Perianal Basal Cell Carcinoma—A Systematic Review and Meta-Analysis of Real-World Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Selection and Quality Assessment
3.2. Clinical Features of the Patients
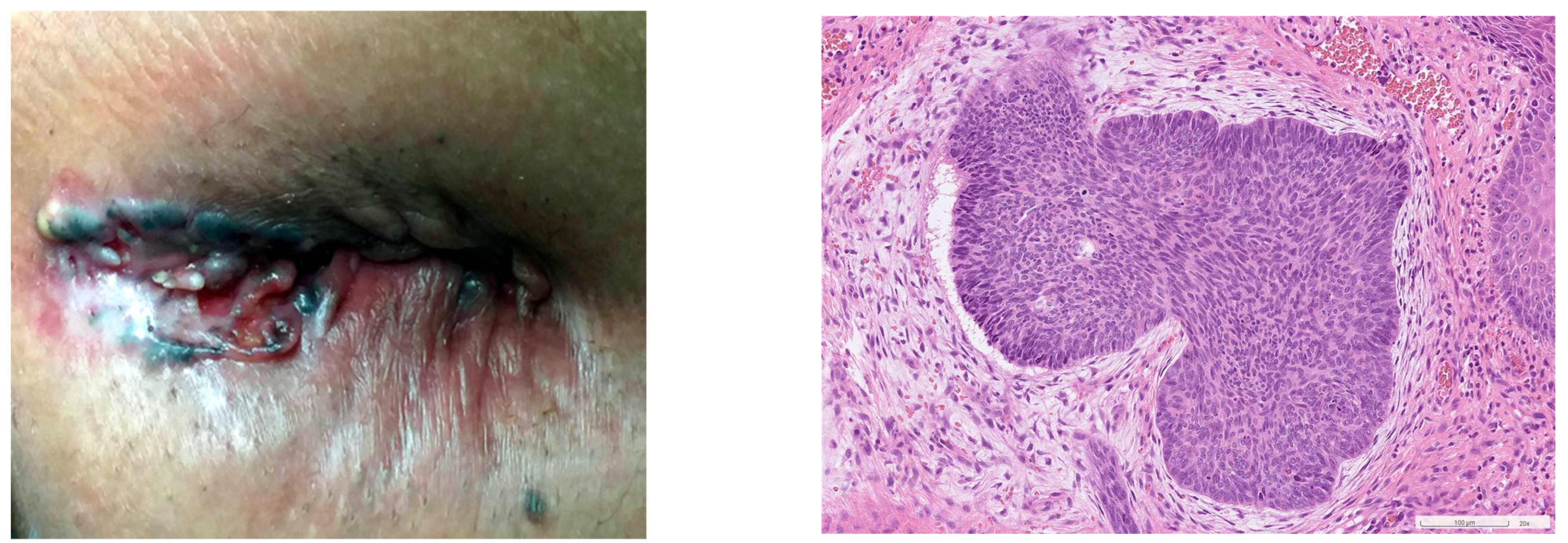
3.3. Characteristics of Perianal BCC
3.4. Diagnosis of Perianal BCC
3.5. Treatment and Outcomes
4. Discussion
4.1. Epidemiology of Perianal BCCs
4.2. Etiology of Perianal BCCs
4.3. Characteristics and Diagnosis of Perianal BCC
4.4. Treatment and Outcomes of Perianal BCC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
| Embase | #1 ‘basal cell carcinoma’/exp OR ‘basal cell carcinoma’ |
| #2 ‘perianal’ OR ‘anus’ | |
| #3 ‘epithelioma’ | |
| #4 ‘basal cell’ | |
| #5 #1 AND #2 | |
| #6 #2 AND #3 AND #4 | |
| #7 #5 OR #6 | |
| Medline (Clarivate Analytics) | (basal cell) AND (carcinoma or epithelioma) AND (perianal or anus or anal) |
| PubMed | #1 ‘basal cell carcinoma’ |
| #2 ‘perianal’ OR ‘anus’ | |
| #3 ‘epithelioma’ | |
| #4 ‘basal cell’ | |
| #5 #1 AND #2 | |
| #6 #2 AND #3 AND #4 | |
| #7 #5 OR #6 |
References
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.I.; Chen, E.H.; Ratner, D. Basal-cell carcinoma. N. Engl. J. Med. 2005, 353, 2262–2269. [Google Scholar] [CrossRef]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Verkouteren, J.; Ramdas, K.; Wakkee, M.; Nijsten, T. Epidemiology of basal cell carcinoma: Scholarly review. Br. J. Dermatol. 2017, 177, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, P.; Olsen, C.; Thompson, B.; Whiteman, D.; Neale, R. Anatomical Distributions of Basal Cell Carcinoma and Squamous Cell Carcinoma in a Population-Based Study in Queensland, Australia. JAMA Dermatol. 2017, 153, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Flohil, S.C.; Seubring, I.; van Rossum, M.M.; Coebergh, J.-W.W.; de Vries, E.; Nijsten, T. Trends in Basal Cell Carcinoma Incidence Rates: A 37-Year Dutch Observational Study. J. Investig. Dermatol. 2013, 133, 913–918. [Google Scholar] [CrossRef]
- Gibson, G.E.; Ahmed, I. Perianal and genital basal cell carcinoma: A clinicopathologic review of 51 cases. J. Am. Acad. Dermatol. 2001, 45, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Bichakjian, C.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; Brown, M.; Busam, K.J.; Eisen, D.B.; Iyengar, V.; Lober, C.; Margolis, D.J.; et al. Guidelines of care for the management of basal cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 540–559. [Google Scholar]
- Patil, D.T.; Goldblum, J.R.; Billings, S.D. Clinicopathological analysis of basal cell carcinoma of the anal region and its distinction from basaloid squamous cell carcinoma. Mod. Pathol. 2013, 26, 1382–1389. [Google Scholar] [CrossRef]
- Micali, G.; Lacarrubba, F.; Nasca, M.R. Advances in the use of topical imiquimod to treat dermatologic disorders. Ther. Clin. Risk Manag. 2008, ume 4, 87–97. [Google Scholar] [CrossRef]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Berg, D.; Bowen, G.M.; Cheney, R.T.; Daniels, G.A.; Glass, L.F.; et al. Basal Cell Skin Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2016, 14, 574–597. [Google Scholar] [CrossRef] [PubMed]
- Keohane, S.G.; Proby, C.M.; Newlands, C.; Motley, R.J.; Nasr, I.; Mustapa, M.F.M.; Slater, D.N. The new 8th edition of TNM staging and its implications for skin cancer: A review by the British Association of Dermatologists and the Royal College of Pathologists, U.K. Br. J. Dermatol. 2018, 179, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Lott, B.D.; Alexander, C.M. Basal cell carcinoma of the anus. Ann. Surg. 1949, 130, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Klippel, A.P.; Tomlinson, W.L. Basal cell carcinoma of the anus: Report of a case. AMA Arch. Surg. 1954, 69, 25–27. [Google Scholar] [CrossRef]
- Manheim, S.D.; Alexander, R.M. Basal cell carcinoma occurring in a fistula-inano. Am. J. Surg. 1955, 90, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Case, T.C.; Caldwell, G.E. Basal cell carcinoma of the anus. Am. J. Surg. 1956, 91, 842–844. [Google Scholar] [CrossRef]
- Bunstock, W. Basal cell carcinoma of the anus. Am. J. Surg. 1958, 95, 822–825. [Google Scholar] [CrossRef]
- Hanley, P.H.; Hines, M.O.; Ray, J.E. Basal cell carcinoma of the anus: Report of three cases. South. Med. J. 1958, 51, 1042–1047. [Google Scholar] [CrossRef]
- Rosenberg, I.; Rosen, S. Basal cell carcinoma of the perianal region. Am. J. Surg. 1958, 95, 1011–1012. [Google Scholar] [CrossRef]
- Turell, R. Basal cell carcinoma: Multiple lesions. Am. J. Surg. 1966, 112, 897–899. [Google Scholar] [CrossRef]
- Kraus, E.W. Perianal basal cell carcinoma. Arch. Dermatol. 1978, 114, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Baruchin, A. Basal cell carcinoma. Dis. Colon. Rectum 1981, 24, 655. [Google Scholar] [CrossRef] [PubMed]
- Espana, A.; Redondo, P.; Idoate, M.A.; Serna, M.J.; Quintanilla, E. Perianal basal cell carcinoma. Clin. Exp. Dermatol. 1992, 17, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Kyzer, S.; Alfandari, C.; Bayer, I.; Gal, R.; Chaimoff, C. Basal cell carcinoma occurring in the perianal region. Plast. Reconstr. Surg. 1992, 89, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Kort, R.; Fazaa, B.; Bouden, S.; Nikkels, A.F.; Pierard, G.E.; Kamoun, M.R. Perianal basal cell carcinoma. Int. J. Dermatol. 1995, 34, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cañas, M.C.; Fernández, F.A.; Rodilla, I.G.; Val-Bernal, J.F. Perianal Basal Cell Carcinoma: A Comparative Histologic, Immunohistochemical, and Flow Cytometric Study with Basaloid Carcinoma of the Anus. Am. J. Dermatopathol. 1996, 18, 371–379. [Google Scholar] [CrossRef]
- Karim, R.B.; Ahmed, A.K.J.; Westerga, J.; Hage, J.J. Pedicled scrotal island skin flap in the treatment of anal basal cell carcinoma. Br. J. Plast. Surg. 2001, 54, 173–176. [Google Scholar] [CrossRef]
- Damin, D.C.; Rosito, M.A.; Gus, P.; Tarta, C.; Weindorfer, M.; Burger, M.B.; Cartell, A. Perianal basal cell carcinoma. J. Cutan. Med. Surg. 2002, 6, 26–28. [Google Scholar] [CrossRef]
- Ruiz-De-Erenchun, R.M.; Rodríguez, N.M.; Hernández, J.L.M.; García-Tutor, E.M. Reconstruction of a perianal defect after basal cell carcinoma using bilateral v-y advancement fasciocutaneous flaps. Plast. Reconstr. Surg. 2003, 111, 1360–1362. [Google Scholar] [CrossRef]
- Misago, N.; Narisawa, Y. Polypoid Basal Cell Carcinoma on the Perianal Region: A Case Report and Review of the Literature. J. Dermatol. 2004, 31, 51–55. [Google Scholar] [CrossRef]
- Naidu, D.N.; Rajakumar, V. Perianal basal cell Carcinoma—An unusual site of occurrence. Indian J. Dermatol. 2010, 55, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.M.; Healy, V.C.; Kavanagh, E.G. A common tumor, an uncommon location. Gastroenterology 2010, 139, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Bechara, F.G.; Stücker, M.; Brockmeyer, N.H.; Altmeyer, P.; Wieland, U. Perianal basal cell carcinoma—Unusual localization of a frequent tumor. JDDG J. Dtsch. Dermatol. Ges. 2011, 10, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Lohana, P.; Creagh, T. Reconstruction for basal cell carcinoma in an anatomical region where the sun does not shine: An attractive option! Ann. R. Coll. Surg. Engl. 2012, 94, 65e–67e. [Google Scholar] [CrossRef]
- Oliphant, T.; Langtry, J.A.A. Mohs micrographic surgery for recurrent perianal basal cell carcinoma. Br. J. Dermatol. 2012, 167, 95. [Google Scholar]
- Yasar, S.; Yasar, B.; Doruk, T.; Abut, E.; Serdar, Z.A. Giant perianal basal cell carcinoma: An uncommon localization: Case report. Turk. Klin. J. Med. Sci. 2012, 32, 1710–1713. [Google Scholar]
- Kahn, S.; Warso, M.A.; Aronson, I.K. A Hyperpigmented Perianal Nodule. Dermatol. Online J. 2013, 19. [Google Scholar] [CrossRef]
- Ng, K.-S.; Stewart, P.; Gladman, M.A. Uncommon site for a common lesion. ANZ J. Surg. 2013, 83, 88–89. [Google Scholar] [CrossRef]
- Bulur, I.; Boyuk, E.; Saracoglu, Z.N.; Arik, D. Perianal Basal Cell Carcinoma. Case Rep. Dermatol. 2015, 7, 25–28. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, S.K. Basal Cell Carcinoma Presenting as a Perianal Ulcer and Treated with Radiotherapy. Ann. Dermatol. 2015, 27, 212–214. [Google Scholar] [CrossRef]
- Rivera-Chavarría, J.P.; Vargas-Villalobos, F.; Riggioni-Víquez, S. Bilateral V-Y flap for a perianal basal cell carcinoma: A case report. Int. J. Surg. Case Rep. 2016, 24, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.V.; Feller, E.; Zakka, F.R.; Griffith, R.C.; Schechter, S. A Case Report of Basal Cell Carcinoma in a Non-Sun-Exposed Area: A Rare Presentation Mimicking Recurrent Perianal Abscess. Case Rep. Surg. 2018, 2018, 9021289. [Google Scholar] [CrossRef]
- Aldana, P.C.; Yfantis, H.G.; John, P.R. Perianal Basal Cell Carcinoma Successfully Managed with Excisional Biopsy. Case Rep. Dermatol. Med. 2019, 2019, 6268354. [Google Scholar] [CrossRef] [PubMed]
- Meeks, M.W.; Grace, S.; Montenegro, G.; Schoen, M.W.; Carpenter, D.; Lai, J.-P.; Poddar, N. Perianal Basal Cell Carcinoma: A Case Report. J. Gastrointest. Cancer 2018, 50, 641–643. [Google Scholar] [CrossRef]
- Hagen, E.R.; Hite, N.; Griffin, J.; Kratz, R. Perianal basal cell carcinoma: A common cancer in an uncommon location. J. Surg. Case Rep. 2020, 2020, rjaa151. [Google Scholar] [CrossRef]
- Sharma, S.; Heerasing, N. A perianal lesion in a patient with chronic diarrhea: The importance of digital rectal examination. J. Gastroenterol. Hepatol. 2020, 35 (Suppl. S1), 203–204. [Google Scholar]
- Imbernón-Moya, A.; Dorado-Fernández, M.; Vargas-Laguna, E. Basal Cell Carcinoma in the Perianal Region: A Challenging Location for Diagnosis. Actas Dermo-Sifiliogr. 2021, 112, 268. [Google Scholar] [CrossRef]
- Lim, M.G.L.; Lopez, M.P.J.; Yu, J.A.O.; Albaño, J.P.P. Perianal basal cell carcinoma managed with wide excision and random flap reconstruction. BMJ Case Rep. 2022, 15, e250493. [Google Scholar] [CrossRef]
- Tung, J.; Lin, B.; Schlenker, J.; Simianu, V.V. A case report of the rarest anal cancer: Basal cell carcinoma. Ann. Med. Surg. 2022, 74, 103291. [Google Scholar] [CrossRef]
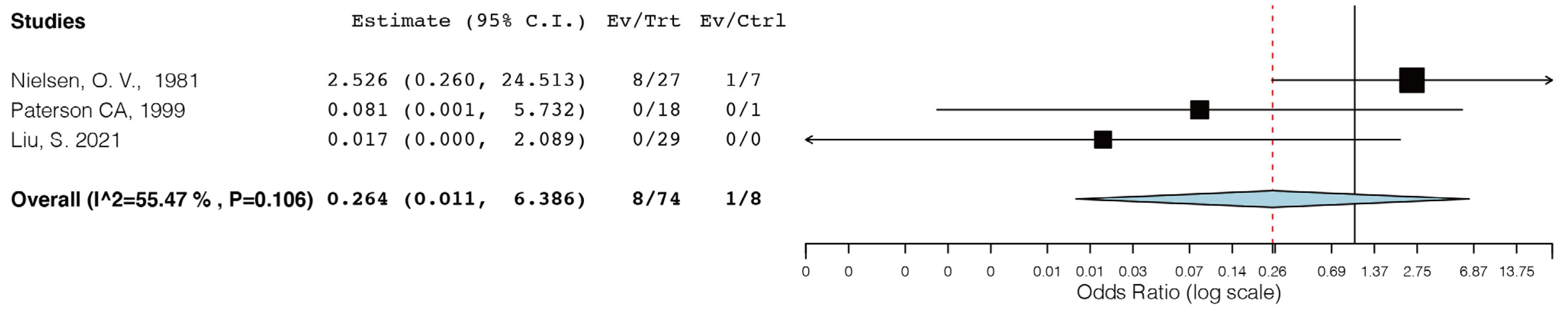
- Nielsen, O.V.; Jensen, S.L. Basal cell carcinoma of the anus—A clinical study of 34 cases. Br. J. Surg. 1981, 68, 856–857. [Google Scholar] [CrossRef]
- Paterson, C.A.; Young-Fadok, T.M.; Dozois, R.R. Basal cell carcinoma of the perianal region: 20-year experience. Dis. Colon. Rectum 1999, 42, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; Mathis, K.L.M.; Graham, R.P.M.; Kelley, S.R.M. Perianal Basal Cell Carcinoma: 35-Year Experience. Dis. Colon. Rectum 2021, 66, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.M.; Coudry, R.; Moniz, C.M.V. Pathology of Anal Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.A.; Suk, R.; Shiels, M.S.; Sonawane, K.; Nyitray, A.G.; Liu, Y.; Gaisa, M.M.; Palefsky, J.M.; Sigel, K. Recent Trends in Squamous Cell Carcinoma of the Anus Incidence and Mortality in the United States, 2001–2015. J. Natl. Cancer Inst. 2020, 112, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Flohil, S.C.; van der Leest, R.J.; Arends, L.R.; de Vries, E.; Nijsten, T. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: A systematic review and meta-analysis. Eur. J. Cancer 2013, 49, 2365–2375. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B Biol. 2001, 63, 8–18. [Google Scholar] [CrossRef]
- Noodleman, F.R.; Pollack, S.V. Trauma as a Possible Etiologic Factor in Basal Cell Carcinoma. J. Dermatol. Surg. Oncol. 1986, 12, 841–846. [Google Scholar] [CrossRef]
- Crowson, A.N. Basal cell carcinoma: Biology, morphology and clinical implications. Mod. Pathol. 2006, 19, S127–S147. [Google Scholar] [CrossRef]
- Steele, S.R.; Hull, T.L.; Hyman, N.; Maykel, J.A.; Read, T.E.; Whitlow, C.B. (Eds.) The ASCRS Textbook of Colon and Rectal Surgery; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Kang, K.W.; Lee, D.L.; Shin, H.K.; Jung, G.Y.; Lee, J.H.; Jeon, M.S. A Retrospective Clinical View of Basal Cell Carcinoma and Squamous Cell Carcinoma in the Head and Neck Region: A Single Institution’s Experience of 247 Cases over 19 Years. Arch. Craniofac. Surg. 2016, 17, 56–62. [Google Scholar] [CrossRef]
- Quazi, S.J.; Aslam, N.; Saleem, H.; Rahman, J.; Khan, S. Surgical Margin of Excision in Basal Cell Carcinoma: A Systematic Review of Literature. Cureus 2020, 12, e9211. [Google Scholar] [CrossRef]
- Van Loo, E.; Mosterd, K.; Krekels, G.A.; Roozeboom, M.H.; Ostertag, J.U.; Dirksen, C.D.; Steijlen, P.M.; Neumann, H.M.; Nelemans, P.J.; Kelleners-Smeets, N.W. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: A randomised clinical trial with 10 year follow-up. Eur. J. Cancer 2014, 50, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.K.; Kopf, A.W.; Gladstein, A.H.; Bart, R.S.; Grin, C.M.; Levenstein, M.J. Recurrence rates of treated basal cell carcinomas. Part 4: X-ray therapy. J. Dermatol. Surg. Oncol. 1992, 18, 549–554. [Google Scholar] [CrossRef] [PubMed]


| Year | Author | Sample Size | Study Type | Gender | Age/Mean or Median (Range) | Quality Score |
|---|---|---|---|---|---|---|
| 1949 | Lott, B.D. [13] | 1 | Case report | F | 62 | 50% |
| 1954 | Klippel [14] | 1 | Case report | M | 65 | 50% |
| 1955 | Manheim, S.D. [15] | 1 | Case report | M | 72 | 50% |
| 1956 | Case, T.C. [16] | 1 | Case report | F | 72 | 63% |
| 1958 | Bunstock, W.H. [17] | 1 | Case report | M | 93 | 40% |
| 1958 | Hanley, P.H. [18] | 3 | Case report | 1M2F | 72, 68, 65 | 53% |
| 1958 | Rosenberg and Rosen [19] | 1 | Case report | F | 49 | 37% |
| 1966 | Turell, R. [20] | 1 | Case report | M | not reported | 53% |
| 1978 | Kraus [21] | 1 | Case report | F | 82 | 53% |
| 1981 | Baruchin [22] | 1 | Letter to the editor | F | 64 | 23% |
| 1992 | Espana, A. [23] | 1 | Case report | F | 72 | 47% |
| 1992 | Kyzer, S. [24] | 1 | Letter to the editor | F | 33 | 37% |
| 1995 | Kort [25] | 1 | Case report | M | 88 | 23% |
| 1996 | Alvarez-Cañas [26] | 5 | Case report | 1M4F | 62.8 (36–84) | 53% |
| 2001 | Karim, R. [27] | 1 | Case report | M | 83 | 53% |
| 2002 | Damin, D.C. [28] | 1 | Case report | F | 77 | 47% |
| 2003 | Ruiz-de-Erenchun, R. [29] | 1 | Letter to the editor | M | 90 | 33% |
| 2004 | Misago, N. [30] | 1 | Case report | F | 88 | 53% |
| 2010 | Naidu, N. [31] | 1 | Case report | M | 69 | 60% |
| 2010 | Shaikh, F.M. [32] | 1 | Image challenge | M | 72 | 43% |
| 2012 | Kreuter, A. [33] | 1 | Case report | M | 88 | 53% |
| 2012 | Lohana, P. [34] | 1 | Case report | M | 58 | 53% |
| 2012 | Oliphant, T. [35] | 1 | Conference abstract | F | 82 | 43% |
| 2012 | Yasar, S [36] | 1 | Case report | F | 50 | 53% |
| 2013 | Kahn, S. [37] | 1 | Case report | F | 62 | 53% |
| 2013 | Ng, K.-S. [38] | 1 | Case report | M | 80 | 33% |
| 2015 | Bulur, I. [39] | 1 | Case report | M | 34 | 60% |
| 2015 | Lee, H.S. [40] | 1 | Letter to the editor | M | 83 | 50% |
| 2016 | Rivera-Chavarrí [41] | 1 | Case report | F | 93 | 73% |
| 2018 | Carr, A.V. [42] | 1 | Case report | M | 66 | 80% |
| 2019 | Aldana, P.C. [43] | 1 | Case report | M | 89 | 53% |
| 2019 | Meeks, M. [44] | 1 | Case report | M | 49 | 50% |
| 2020 | Hagen, E.R. [45] | 1 | Case report | M | 67 | 57% |
| 2020 | Sharma, S. [46] | 1 | Conference abstract | F | 73 | 43% |
| 2021 | Imbernon-Moya, A. [47] | 1 | Case report | F | 68 | 43% |
| 2022 | Lim, M.G. [48] | 1 | Case report | F | 60 | 70% |
| 2022 | Tung, J. [49] | 1 | Case report | M | 69 | 90% |
| 1981 | Nielsen, O.V. [50] | 34 | Retrospective study | 18M16F | 68 (43–86) | 64% |
| 1999 | Paterson, C.A. [51] | 19 | Retrospective study | 15M4F | 67(43–81) | 64% |
| 2001 | Gibson [7] | 15 | Retrospective study | 9M6F | 73(45–100) | 64% |
| 2021 | Liu, S. [52] | 29 | Retrospective study | 23M6F | 70 (43–90) | 73% |
| Mean | |
|---|---|
| Age at diagnosis (n = 124) | 68.8 ± 8.9 |
| Gender (n = 140) | |
| Male | 86 (61.4) |
| Female | 54 (38.6) |
| Clinical presentation at diagnosis | Reported cases (% out of 70) |
| No symptoms | 9 (12.9) |
| Pain | 21 (30.0) |
| Bleeding | 24 (24.3) |
| Pruritus | 13 (18.6) |
| Change in bowel habits | 9 (12.9) |
| Anal discharge | 3 (4.3) |
| History of BCCs at other anastomotic sites | Reported cases (% out of 70) |
| Yes | 19 (27.1) |
| No | 51 (72.9) |
| History of other anal diseases | Reported cases (% out of 70) |
| Yes | 6 (8.6) |
| No | 64 (91.4) |
| Tumor Morphology | n (% Out of 42) |
|---|---|
| Polypoid/Nodular | 2 (4.8) |
| Pedunculated | 1 (2.4) |
| Induration | 6 (14.3) |
| Ulceration | 28 (66.7) |
| Fungating | 4 (9.5) |
| Raised edge | 16 (38.1) |
| Hyper-/Hypopigmentation | 13 (31.0) |
| Tumor size | n (% out of 37) |
| ≤20 mm | 10 (27.0) |
| >20 mm to ≤40 mm | 14 (37.8) |
| >40 mm | 13 (35.1) |
| Tumor stage | n (% out of 37) |
| Stage I | 10 (27.0) |
| Stage II | 13 (35.1) |
| Stage III | 14 (37.8) |
| Stage IV | 0 (0) |
| Tumor location | n (% of 69) |
| Anterior | 5 (7.2) |
| Right anterior | 3 (4.3) |
| Right | 9 (13.0) |
| Right posterior | 3 (4.3) |
| Posterior | 19 (27.5) |
| Left posterior | 1 (1.4) |
| Left | 26 (37.7) |
| Left anterior | 1 (1.4) |
| Circumferential | 2 (2.9) |
| Sphincter invasion | n (% of 140) |
| Yes | 4 (2.9) |
| No | 30 (21.4) |
| Not reported | 106 (75.7) |
| Studies | Case Number | Treatment | Follow Up Times | Outcomes |
|---|---|---|---|---|
| Nielsen, O.V., 1981 [50] | 34 | Local excisions (n = 27) APR (n = 4) Colostomy (n = 1) | Not reported | Five-year OS 72.6%. Eight (23%) patients had a recurrence after local excision. One patient had an inguinal recurrence. Sixteen mortalities of other causes. |
| Paterson C.A., 1999 [51] | 19 | Local excisions (n = 17) Mohs microsurgery (n = 1) Electrodesiccation (n = 1) | 72 months (2–214) | No recurrence. Four mortalities of other causes. |
| Gibson, 2001 [7] | 51 (15 perianal, 36 genital) | Wide excision (n = 32) Electrodesiccation and curettage (n = 10), Mohs micrographic surgery (n = 8) Carbon dioxide laser (n = 1) | 5 years or more | One recurrence of a superficial BCC of the vulva. |
| Liu, S., 2021 [52] | 29 | Local excisions (n = 29) | 5.5 ± 4.6 years | No recurrence. No mortality. |
| n | Recurrence | p Value | |
|---|---|---|---|
| Radiation therapy only | 5 | 0 | 0.94 |
| Radiation therapy followed by cryotherapy | 1 | 0 | |
| Electrodesiccation | 1 | 0 | |
| Local excision only | 102 | 8 | |
| Local excision plus adjuvant RT | 4 | ||
| Radiation therapy followed by Mohs microscopic surgery | 1 | 0 | |
| Mohs microscopic surgery | 3 | 0 | |
| Radiation therapy followed by APR | 1 | 0 | |
| APR | 5 | 0 | |
| Others | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, T.-Y.; Liao, C.-K.; Zhang, B.-Y.; Huang, Y.-L.; Tsai, W.-S.; You, J.-F.; Yeh, C.-Y.; Hsieh, P.-S. Perianal Basal Cell Carcinoma—A Systematic Review and Meta-Analysis of Real-World Data. Diagnostics 2023, 13, 1650. https://doi.org/10.3390/diagnostics13091650
Tsai T-Y, Liao C-K, Zhang B-Y, Huang Y-L, Tsai W-S, You J-F, Yeh C-Y, Hsieh P-S. Perianal Basal Cell Carcinoma—A Systematic Review and Meta-Analysis of Real-World Data. Diagnostics. 2023; 13(9):1650. https://doi.org/10.3390/diagnostics13091650
Chicago/Turabian StyleTsai, Tzong-Yun, Chun-Kai Liao, Bang-Yan Zhang, Yen-Lin Huang, Wen-Sy Tsai, Jeng-Fu You, Chien-Yuh Yeh, and Pao-Shiu Hsieh. 2023. "Perianal Basal Cell Carcinoma—A Systematic Review and Meta-Analysis of Real-World Data" Diagnostics 13, no. 9: 1650. https://doi.org/10.3390/diagnostics13091650
APA StyleTsai, T.-Y., Liao, C.-K., Zhang, B.-Y., Huang, Y.-L., Tsai, W.-S., You, J.-F., Yeh, C.-Y., & Hsieh, P.-S. (2023). Perianal Basal Cell Carcinoma—A Systematic Review and Meta-Analysis of Real-World Data. Diagnostics, 13(9), 1650. https://doi.org/10.3390/diagnostics13091650