer Treated with Abiraterone Acetate plus Prednisone or Placebos Added to Androgen Deprivation Therapy: Post Hoc Analysis of Phase 3 LATITUDE Study. Eur. Urol. 2020, 77, 494–500. [Google Scholar]
- Tomioka, A.; Tanaka, N.; Yoshikawa, M.; Miyake, M.; Anai, S.; Chihara, Y.; Okajima, E.; Hirayama, A.; Hirao, Y.; Fujimoto, K. Nadir PSA level and time to nadir PSA are prognostic factors in patients with metastatic prostate cancer. BMC Urol. 2014, 14, 33. [Google Scholar] [CrossRef]
- Koo, K.C.; Park, S.U.; Kim, K.H.; Rha, K.H.; Hong, S.J.; Yang, S.C.; Chung, B.H. Predictors of survival in prostate cancer patients with bone metastasis and extremely high prostate-specific antigen levels. Prostate Int. 2015, 3, 10–15. [Google Scholar] [CrossRef]
| Variables | All | Abiraterone | Apalutamide | Enzalutamide | p-Value |
|---|---|---|---|---|---|
| Number of patients | 276 | 163 | 70 | 43 | |
| Age (years, median, IQR) | 73.0 (68.0–79.0) | 73.0 (68.0–79.0) | 73.5 (69.0–78.7) | 73.0 (65.5–76.0) | 0.575 |
| ECOG-PS (number, %) | 0.792 | ||||
| 0 | 198 (71.7) | 117 (71.8) | 51 (72.9) | 30 (69.8) | |
| 1 | 46 (16.7) | 24 (14.7) | 13 (18.6) | 9 (20.9) | |
| 2 | 27 (9.8) | 19 (11.7) | 5 (7.1) | 3 (7.0) | |
| 3 | 3 (1.1) | 2 (1.2) | 0 (0.0) | 1 (2.3) | |
| 4 | 2 (0.7) | 1 (0.6) | 1 (1.4) | 0 (0.0) | |
| Initial PSA (ng/mL, median, IQR) | 286.5 (68.7–1163.2) | 409.0 (78.8–1656.0) | 185.9 (57.5–598.3) | 380.4 (92.5–1011.9) | 0.05 |
| Gleason score (number, %) | 0.002 | ||||
| ≤6 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 7 | 12 (4.3) | 1 (0.6) | 8 (11.6) | 3 (7.0) | |
| ≥8 | 262 (95.0) | 161 (99.4) | 61 (88.4) | 40 (93.0) | |
| Clinical T stage (number, %) | 0.927 | ||||
| 1c | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 2 | 42 (15.2) | 24 (14.7) | 11 (15.7) | 7 (16.3) | |
| 3 | 134 (48.6) | 81 (49.6) | 35 (50.0) | 18 (41.9) | |
| 4 | 92 (33.3) | 55 (33.7) | 21 (30.0) | 16 (37.2) | |
| x | 8 (2.8) | 3 (1.8) | 3 (4.3) | 2 (4.7) | |
| Lymph node involvement (number, %) | 0.461 | ||||
| Negative | 111 (40.2) | 62 (38.0) | 30 (42.9) | 19 (44.2) | |
| positive | 165 (59.8) | 101 (62.0) | 40 (57.1) | 24 (55.8) | |
| Number of bone metastasis (number, %) | <0.001 | ||||
| 1 | 18 (6.5) | 5 (3.1) | 11 (16.2) | 2 (5.0) | |
| 2 | 11 (3.9) | 2 (1.2) | 7 (10.3) | 2 (5.0) | |
| ≥3 | 225 (81.5) | 144 (88.9) | 45 (66.2) | 36 (90.0) | |
| Visceral metastasis (number, %) | 93 (33.7) | 59 (36.2) | 23 (32.9) | 11 (25.6) | 0.418 |
| Patients who met the criteria for high-risk PCa in the LATITTUDE trial (number, %) | 245 (89.2) | 160 (98.2) | 50 (71.4) | 35 (81.4) | <0.001 |
| WBC (count/μL, median, IQR) | 6680 (5500–7860) | 6600 (5470–7810) | 6690 (5500–8280) | 6800 (5800–7800) | 0.668 |
| Neutrophil (count/μL, median, IQR) | 4100 (3235–5311) | 4100 (3200–5140) | 4200 (3235–5444) | 4000 (3373–5587) | 0.816 |
| Lymphocyte (count/μL, median, IQR) | 1500 (1191–1892) | 1500 (1200–1803) | 1400 (1096–1911) | 1440 (1247–2229) | 0.584 |
| NLR | 2.74 (2.00– 3.90) | 2.73 (2.00–3.82) | 2.77 (1.76–4.49) | 2.54 (1.92–3.38) | 0.860 |
| ALP (U/L, median, IQR) | 275 (174– 605) | 401 (235–923) | 213 (101–310) | 158 (102–299) | <0.001 |
| LDH (U/L, median, IQR) | 204 (178– 243) | 205 (178–248) | 198 (169–229) | 206 (182–242) | 0.397 |
| Albumin (g/dL, median, IQR) | 4.0 (3.6– 4.3) | 3.9 (3.5–4.2) | 4.1 (3.7–4.3) | 4.0 (3.8–4.3) | 0.099 |
| CRP (mg/dL, median, IQR) | 0.23 (0.06–1.06) | 0.26 (0.08–1.27) | 0.20 (0.04–1.15) | 0.19 (0.06–0.81) | 0.487 |
| Follow-up period(months, median, IQR) | 12.0 (6.0–22.5) | 19.0 (8.5–27.0) | 9.0 (7.0–12.0) | 7.0 (4.5–12.5) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (≥73 vs. <73 years) | 3.040 | 0.847–10.900 | 0.087 | - | - | - |
| ECOG-PS (≥1 vs. 0) | 2.140 | 0.741–6.176 | 0.159 | - | - | - |
| Albumin (≥3.8 vs. <3.8 g/dL) | 0.351 | 0.120–1.077 | 0.068 | - | - | - |
| NLR (≥2.76 vs. <2.76) | 11.690 | 1.495–91.480 | 0.019 | 9.700 | 1.2010–78.360 | 0.033 |
| Hemoglobin (≥13.1 vs. <13.1 g/dL) | 0.450 | 0.150–1.344 | 0.153 | - | - | - |
| Gleason score 5 (yes vs. no) | 1.473 | 0.461–4.703 | 0.513 | - | - | - |
| Initial PSA (≥413 vs. <413 ng/mL) | 0.745 | 0.249–2.226 | 0.599 | - | - | - |
| Time to PSA nadir (≥ 6 vs. <6 months) | 0.161 | 0.042–0.602 | 0.007 | - | - | - |
| PSA level 3 months after the start of first-line treatment (≥0.55 vs. <0.55 ng/mL) | 7.191 | 1.608–32.170 | <0.001 | 10.840 | 1.3620–86.360 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakane, K.; Watanabe, H.; Naiki, T.; Takahara, K.; Yasui, T.; Miyake, H.; Shiroki, R.; Koie, T. Trends in the Use of Second-Generation Androgen Receptor Axis Inhibitors for Metastatic Hormone-Sensitive Prostate Cancer and Clinical Factors Predicting Biological Recurrence. Diagnostics 2023, 13, 1661. https://doi.org/10.3390/diagnostics13091661
Nakane K, Watanabe H, Naiki T, Takahara K, Yasui T, Miyake H, Shiroki R, Koie T. Trends in the Use of Second-Generation Androgen Receptor Axis Inhibitors for Metastatic Hormone-Sensitive Prostate Cancer and Clinical Factors Predicting Biological Recurrence. Diagnostics. 2023; 13(9):1661. https://doi.org/10.3390/diagnostics13091661
Chicago/Turabian StyleNakane, Keita, Hiromitsu Watanabe, Taku Naiki, Kiyoshi Takahara, Takahiro Yasui, Hideaki Miyake, Ryoichi Shiroki, and Takuya Koie. 2023. "Trends in the Use of Second-Generation Androgen Receptor Axis Inhibitors for Metastatic Hormone-Sensitive Prostate Cancer and Clinical Factors Predicting Biological Recurrence" Diagnostics 13, no. 9: 1661. https://doi.org/10.3390/diagnostics13091661
APA StyleNakane, K., Watanabe, H., Naiki, T., Takahara, K., Yasui, T., Miyake, H., Shiroki, R., & Koie, T. (2023). Trends in the Use of Second-Generation Androgen Receptor Axis Inhibitors for Metastatic Hormone-Sensitive Prostate Cancer and Clinical Factors Predicting Biological Recurrence. Diagnostics, 13(9), 1661. https://doi.org/10.3390/diagnostics13091661








