Marginal Bone Level and Clinical Parameter Analysis Comparing External Hexagon and Morse Taper Implants: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Inclusion and Exclusion Criteria
2.3. Study Search and Strategy of Selection
2.4. Data Extraction
2.5. Quality Assessment and Statistical Analysis
3. Results
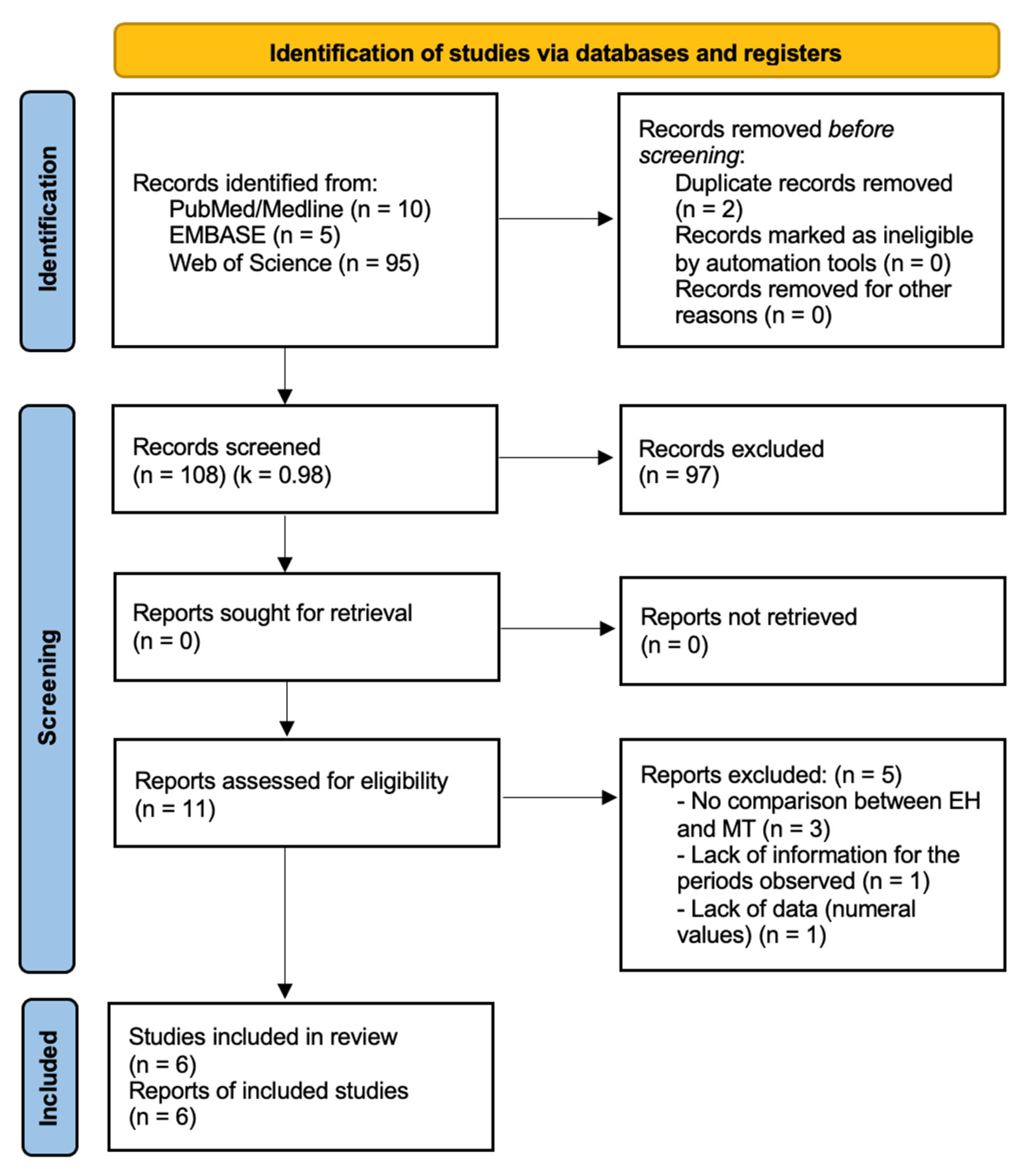
3.1. Selection of Studies
3.2. Characteristics of the Patients Observed (Table 2)
3.3. Characteristics of the Implants and Survival Rate (Table 3)
3.4. Clinical Findings (Table 4 and Table 5)
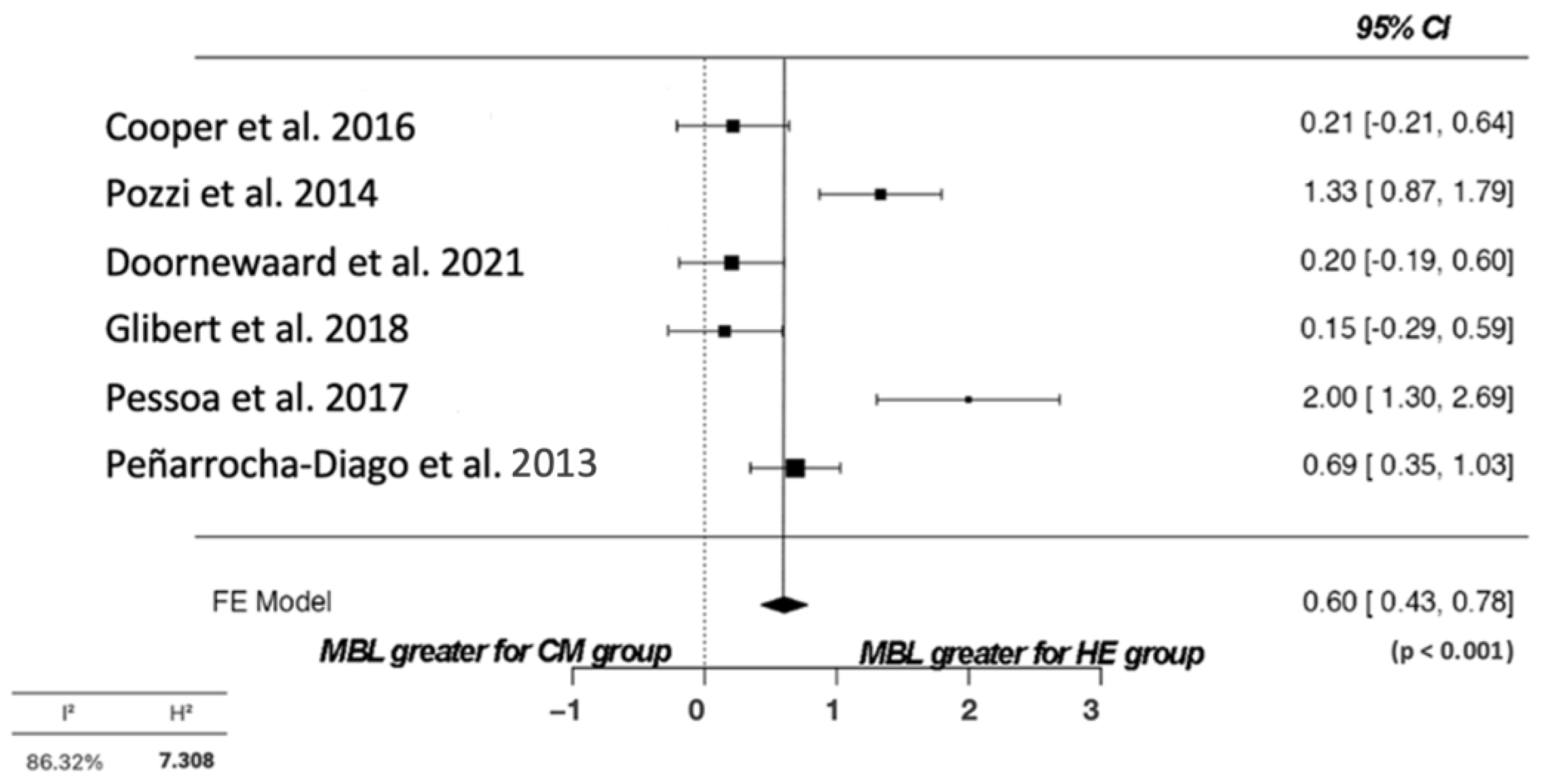
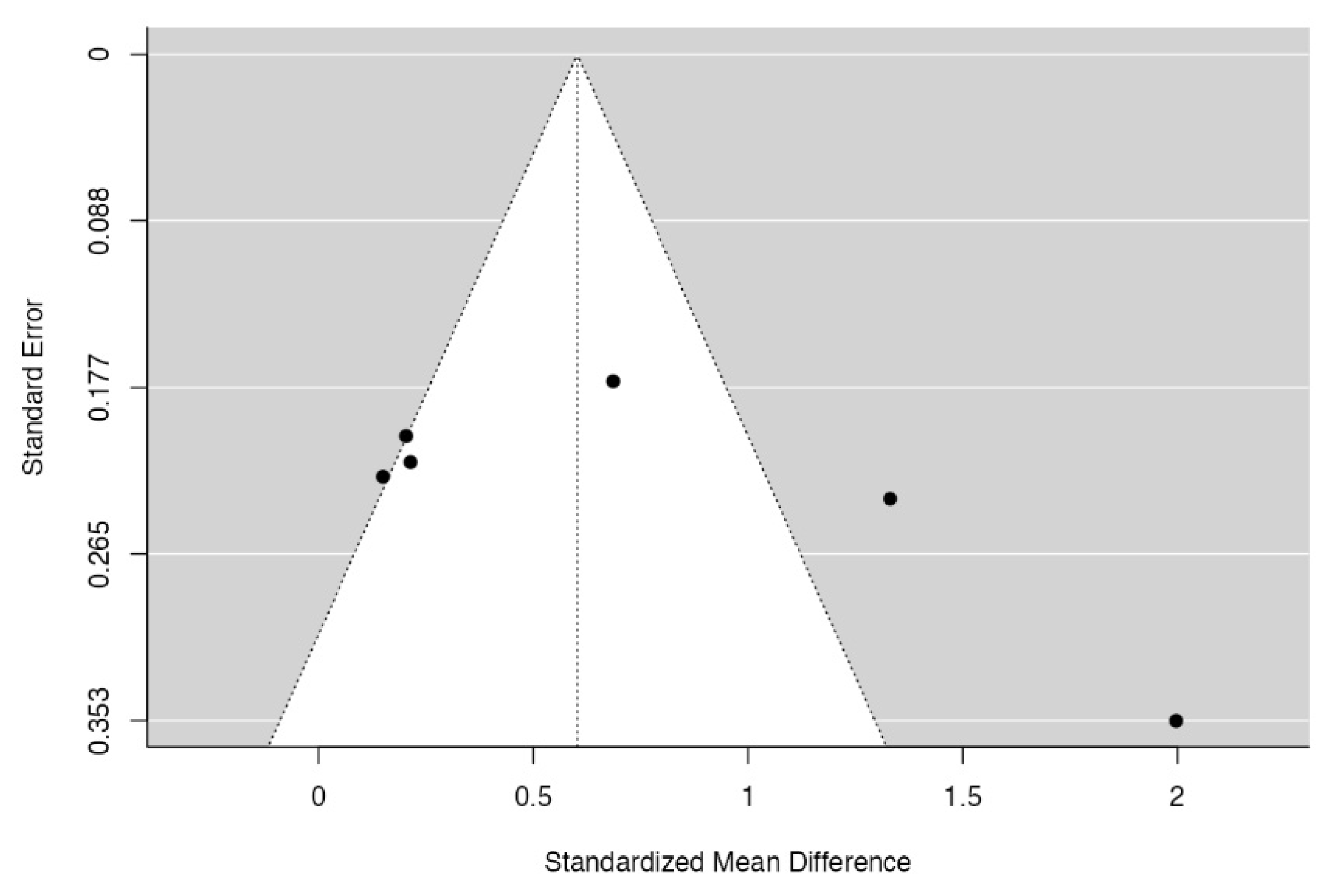
3.5. Quality Assessment and Statistical Analysis
4. Discussion
4.1. Other Clinical Parameters
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2020, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Borges, H.; Correia, A.R.M.; Castilho, R.M.; Fernandes, G.V.O. Zirconia Implants and Marginal Bone Loss: A Systematic Review and Meta-Analysis of Clinical Studies. Int. J. Oral. Maxillofac. Implant. 2020, 35, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Cortellari, G.C.; Fernandes, G.V.O.; Scarano, A.; Martins, R.G.; Cançado, R.M.; Mesquita, A.M.M. Randomized Clinical Trial Comparing Insertion Torque and Implant Stability of Two Different Implant Macrogeometries in the Initial Periods of Osseointegration. Medicina 2023, 59, 168. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Naveau, A.; Shinmyouzu, K.; Moore, C.; Avivi-Arber, L.; Jokerst, J.; Koka, S. Etiology and measurement of peri-implant crestal bone loss (CBL). J. Clin. Med. 2019, 8, 166. [Google Scholar] [CrossRef]
- Kowalski, J.; Lapinska, B.; Nissan, J.; Lukomska-Szymanska, M. Factors influencing marginal bone loss around dental implants: A narrative review. Coatings 2021, 11, 865. [Google Scholar] [CrossRef]
- Martins, B.G.S.; Fernandes, J.C.H.; Martins, A.G.; Castilho, R.M.; Fernandes, G.V.O. Surgical and Nonsurgical Treatment Protocols for Peri-implantitis: An Overview of Systematic Reviews. Int. J. Oral. Maxillofac. Implant. 2022, 37, 660–676. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Caricasulo, R.; Malchiodi, L.; Ghensi, P.; Fantozzi, G.; Cucchi, A. The influence of implant-abutment connection to peri-implant bone loss: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 653–664. [Google Scholar] [CrossRef]
- Carossa, M.; Alovisi, M.; Crupi, A.; Ambrogio, G.; Pera, F. Full-Arch Rehabilitation Using Trans-Mucosal Tissue-Level Implants with and without Implant-Abutment Units: A Case Report. Dent. J. 2022, 10, 116. [Google Scholar] [CrossRef]
- Arvidson, K.; Fartash, B.; Hilliges, M.; Kondell, P.A. Histological characteristics of peri-implant mucosa around branemark and 429 single-crystal sapphire implants. Clin. Oral Implant. Res. 1996, 7, 1–10. [Google Scholar] [CrossRef]
- Qian, J.; Wennerberg, A.; Albrektsson, T. Reasons for Marginal Bone Loss around Oral Implants. Clin. Implant. Dent. Relat. Res. 2012, 14, 792–807. [Google Scholar] [CrossRef]
- Bio-Joachim SHermann, C.D.; Higginbottom, F.L.; Cochran, D.L. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin. Oral Implant. Res. 2000, 11, 1–11. [Google Scholar]
- Vigolo, P.; Gracis, S.; Carboncini, F.; Mutinelli, S. Internal- vs. External-Connection Single Implants: A Retrospective Study in an Italian Population Treated by Certified Prosthodontists. Int. J. Oral Maxillofac. Implant. 2016, 31, 1385–1396. [Google Scholar] [CrossRef]
- Lemos, C.A.A.; Verri, F.R.; Bonfante, E.A.; Santiago Júnior, J.F.; Pellizzer, E.P. Comparison of external and internal implant-abutment connections for implant supported prostheses. A systematic review and meta-analysis. J. Dent. 2018, 70, 14–22. [Google Scholar] [CrossRef]
- Sasada, Y.; Cochran, D. Implant-Abutment Connections: A Review of Biologic Consequences and Peri-implantitis Implications. Int. J. Oral Maxillofac. Implant. 2017, 32, 1296–1307. [Google Scholar] [CrossRef]
- Canullo, L.; Penarrocha-Oltra, D.; Soldini, C.; Mazzocco, F.; Penarrocha, M.; Covani, U. Microbiological assessment of the implant-abutment interface in different connections: Cross-sectional study after 5 years of functional loading. Clin. Oral Implant. Res. 2015, 26, 426–434. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Branemark, P.-I.; Lindhe, J.; Eriksson, B.; Sbordone, L. Marginal tissue reactions at osseoin- 427 tegrated titanium fixtures: A 3-year longitudinal prospective study. Int. J. Oral Maxillofac. Surg. 1986, 15, 39–52. [Google Scholar] [CrossRef]
- Gupta, S.; Sabharwal, R.; Nazeer, J.; Taneja, L.; Choudhury, B.; Sahu, S. Platform switching technique and crestal bone loss around the dental implants: A systematic review. Ann. Afr. Med. 2019, 18, 1–6. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J.; Jonsson, K.; Ericsson, I. The topography of the vascular systems in the periodontal and peri-implant 433 tissues in the dog. J. Clin. Periodontol. 1994, 21, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.P.R.; Fernandes, F.S.F.; Straioto, F.G.; Silva, W.J.; Del Bel Cury, A.A. Preload Loss and Bacterial Penetration on Different Implant-Abutment Connection Systems. Braz. Dent. J. 2010, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Forrest, J.L. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J. Evid. Based Dent. Pract. 2001, 1, 136–141. [Google Scholar] [CrossRef]
- Jadad, A.R.; Andrew Moore, R.; Carroll, D.; Jenkinson, C.; John Reynolds, D.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Pozzi, A.; Agliardi, E.; Tallarico, M.; Barlattani, A. Clinical and radiological outcomes of two implants with different prosthetic interfaces and neck configurations: Randomized, controlled, split-mouth clinical trial. Clin. Implant. Dent. Relat. Res. 2014, 16, 96–106. [Google Scholar] [CrossRef]
- Doornewaard, R.; Sakani, S.; Matthys, C.; Glibert, M.; Bronkhorst, E.; Vandeweghe, S.; Vervaeke, S.; De Bruyn, H. Four-implant-supported overdenture treatment in the maxilla. Part I: A randomized controlled split mouth trial assessing the effect of microthreads and abutment connection type on 4 years peri-implant health. Clin. Implant. Dent. Relat. Res. 2021, 23, 671–679. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Sousa, R.M.; Pereira, L.M.; Neves, F.D.; Bezerra, F.J.B.; Jaecques, S.V.N.; Sloten, J.V.; Quirynen, M.; Teughels, W.; Spin-Neto, R. Bone Remodeling Around Implants with External Hexagon and Morse-Taper Connections: A Randomized, Controlled, Split-Mouth, Clinical Trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 97–110. [Google Scholar] [CrossRef]
- Glibert, M.; Vervaeke, S.; Jacquet, W.; Vermeersch, K.; Östman, P.O.; de Bruyn, H. A randomized controlled clinical trial to assess crestal bone remodeling of four different implant designs. Clin. Implant. Dent. Relat. Res. 2018, 20, 455–462. [Google Scholar] [CrossRef]
- Cooper, L.; Tarnow, D.; Froum, S.; Moriarty, J.; de Kok, I. Comparison of Marginal Bone Changes with Internal Conus and External Hexagon Design Implant Systems: A Prospective, Randomized Study. Int. J. Periodontics Restor. Dent. 2016, 36, 631–642. [Google Scholar] [CrossRef]
- Peñarrocha-Diago, M.A.; Flichy-Fernández, A.J.; Alonso-González, R.; Peñarrocha-Oltra, D.; Balaguer-Martínez, J.; Peñarrocha-Diago, M. Influence of implant neck design and implant-abutment connection type on peri-implant health. Radiological study. Clin. Oral Implant. Res. 2013, 24, 1192–1200. [Google Scholar] [CrossRef]
- Fernandes, G.V.O.; Costa, B.M.G.N.; Trindade, H.F.; Castilho, R.M.; Fernandes, J.C.H. Comparative analysis between extra-short implants (≤6 mm) and 6 mm-longer implants: A meta-analysis of randomized controlled trial. Aust. Dent. J. 2022, 67, 194–211. [Google Scholar] [CrossRef]
- Fernandes, P.R.E.; Otero, A.I.P.; Fernandes, J.C.H.; Nassani, L.M.; Castilho, R.M.; Fernandes, G.V.O. Clinical Performance Comparing Titanium and Titanium–Zirconium or Zirconia Dental Implants: A Systematic Review of Randomized Controlled Trials. Dent. J. 2022, 10, 83. [Google Scholar] [CrossRef]
- Fernandes, G.V.O. Peri-implantitis matter: Possibilities of treatment but without a strong predictability for solution. Environ. Dent. J. 2021, 3, 1. [Google Scholar]
- Srimaneepong, V.; Heboyan, A.; Zafar, M.S.; Khurshid, Z.; Marya, A.; Fernandes, G.V.O.; Rokaya, D. Fixed Prosthetic Restorations and Periodontal Health: A Narrative Review. J. Funct. Biomater. 2022, 13, 15. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, R.A. The long-term efficacy of currently used dental implants: A review and proposed criteria for success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Lesaffre, E.; Philstrom, B.; Needleman, I.; Worthington, H. The design and analysis of split-mouth studies: What statisticians and clinicians should know. Stat. Med. 2009, 28, 3470–3482. [Google Scholar] [CrossRef]
- Palaska, I.; Tsaousoglou, P.; Vouros, I.; Konstantinidis, A.; Menexes, G. Influence of placement depth and abutment connection pattern on bone remodeling around 1-stage implants: A prospective randomized controlled clinical trial. Clin. Oral Implant. Res. 2016, 27, e47–e56. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Cho, S.C.; Wallace, S.S. The effect of inter-implant distance on the height of inter-implant bone crest. J. Periodontol. 2000, 71, 546–549. [Google Scholar] [CrossRef]
- Quirynen, M.; van Steenberghe, D. Bacterial colonization of the internal part of two-stage implants. An in vivo study. Clin. Oral Implant. Res. 1993, 4, 158–161. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Eyssen, H.; van Steenberghe, D. Microbial penetration along the implant components of the Brånemark system. An in vitro study. Clin. Oral Implant. Res. 1994, 5, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, I.; Persson, L.G.; Berglundh, T.; Marinello, C.P.; Lindhe, J.; Klinge, B. Different types of inflammatory reactions in peri-implant soft tissues. J. Clin. Periodontol. 1995, 22, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Persson, L.G.; Lekholm, U.; Leonhardt, A.; Dahlén, G.; Lindhe, J. Bacterial colonization on internal surfaces of Brånemark system implant components. Clin. Oral Implant. Res. 1996, 7, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Camps-Font, O.; Rubianes-Porta, L.; Valmaseda-Castellón, E.; Jung, R.E.; Gay-Escoda, C.; Figueiredo, R. Comparison of external, internal flat-to-flat, and conical implant abutment connections for implant-supported prostheses: A systematic review and network meta-analysis of randomized clinical trials. J. Prosthet. Dent. 2021, S0022-3913(21)00529-1. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, H.J.; Kim, S.; Koo, K.T.; Kim, T.I.; Seol, Y.J.; Lee, Y.-M.; Ku, Y.; Rhyu, I.-C. Comparison of marginal bone loss between internal- and external-connection dental implants in posterior areas without periodontal or peri-implant disease. J. Periodontal Implant. Sci. 2018, 48, 103–113. [Google Scholar] [CrossRef]
- Meloni, S.M.; Lolli, F.M.; de Riu, N. Platform switching vs. regular platform implants: Nine-month post-loading results from a randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 257–265. [Google Scholar]
- Enkling, N.; Jöhren, P.; Klimberg, V.; Bayer, S.; Mericske-Stern, R.; Jepsen, S. Effect of platform switching on peri-implant bone levels: A randomized clinical trial. Clin. Oral Implant. Res. 2011, 22, 1185–1192. [Google Scholar] [CrossRef]
- Hermann, J.S.; Schoolfield, J.D.; Schenk, R.K.; Buser, D.; Cochran, D.L. Influence of the size of the microgap on crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged implants in the canine mandible. J. Periodontol. 2001, 72, 1372–1383. [Google Scholar] [CrossRef]
- Gardner, D.M. Platform switching as a means to achieving implant esthetics. N. Y. State Dent. J. 2005, 71, 34–37. [Google Scholar]
- Lazzara, R.J.; Porter, S.S. Platform switching: A new concept in implant dentistry for controlling postrestorative crestal bone levels. Int. J. Periodontics Restor. Dent. 2006, 26, 9–17. [Google Scholar]
- Baumgarten, H.; Cocchetto, R.; Testori, T.; Meltzer, A.; Porter, S. A new implant design for crestal bone preservation: Initial observations and case report. Pract. Proced. Aesthet. Dent. 2005, 17, 735–740. [Google Scholar] [PubMed]
- Hahn, J.A. Clinical and radiographic evaluation of one-piece implants used for immediate function. J. Oral Implantol. 2007, 33, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, D.P.; Magner, A.W.; Fletcher, P. The effect of the distance from the contact point to the crest of the bone on the presence or absence of the interproximal dental papilla. J. Periodontol. 1992, 63, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, D.P.; Elian, N.; Fletcher, P.; Froum, S.; Magner, A.; Cho, S.-C.; Salama, M.; Salama, H.; Garber, D.A. Vertical distance from the crest of bone to the height of the interproximal papilla between adjacent implants. J. Periodontol. 2003, 74, 1785–1788. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Luraghi, G.; Scribante, A. Ozonized Water Administration in Peri-Implant Mucositis Sites: A Randomized Clinical Trial. Appl. Sci. 2021, 11, 7812. [Google Scholar] [CrossRef]
- Bonato, R.S.; Fernandes, G.V.O.; Calasans-Maia, M.D.; Mello, A.; Rossi, A.M.; Carreira, A.C.O.; Sogayar, M.C.; Granjeiro, J.M. The Influence of rhBMP-7 Associated with Nanometric Hydroxyapatite Coatings Titanium Implant on the Osseointegration: A Pre-Clinical Study. Polymers 2022, 14, 4030. [Google Scholar] [CrossRef]
- Bratu, E.A.; Tandlich, M.; Shapira, L. A rough surface implant neck with microthreads reduces the amount of marginal bone loss: A prospective clinical study. Clin. Oral Implant. Res. 2009, 20, 827–832. [Google Scholar] [CrossRef]
- Piao, C.M.; Lee, J.E.; Koak, J.Y.; Kim, S.K.; Rhyu, I.C.; Han, C.H.; Herr, Y.; Heo, S.J. Marginal bone loss around three different implant systems: Radiographic evaluation after 1 year. J. Oral Rehabil. 2009, 36, 748–754. [Google Scholar] [CrossRef]
- Puchades-Roman, L.; Palmer, R.M.; Palmer, P.J.; Howe, L.C.; Ide, M.; Wilson, R.F. A clinical, radiographic, and microbiologic comparison of astra tech and Branemark single tooth implants. Clin. Implant. Dent. Relat. Res. 2000, 2, 78–84. [Google Scholar] [CrossRef]
- Al-Thobity, A.M.; Kutkut, A.; Almas, K. Microthreaded Implants and Crestal Bone Loss: A Systematic Review. J. Oral Implantol. 2017, 43, 157–166. [Google Scholar] [CrossRef]
- Palacios-Garzón, N.; Mauri-Obradors, E.; Roselló-LLabrés, X.; Estrugo-Devesa, A.; Jané-Salas, E.; López-López, J. Comparison of Marginal Bone Loss Between Implants with Internal and External Connections: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2018, 33, 580–589. [Google Scholar] [CrossRef]
- Cha, J.Y.; Pereira, M.D.; Smith, A.A.; Houschyar, K.S.; Yin, X.; Mouraret, S.; Brunski, J.; Helms, J. Multiscale analyses of the bone-implant interface. J. Dent. Res. 2015, 94, 482–490. [Google Scholar] [CrossRef]
- Norton, M. The Influence of Low Insertion Torque on Primary Stability, Implant Survival, and Maintenance of Marginal Bone Levels: A Closed-Cohort Prospective Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 849–857. [Google Scholar] [CrossRef]
- Esposito, M.; Maghaireh, H.; Pistilli, R.; Gabriella Grusovin, M.; Taek Lee, S.; Trullenque-Eriksson, A.; Gualini, F. Dental implants with internal versus external connections: 5-year post-loading results from a pragmatic multicenter randomised controlled trial. Eur. J. Oral Implantol. 2016, 9 (Suppl. S1), 129–141. [Google Scholar] [CrossRef]
- Augusto, G.; Barbosa, S.; Almeida De Melo, L.; Bezerra De Farias, D.; Bezerra De Medeiros, A.K.; Dantas, M.; Carreiro, A. Comparative evaluation of peri-implant tissues in patients wearing mandibular overdenture with different implant platforms. J. Indian. Soc. Periodontol. 2017, 21, 473–477. [Google Scholar] [CrossRef]
- Castro, F.M.C.; Martins, G.Z.; Oliveira, H.F.P.; Hernández, P.B.; Gavinha, S.; Fernandes, G.V.O. Comparison of Stud- Retentor Versus Bar-Clip Attachment as Implant- Supported Systems Used in Overdentures: A Systematic Review and Meta-Analysis. Eur. J. Prosthodont. Restor. Dent. 2022, 30, 169–187. [Google Scholar] [CrossRef]
- Desai, S.R.; Koulgikar, K.D.; Alqhtani, N.R.; Alqahtani, A.R.; Alqahtani, A.S.; Alenazi, A.; Heboyan, A.; Fernandes, G.V.O.; Mustafa, M. Three-Dimensional FEA Analysis of the Stress Distribution on Titanium and Graphene Frameworks Supported by 3 or 6-Implant Models. Biomimetics 2023, 8, 15. [Google Scholar] [CrossRef]
| Population | Patients Treated with Dental Implants |
|---|---|
| Intervention | Implants with external connection |
| Control | Implants with Morse taper |
| Outcomes | Differences in the marginal crestal bone (maintenance) after at least three months of function |
| Author/Year | Study Design | Objective | n | Age | Gender | Smokers |
|---|---|---|---|---|---|---|
| Mean/Range | Male/Female | |||||
| Pozzi et al., 2014 [26] | RCT (split-mouth) | Compare clinical and radiological outcomes of two implant designs with different prosthetic interfaces and neck configurations in a randomized, controlled, split-mouth clinical trial. | 34 | 52.20/39–59 | NR | 4 patients smoke less than 10 cigarettes/day |
| Doornewaard et al., 2021 [27] | RCT (split-mouth) | They assessed the effect of implant neck (microthreaded vs. non-microthreaded) and the type of abutment connection (internal conical vs. external flat-to-flat) on peri-implant bone stability and peri-implant health after at least 36 months. | 27 | 62/42–83 | M:15 F:12 | Limited to patients smoking less than 10 cigarettes/day |
| Pessoa et al., 2017 [28] | RCT (split-mouth) | To evaluate clinical, radiographic, microbiologic, and biomechanical parameters related to bone remodeling around implants with external hexagon (EH) and Morse taper (MT) connections. | 12 | 63/18–75 | M:3 F:9 | 0 |
| Glibert et al., 2018 [29] | RCT | This RCT assesses whether a coronal microthreaded design and an internal abutment connection affect crestal bone loss up to one year of function. | 21 | 65/44–66 | M:12 F:9 | Limited to patients smoking less than 10 cigarettes/day |
| Cooper et al., 2016 [30] | PS | Over 3 years, compare the proximal marginal bone responses at external hex interface (EXI) versus internal conus interface (ICI) implants. | 36 | 53.1/18–75 | M:13 F:23 | 14 previous smokers |
| Peñarrocha-Diago et al., 2013 [31] | PS | To conduct a comparative study of two implants with different neck features and prostheses platform connection (machined with external connection and rough-surfaced with switching platform) upon peri-implant marginal bone loss, before and after functional loading. | 15 | 56.9/44–77 | M:4 F:11 | 3 smoking patients, less than 10 cigarettes/day |
| Author/Year | Surgical Site | Implant Loading | Implant/Abutment Loading | Follow-Up (Months) | Implants | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Location | Groups Treated | Threads | Length (mm) | Width (mm) | Manufacturer | Success Rate (%) | Survival Rate (%) | |||||
| Pozzi et al., 2014 [26] | Any partially edentate patient in the lower jaw, aged 25 years or more, requiring at least two single implant-supported crowns; sufficient bone volumes to accommodate dental implants without augmentation procedure. | D | 2nd stage | 8 weeks; 4; 16 | 88 | Max: 0 Mand: 88 | Test: ICC Control: EH | MT MT | 10–13 10–13 | 4.3 4 | Nobel Active, Nobel Biocare AB Nobel Speedy Groovy, Nobel Biocare AB | NR | 100 |
| Doornewaard et al., 2021 [27] | Fully edentulous patients in the maxilla in need of a four-implant-supported overdenture. The preferred implant locations were the canine and first-molar regions. In case of insufficient bone in the molar region, the second premolar site was chosen. | I | 1st stage | 3; 6; 12; 24; 36 | 98 | Max: 98 Mand: 0 | I-MT I-NMT E-MT I-NMT | MT NMT MT NMT | 9–11 9–11 9–11 9–11 | 4 4 4 4 | DCC, Southern implants Southern implants | 98.4 | 95.9 |
| Pessoa et al., 2017 [28] | Edentulous patients should also have adequate bone quantity for the placement of 4 3.8 and 3 13 mm implants in the interforaminal region of the mandible. | I | 1st stage | 1; 3; 6; 12 | 48 | Max: 0 Mand: 48 | Test: Morse taper connection Control: External hexagon connection | MT MT | NR NR | NR NR | UNITITE®, SIN | NR | 100 |
| Glibert et al., 2018 [29] | Totally edentulous patients in the upper jaw for at least 4 months; the presence of sufficient residual bone volume to install 4 implants with a 4 mm diameter and 9–11 mm in length. | I | 1st stage | 3; 6; 12; 21 | 83 | Max: 83 Mand: 0 | I-MT I-NMT E-MT I-NMT | MT NMT MT NMT | 9–11 9–11 9–11 9–11 | 4 4 4 4 | SICace®; SIC invent Southern implants | NR | 96.4 |
| Cooper et al., 2016 [30] | Individuals classified as Kennedy class I or II for mandibular or maxillary arches involving the left, the right, or both quadrants were eligible for enrollment. | D | 1st stage | 6; 12; 36 | 86 | Max: 36 Mand: 50 | Test: ICI Control: EXI | NR | NR | NR | Osseotite Standard, Biomet 3i Astra Tech Fixture ST, Dentsply | NR | 96 |
| Penarrocha-Diago et al., 2013 [31] | Completely edentulous arch requiring implant placement for a fixed prosthesis, bar overdenture, locator overdenture; bone with a minimum width of 7 mm and a minimum height of 6 mm. | D | 2nd stage | Implant placement and prosthesis placement: 6; 12 | 141 | NR | Group A: External hexagon Group B: Internal connection and platform switching | NMT MT | 10–13 10–13 | 3.75–4.25 3.75–4.25 | Osseous®, Mozo-Grau Inhex®, Mozo-Grau | 97.2 | 98.6 |
| Author/Year | Patients (n) | Implants (n) | Morse Taper | ||||
|---|---|---|---|---|---|---|---|
| 3 m | 6 m | 12 m | 21 m | 36 m | |||
| Pozzi et al., 2014 [26] | 34 | 88 | −0.37 ± 0.23 | NR | −0.51 ± 0.34 | NR | NR |
| Doornewaard et al., 2021 [27] | 27 | 98 | NR | I-MT −0.45 ± 0.61 t0–t1 | I-MT −0.01 ± 0.47, t1–t2 | NR | I-MT −0.01 ± 0.47, t1–t2 |
| I-NMT −0.33 ± 0.61 t0–t1 | I-NMT −0.07 ± 0.60, t1–t2 | I-NMT −0.07 ± 0.60, t1–t2 | |||||
| Pessoa et al., 2017 [28] | 12 | 48 | NR | NR | −0.17 ± 0.54 | NR | NR |
| Glibert et al., 2018 [29] | 21 | 83 | I-MT −0.27 ± 0.65 | I-MT −0.34 ± 0.47 | I-MT −0.22 ± 0.32 | I-MT −0.26 ± 0.32 | NR |
| I-NMT −0.15 ± 0.29 | I-NMT −0.26 ± 0.39 | I-NMT −0.27 ± 0.42 | I-NMT −0.24 ± 0.36 | ||||
| Cooper et al., 2016 [30] | 36 | 86 | NR | NR | −0.48 ± 0.55 | NR | −0.25 ± 0.60 |
| Penarrocha-Diago et al., 2013 [31] | 15 | 141 | NR | −0.07 ± 0.13 mm | −0.12 ± 0.17 mm | NR | NR |
| External Hexagon | |||||||
| 3 m | 6 m | 12 m | 21 m | 36 m | |||
| −0.95 ± 0.56 | NR | −1.10 ± 0.52 | NR | NR | |||
| NR | E-MT −0.45 ± 0.77, t0–t1 | E-MT −0.10 ± 0.58 t1–t2 | NR | E-MT −0.10 ± 0.58, t1–t2 | |||
| E-NMT −0.34 ± 0.51, t0–t1 | E-NMT −0.19 ± 0.48, t1–t2 | E-NMT −0.19 ± 0.48, t1–t2 | |||||
| NR | NR | −1.17 ± 0.44 | NR | NR | |||
| E-MT −0.24 ± 0.36 | E-MT −0.32 ± 0.42 | E-MT −0.32 ± 0.45 | E-MT −0.22 ± 0.33 | NR | |||
| E-NMT −0.16 ± 0.25 | E-NMT −0.29 ± 0.36 | E-NMT −0.29 ± 0.38 | E-NMT −0.19 ± 0.23 | ||||
| NR | NR | −0.68 ± 1.2 | NR | −0.5 ± 0.93 | |||
| NR | −0.27 ± 0.43 | −0.38 ± 0.51 | NR | NR | |||
| Author/Year | Patients (n) | Connection (n) | BoP | PD (mm) | Plaque | Follow-Up (Months) | Conclusions |
|---|---|---|---|---|---|---|---|
| Pozzi et al., 2014 [26] | 34 | ICC (44) | Not detected around any implants | NR | Low presence | 16 | The MBL was statistically significantly lower in the back-tapered neck configuration with CC and built-in platform shifting compared with the straight neck configuration with the flat-to-flat implant–abutment interface and external hexagonal connection. |
| EH (44) | Not detected around any implants | Low presence | |||||
| Doornewaard et al., 2021 [27] | 27 | I-MT (24) | Positive in 33 implants | Mean of 4.5 | No significant impact between implant type and position | 36 | The implant–abutment connection (internal vs. external), implant neck design (microthreaded vs. non-microthreaded), and implant position (anterior vs. posterior) have no influence on peri-implant bone remodeling after implant placement, no impact on peri-implant bone level after initial remodeling, and no effect on peri-implant health parameters. |
| I-NMT (25) | |||||||
| E-MT (25) | |||||||
| E-NMT (24) | |||||||
| Platform matching | Positive in 8 sites | Mean of 2.1 | 36 implants with plaque | ||||
| Pessoa et al., 2017 [28] | 12 | External hexagon (24) | No bleeding | 1.57 ± 0.9 | NR | 12 | Within the limitations of this study, it can be concluded that varying implant–abutment connection types will result in diverse early peri-implant bone remodeling. The present findings suggest that MT connections are more efficient in preventing early peri-implant bone loss compared to EH connections. |
| Morse Taper (24) | No bleeding | 1.36 ± 0.7 | |||||
| Glibert et al., 2018 [29] | 21 | I-MT (20) | 23.4% was recorded | Mean of 3.26 | 39.5% of implants presented the plaque | 12–21 | From this RCT, it is concluded that crestal bone remodeling is not affected by the implant–abutment connection or microthreads. Bone remodeling is a multifactorial process and might be more dependent on other factors than implant design itself. |
| I-NMT (21) | |||||||
| E-MT (20) | |||||||
| E-NMT (19) | |||||||
| Cooper et al., 2016 [30] | 36 | ICI (44) | Less than 2% | NR | Low presence | 36 | Comparing two implant designs revealed minor differences in marginal bone responses from permanent restoration to 3 years. Significantly more apical MBLs were recorded for EXI implants. Furthermore, more positive papilla scores were found between adjacent ICI implants than between adjacent EXI implants. EXI implant displayed more abutment complications than the ICI implant. |
| EXI (42) | Less than 2% | Low presence | |||||
| Penarrocha-Diago et al., 2013 [31] | 15 | EH (69) | NR | NR | NR | 12 | Bone loss after 6 and 12 months proved statistically significant between the two groups, with comparatively greater loss in the case of the Osseous® implants vs. the Inhex® implants. Regardless of the heterogeneity of the two groups (neck shape, microthreads, surface texture), the implant–abutment connection appears to be a significant factor in peri-implant crestal bone levels. |
| IC (72) |
| Author/Year | Randomization | Appropriateness of Randomization | Blinding | Appropriateness of Blinding | An Account of All Patients or Description of Withdrawal or Drop-Out | Total |
|---|---|---|---|---|---|---|
| Pozzi et al., 2014 [26] | 1 | 1 | 1 | 1 | 1 | 5 |
| Doornewaard et al., 2021 [27] | 1 | 1 | 0 | 0 | 1 | 3 |
| Pessoa et al., 2017 [28] | 1 | 0 | 1 | 1 | 1 | 4 |
| Glibert et al., 2018 [29] | 1 | 1 | 0 | 0 | 1 | 3 |
| Cooper et al., 2016 [30] | 1 | 1 | 0 | 0 | 1 | 3 |
| Peñarrocha-Diago et al., 2013 [31] | 1 | 1 | 0 | 0 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuda, S.; Martins, B.G.d.S.; Castro, F.C.d.; Heboyan, A.; Gehrke, S.A.; Fernandes, J.C.H.; Mello-Moura, A.C.V.; Fernandes, G.V.O. Marginal Bone Level and Clinical Parameter Analysis Comparing External Hexagon and Morse Taper Implants: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 1587. https://doi.org/10.3390/diagnostics13091587
Fuda S, Martins BGdS, Castro FCd, Heboyan A, Gehrke SA, Fernandes JCH, Mello-Moura ACV, Fernandes GVO. Marginal Bone Level and Clinical Parameter Analysis Comparing External Hexagon and Morse Taper Implants: A Systematic Review and Meta-Analysis. Diagnostics. 2023; 13(9):1587. https://doi.org/10.3390/diagnostics13091587
Chicago/Turabian StyleFuda, Samuele, Bruno Gomes dos Santos Martins, Filipe Correia de Castro, Artak Heboyan, Sergio Alexandre Gehrke, Juliana Campos Hasse Fernandes, Anna Carolina Volpi Mello-Moura, and Gustavo Vicentis Oliveira Fernandes. 2023. "Marginal Bone Level and Clinical Parameter Analysis Comparing External Hexagon and Morse Taper Implants: A Systematic Review and Meta-Analysis" Diagnostics 13, no. 9: 1587. https://doi.org/10.3390/diagnostics13091587
APA StyleFuda, S., Martins, B. G. d. S., Castro, F. C. d., Heboyan, A., Gehrke, S. A., Fernandes, J. C. H., Mello-Moura, A. C. V., & Fernandes, G. V. O. (2023). Marginal Bone Level and Clinical Parameter Analysis Comparing External Hexagon and Morse Taper Implants: A Systematic Review and Meta-Analysis. Diagnostics, 13(9), 1587. https://doi.org/10.3390/diagnostics13091587










