The GRPR Antagonist [99mTc]Tc-maSSS-PEG2-RM26 towards Phase I Clinical Trial: Kit Preparation, Characterization and Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
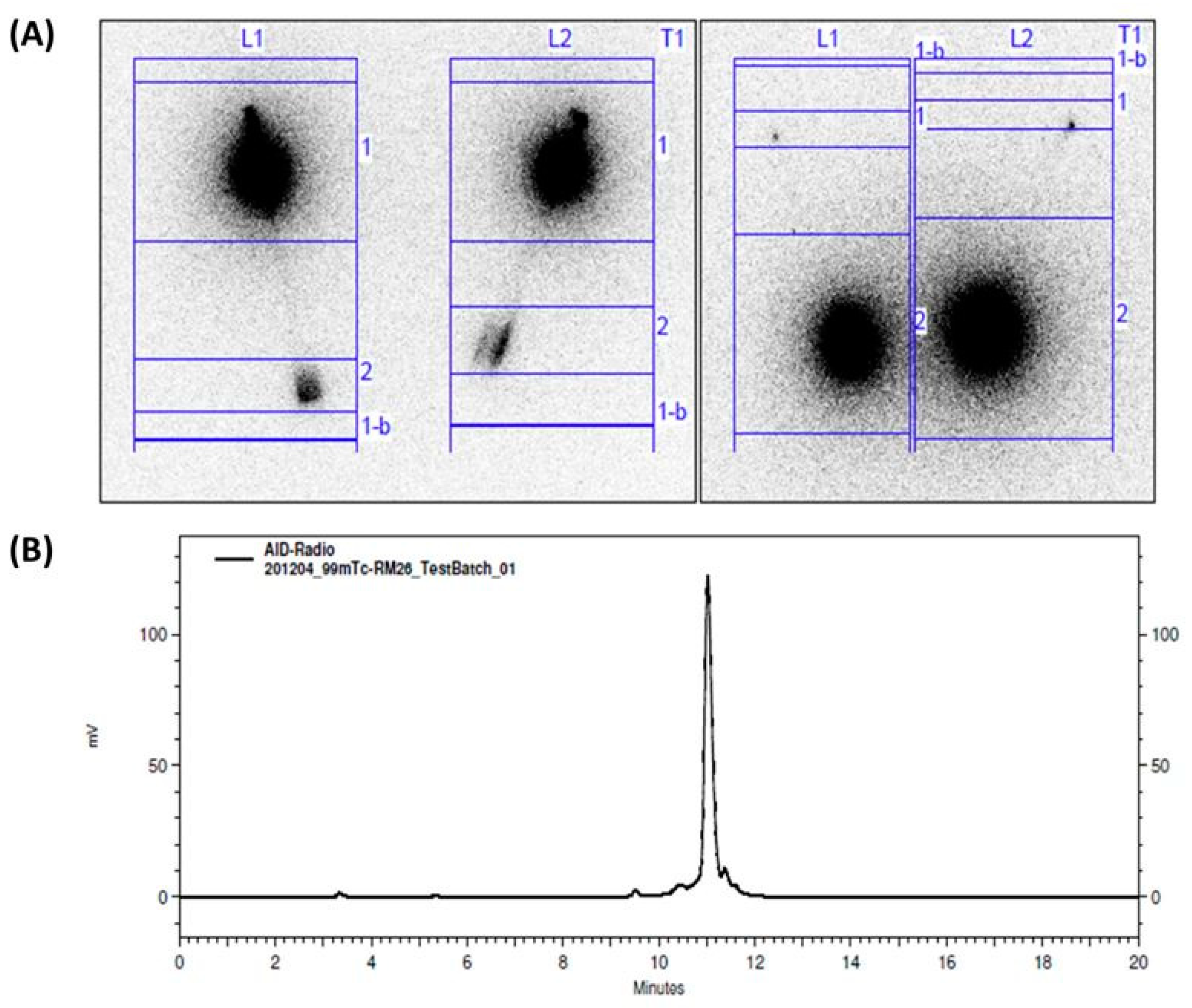
2.2. Kit Preparation and Radiolabeling
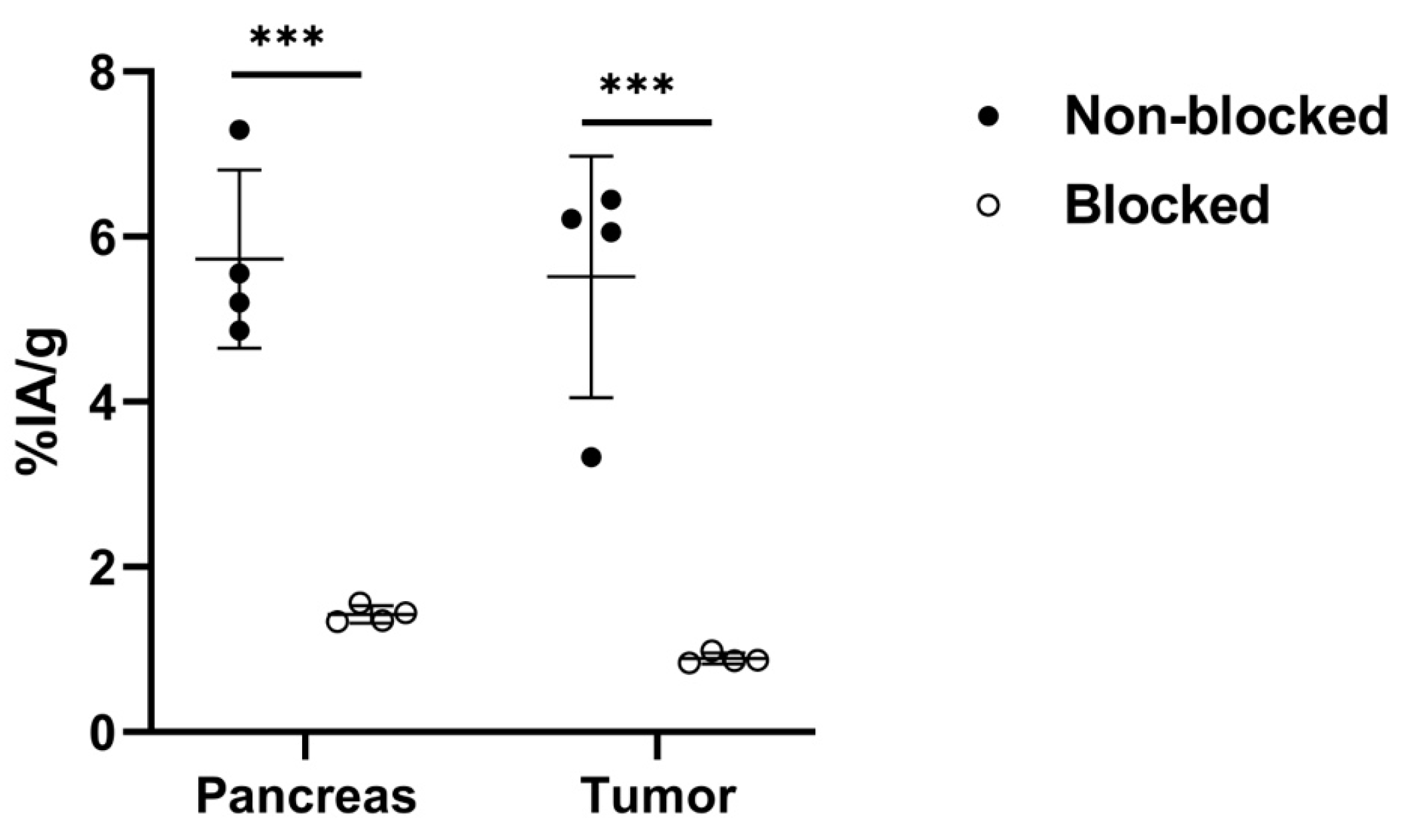
2.3. Cell Binding
2.4. Validation of Kit Sterility
2.5. Toxicity Study
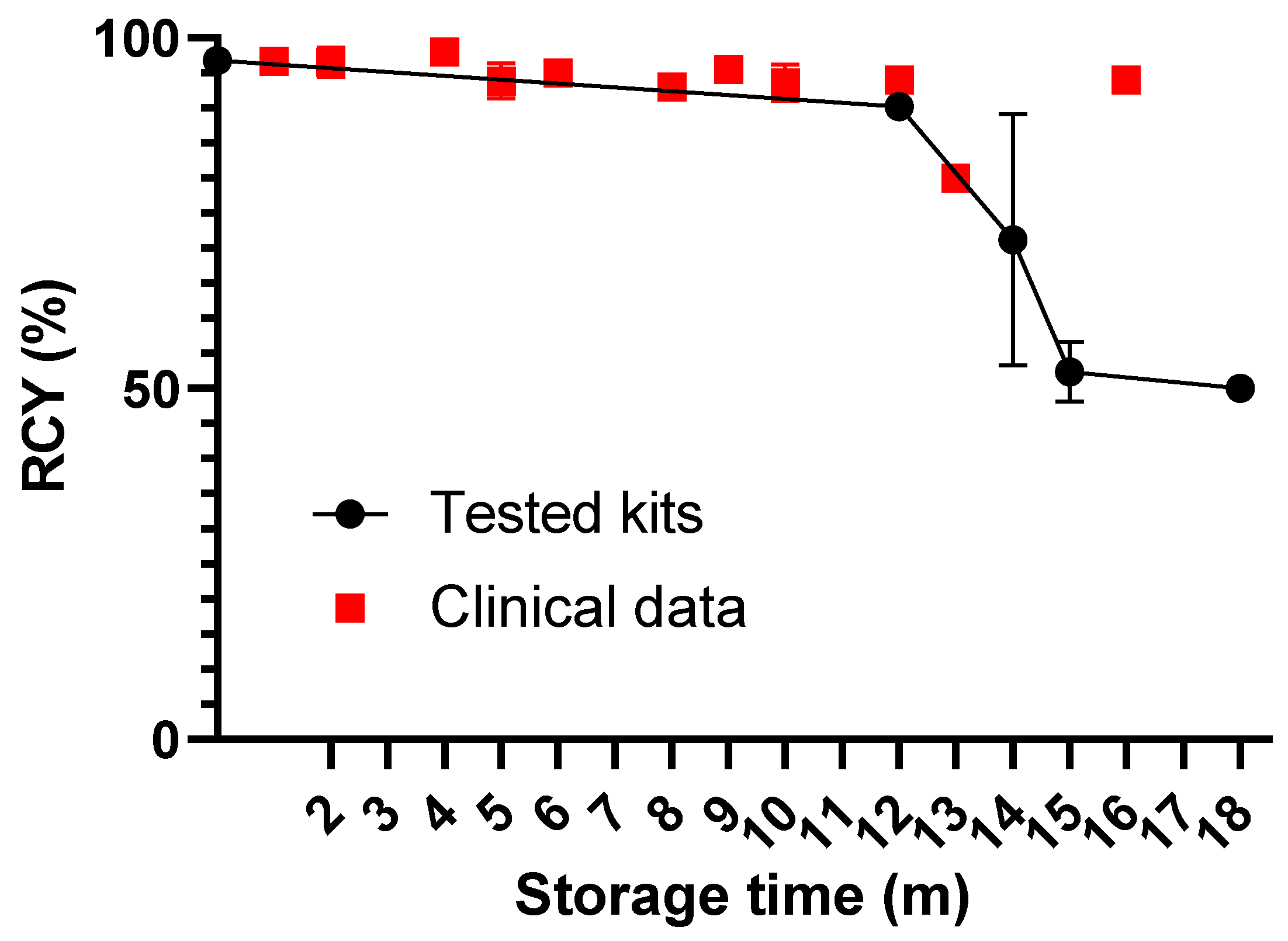
2.6. Radiolabeling, In Vitro, and In Vivo Evaluation after Long-Term Storage
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Organ | Non-Blocked | Tumor-to-Non-Tumor Ratios for Non-Blocked Group | Blocked |
|---|---|---|---|
| Blood | 0.3 ± 0.2 | 30 ± 4 | 0.3 ± 0.4 |
| Liver | 2.9 ± 0.8 | 2.0 ± 0.4 | 1.7 ± 0.4 |
| Spleen | 0.13 ± 0.05 | 51 ± 18 | 0.13 ± 0.06 |
| Pancreas | 6 ± 1 | 1.1 ± 0.2 | 1.4 ± 0.1 |
| Kidneys | 5.0 ± 0.4 | 1.3 ± 0.2 | 5.0 ± 0.6 |
| Tumor | 6 ± 1 | ––– | 0.89 ± 0.07 |
| Muscle | 0.05 ± 0.02 | 125 ± 50 | 0.032 ± 0.008 |
| Bone | 0.09 ± 0.05 | 78 ± 42 | 0.042 ± 0.009 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Barve, A.; Jin, W.; Cheng, K. Prostate Cancer Relevant Antigens and Enzymes for Targeted Drug Delivery. J. Control. Release 2014, 187, 118–132. [Google Scholar] [CrossRef]
- Tolkach, Y.; Gevensleben, H.; Bundschuh, R.; Koyun, A.; Huber, D.; Kehrer, C.; Hecking, T.; Keyver-Paik, M.-D.; Kaiser, C.; Ahmadzadehfar, H.; et al. Prostate-Specific Membrane Antigen in Breast Cancer: A Comprehensive Evaluation of Expression and a Case Report of Radionuclide Therapy. Breast Cancer Res. Treat. 2018, 169, 447–455. [Google Scholar] [CrossRef]
- Moreno, P.; Ramos-Álvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin Related Peptides/Receptors and Their Promising Therapeutic Roles in Cancer Imaging, Targeting and Treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot Comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2016, 57, 557–562. [Google Scholar] [CrossRef]
- Sun, B.; Halmos, G.; Schally, A.V.; Wang, X.; Martinez, M. Presence of Receptors for Bombesin/ Gastrin-Releasing Peptide and MRNA for Three Receptor Subtypes in Human Prostate Cancers. Prostate 2000, 42, 295–303. [Google Scholar] [CrossRef]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.-C.; Gugger, M. Bombesin Receptor Subtypes in Human Cancers: Detection with the Universal Radioligand 125I-[D-TYR6, β-ALA11, PHE13, NLE14] Bombesin(6–14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar]
- Ananias, H.J.K.; van den Heuvel, M.C.; Helfrich, W.; de Jong, I.J. Expression of the Gastrin-Releasing Peptide Receptor, the Prostate Stem Cell Antigen and the Prostate-Specific Membrane Antigen in Lymph Node and Bone Metastases of Prostate Cancer. Prostate 2009, 69, 1101–1108. [Google Scholar] [CrossRef]
- Markwalder, R.; Reubi, J.C. Gastrin-Releasing Peptide Receptors in the Human Prostate: Relation to Neoplastic Transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar]
- Pansky, A.; De Weerth, A.; Fasler-Kan, E.; Boulay, J.-L.; Schulz, M.; Ketterer, S.; Selck, C.; Beglinger, C.; Von Schrenck, T.; Hildebrand, P. Gastrin Releasing Peptide-Preferring Bombesin Receptors Mediate Growth of Human Renal Cell Carcinoma. J. Am. Soc. Nephrol. 2000, 11, 1409–1418. [Google Scholar] [CrossRef]
- Millar, J.B.; Rozengurt, E. Chronic Desensitization to Bombesin by Progressive Down-Regulation of Bombesin Receptors in Swiss 3T3 Cells. Distinction from Acute Desensitization. J. Biol. Chem. 1990, 265, 12052–12058. [Google Scholar] [CrossRef]
- Cescato, R.; Maina, T.; Nock, B.; Nikolopoulou, A.; Charalambidis, D.; Piccand, V.; Reubi, J.C. Bombesin Receptor Antagonists May Be Preferable to Agonists for Tumor Targeting. J. Nucl. Med. 2008, 49, 318–326. [Google Scholar] [CrossRef]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef]
- IAEA. IMAGINE—Medical ImAGIng and Nuclear MEdicine Global Resources Database. Available online: https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINEMaps.html (accessed on 5 April 2023).
- Eurostat. Healthcare Resource Statistics-Technical Resources and Medical Technology. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Healthcare_resource_statistics_technical_resources_and_medical_technology&oldid=460272#Availability_of_technical_resources_in_hospitals (accessed on 5 April 2023).
- Abiraj, K.; Mansi, R.; Tamma, M.-L.; Forrer, F.; Cescato, R.; Reubi, J.C.; Akyel, K.G.; Maecke, H.R. Tetraamine-Derived Bifunctional Chelators for Technetium-99m Labelling: Synthesis, Bioconjugation and Evaluation as Targeted SPECT Imaging Probes for GRP-Receptor-Positive Tumours. Chem. Eur. J. 2010, 16, 2115–2124. [Google Scholar] [CrossRef]
- Nock, B.; Nikolopoulou, A.; Chiotellis, E.; Loudos, G.; Maintas, D.; Reubi, J.; Maina, T. [99mTc]Demobesin 1, a Novel Potent Bombesin Analogue for GRP Receptor-Targeted Tumour Imaging. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 247–258. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Kanellopoulos, P.; Janota, B.; Bromińska, B.; Iżycki, D.; Mikołajczak, R.; Czepczynski, R.; Maina, T. [99mTc]Tc-DB15 in GRPR-Targeted Tumor Imaging with SPECT: From Preclinical Evaluation to the First Clinical Outcomes. Cancers 2021, 13, 5093. [Google Scholar] [CrossRef]
- Abouzayed, A.; Rinne, S.S.; Sabahnoo, H.; Sörensen, J.; Chernov, V.; Tolmachev, V.; Orlova, A. Preclinical Evaluation of 99mTc-Labeled GRPR Antagonists MaSSS/SES-PEG2-RM26 for Imaging of Prostate Cancer. Pharmaceutics 2021, 13, 182. [Google Scholar] [CrossRef]
- Llinares, M.; Devin, C.; Chaloin, O.; Azay, J.; Noel-Artis, A.M.; Bernad, N.; Fehrentz, J.A.; Martinez, J. Syntheses and Biological Activities of Potent Bombesin Receptor Antagonists: Bombesin Receptor Antagonists. J. Pept. Res. 1999, 53, 275–283. [Google Scholar] [CrossRef]
- Ahlgren, S.; Andersson, K.; Tolmachev, V. Kit Formulation for 99mTc-Labeling of Recombinant Anti-HER2 Affibody Molecules with a C-Terminally Engineered Cysteine. Nucl. Med. Biol. 2010, 37, 539–546. [Google Scholar] [CrossRef]
- Engfeldt, T.; Tran, T.; Orlova, A.; Widström, C.; Feldwisch, J.; Abrahmsen, L.; Wennborg, A.; Karlström, A.E.; Tolmachev, V. 99mTc-Chelator Engineering to Improve Tumour Targeting Properties of a HER2-Specific Affibody Molecule. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Noto, B.; Auf der Springe, K.; Huss, S.; Allkemper, T.; Stegger, L. Prostate-Specific Membrane Antigen–Negative Metastases—A Potential Pitfall in Prostate-Specific Membrane Antigen PET. Clin. Nucl. Med. 2018, 43, e186–e188. [Google Scholar] [CrossRef]
- Sasikumar, A.; Joy, A.; Nanabala, R.; Pillai, M.R.A.; Hari, T.A. 68Ga-PSMA PET/CT False-Positive Tracer Uptake in Paget Disease. Clin. Nucl. Med. 2016, 41, e454–e455. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Bode, A.; Weckesser, M.; Avramovic, N.; Claesener, M.; Stegger, L.; Bögemann, M. Radioligand Therapy With 177Lu-PSMA-617 as A Novel Therapeutic Option in Patients With Metastatic Castration Resistant Prostate Cancer. Clin. Nucl. Med. 2016, 41, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Chernov, V.; Rybina, A.; Zelchan, R.; Medvedeva, A.; Bragina, O.; Lushnikova, N.; Doroshenko, A.; Usynin, E.; Tashireva, L.; Vtorushin, S.; et al. Phase I Trial of [99mTc]Tc-MaSSS-PEG2-RM26, a Bombesin Analogue Antagonistic to Gastrin-Releasing Peptide Receptors (GRPRs), for SPECT Imaging of GRPR Expression in Malignant Tumors. Cancers 2023, 15, 1631. [Google Scholar] [CrossRef]
- Beer, M.; Montani, M.; Gerhardt, J.; Wild, P.J.; Hany, T.F.; Hermanns, T.; Müntener, M.; Kristiansen, G. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate 2012, 72, 318–325. [Google Scholar] [CrossRef]
- Dalm, S.U.; Martens, J.W.; Sieuwerts, A.M.; van Deurzen, C.H.; Koelewijn, S.J.; de Blois, E.; Maina, T.; Nock, B.A.; Brunel, L.; Fehrentz, J.-A.; et al. In vitro and in vivo application of radiolabeled gastrin-releasing peptide receptor ligands in breast cancer. J. Nucl. Med. 2015, 56, 752–757. [Google Scholar] [CrossRef]
- Morgat, C.; MacGrogan, G.; Brouste, V.; Vélasco, V.; Sévenet, N.; Bonnefoi, H.; Fernandez, P.; Debled, M.; Hindié, E. Expression of gastrin-releasing peptide receptor in breast cancer and its association with pathologic, biologic and clinical parameters: A study of 1.432 primary tumors. J. Nucl. Med. 2017, 58, 1401–1407. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abouzayed, A.; Borin, J.; Lundmark, F.; Rybina, A.; Hober, S.; Zelchan, R.; Tolmachev, V.; Chernov, V.; Orlova, A. The GRPR Antagonist [99mTc]Tc-maSSS-PEG2-RM26 towards Phase I Clinical Trial: Kit Preparation, Characterization and Toxicity. Diagnostics 2023, 13, 1611. https://doi.org/10.3390/diagnostics13091611
Abouzayed A, Borin J, Lundmark F, Rybina A, Hober S, Zelchan R, Tolmachev V, Chernov V, Orlova A. The GRPR Antagonist [99mTc]Tc-maSSS-PEG2-RM26 towards Phase I Clinical Trial: Kit Preparation, Characterization and Toxicity. Diagnostics. 2023; 13(9):1611. https://doi.org/10.3390/diagnostics13091611
Chicago/Turabian StyleAbouzayed, Ayman, Jesper Borin, Fanny Lundmark, Anastasiya Rybina, Sophia Hober, Roman Zelchan, Vladimir Tolmachev, Vladimir Chernov, and Anna Orlova. 2023. "The GRPR Antagonist [99mTc]Tc-maSSS-PEG2-RM26 towards Phase I Clinical Trial: Kit Preparation, Characterization and Toxicity" Diagnostics 13, no. 9: 1611. https://doi.org/10.3390/diagnostics13091611
APA StyleAbouzayed, A., Borin, J., Lundmark, F., Rybina, A., Hober, S., Zelchan, R., Tolmachev, V., Chernov, V., & Orlova, A. (2023). The GRPR Antagonist [99mTc]Tc-maSSS-PEG2-RM26 towards Phase I Clinical Trial: Kit Preparation, Characterization and Toxicity. Diagnostics, 13(9), 1611. https://doi.org/10.3390/diagnostics13091611










