Obesity-Related Pitfalls of Virtual versus True Non-Contrast Imaging—An Intraindividual Comparison in 253 Oncologic Patients
Abstract
1. Introduction
2. Materials and Methods
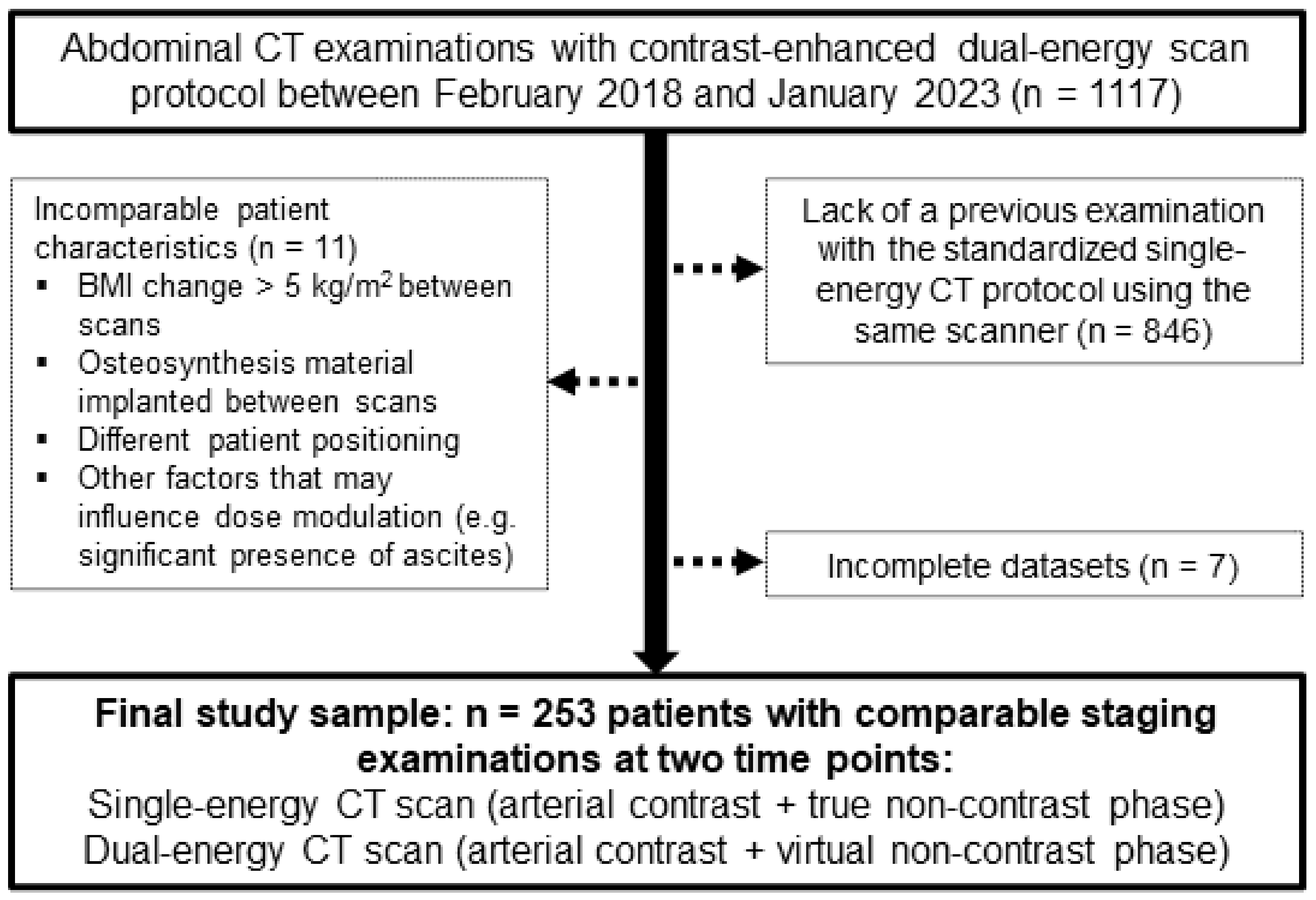
2.1. Study Population
2.2. Phantom Scan
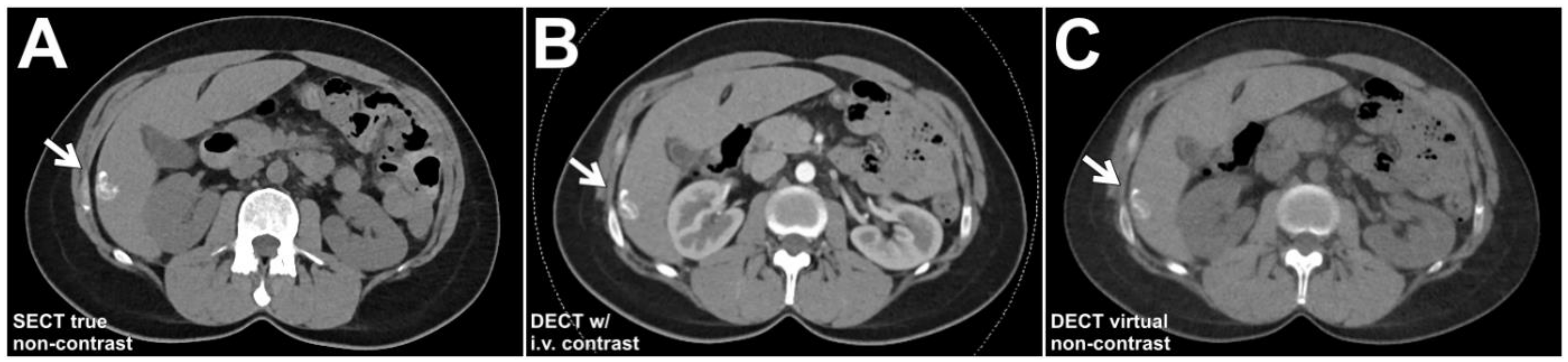
2.3. Patient Examinations
2.4. Dual-Energy Coverage of Liver Parenchyma
2.5. Statistical Analysis
3. Results
3.1. Patient Sample
3.2. Radiation Dose
3.2.1. Phantom Scan
3.2.2. Patient Examinations
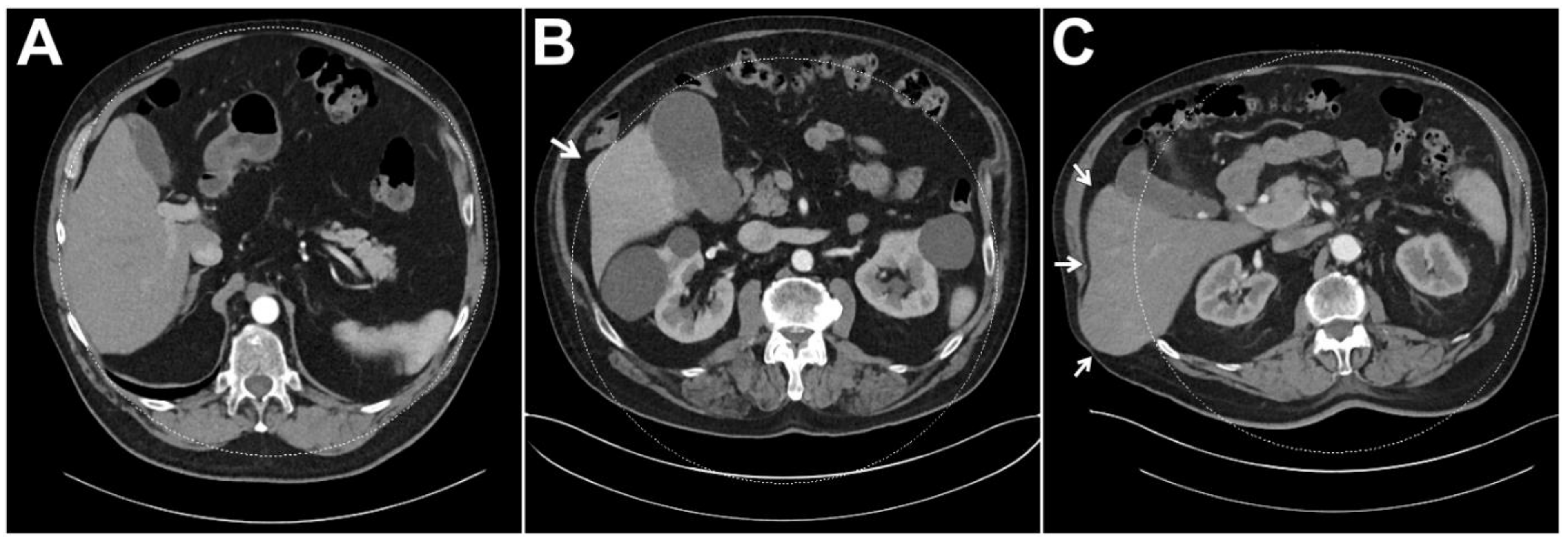
3.3. Dual-Energy Coverage of Liver Parenchyma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTDIvol | Volume computed tomography dose index |
| DECT | Dual-energy computed tomography |
| FOV | Field of view |
| HU | Hounsfield units |
| ROI | Region of interest |
| SECT | Single-energy CT |
References
- Gruschwitz, P.; Petritsch, B.; Schmid, A.; Schmidt, A.; Grunz, J.-P.; Kuhl, P.; Heidenreich, J.; Huflage, H.; Bley, T.; Kosmala, A. Noise-optimized virtual monoenergetic reconstructions of dual-energy CT angiographies improve assessability of the lower leg arterial segments in peripheral arterial occlusive disease. Radiography 2023, 29, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Grunz, J.-P.; Sailer, L.; Lang, P.; Schüle, S.; Kunz, A.S.; Beer, M.; Hackenbroch, C. Dual-energy CT in sacral fragility fractures: Defining a cut-off Hounsfield unit value for the presence of traumatic bone marrow edema in patients with osteoporosis. BMC Musculoskelet. Disord. 2022, 23, 724. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.S.; Grunz, J.-P.; Halt, D.; Kalogirou, C.; Luetkens, K.S.; Patzer, T.S.; Christner, S.A.; Sauer, S.T.; Bley, T.A.; Huflage, H. Tin-filtered 100 kV Ultra-low-dose Abdominal CT for Calculi Detection in the Urinary Tract: A Comparative Study of 510 Cases. Acad. Radiol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Petritsch, B.; Pannenbecker, P.; Weng, A.M.; Veldhoen, S.; Grunz, J.-P.; Bley, T.A.; Kosmala, A. Comparison of Dual- and Single-Source Dual-Energy CT for Diagnosis of Acute Pulmonary Artery Embolism. RöFo-Fortschr. Auf Dem Geb. Der Röntgenstrahlen Und Der Bildgeb. Verfahr. 2021, 193, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Fulwadhva, U.P.; Wortman, J.R.; Sodickson, A.D. Use of Dual-Energy CT and Iodine Maps in Evaluation of Bowel Disease. Radiographics 2016, 36, 393–406. [Google Scholar] [CrossRef]
- May, M.S.; Wiesmueller, M.; Heiss, R.; Brand, M.; Bruegel, J.; Uder, M.; Wuest, W. Comparison of dual- and single-source dual-energy CT in head and neck imaging. Eur. Radiol. 2019, 29, 4207–4214. [Google Scholar] [CrossRef]
- Hackenbroch, C.; Riesner, H.-J.; Lang, P.; Stuby, F.; Danz, B.; Friemert, B.; Palm, H.-G. Dual Energy CT—A Novel Technique for Diagnostic Testing of Fragility Fractures of the Pelvis. Z. Für Orthopädie Und Unf. 2017, 155, 27–34. [Google Scholar] [CrossRef]
- Thiravit, S.; Brunnquell, C.; Cai, L.M.; Flemon, M.; Mileto, A. Building a dual-energy CT service line in abdominal radiology. Eur. Radiol. 2021, 31, 4330–4339. [Google Scholar] [CrossRef]
- McCollough, C.H.; Boedeker, K.; Cody, D.; Duan, X.; Flohr, T.; Halliburton, S.S.; Hsieh, J.; Layman, R.R.; Pelc, N.J. Principles and applications of multienergy CT: Report of AAPM Task Group 291. Med. Phys. 2020, 47, e881–e912. [Google Scholar] [CrossRef]
- Krauss, B.; Grant, K.L.; Schmidt, B.T.; Flohr, T.G. The Importance of Spectral Separation. Investig. Radiol. 2015, 50, 114–118. [Google Scholar] [CrossRef]
- Lv, P.; Zhou, Z.; Liu, J.; Chai, Y.; Zhao, H.; Guo, H.; Marin, D.; Gao, J. Can virtual monochromatic images from dual-energy CT replace low-kVp images for abdominal contrast-enhanced CT in small- and medium-sized patients? Eur. Radiol. 2019, 29, 2878–2889. [Google Scholar] [CrossRef]
- Cester, D.; Eberhard, M.; Alkadhi, H.; Euler, A. Virtual monoenergetic images from dual-energy CT: Systematic assessment of task-based image quality performance. Quant. Imaging Med. Surg. 2022, 12, 726–741. [Google Scholar] [CrossRef]
- Vogl, T.J.; Schulz, B.; Bauer, R.W.; Stöver, T.; Sader, R.; Tawfik, A.M. Dual-Energy CT Applications in Head and Neck Imaging. Am. J. Roentgenol. 2012, 199, S34–S39. [Google Scholar] [CrossRef]
- Sodickson, A.D.; Keraliya, A.; Czakowski, B.; Primak, A.; Wortman, J.; Uyeda, J.W. Dual energy CT in clinical routine: How it works and how it adds value. Emerg. Radiol. 2021, 28, 103–117. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, X.; Deng, Y.; Chen, J.; Zhang, L.J. Can virtual non-enhanced CT be used to replace true non-enhanced CT for the detection of palpable cervical lymph nodes? A preliminary study. Jpn. J. Radiol. 2014, 32, 324–330. [Google Scholar] [CrossRef]
- Rajiah, P.; Parakh, A.; Kay, F.; Baruah, D.; Kambadakone, A.R.; Leng, S. Update on Multienergy CT: Physics, Principles, and Applications. Radiographics 2020, 40, 1284–1308. [Google Scholar] [CrossRef]
- Boone, J.; Strauss, K.; Cody, D.; McCollough, C.; McNitt-Gray, M.; Toth, T.; Goske, M.; Frush, D. Size-Specific Dose Estimates (SSDE) in Pediatric and Adult Body CT Examinations; American Association of Physicists in Medicine: Alexandria, VA, USA, 2011. [Google Scholar] [CrossRef]
- Deak, P.D.; Smal, Y.; Kalender, W.A. Multisection CT Protocols: Sex- and Age-specifi c Conversion Dose from Dose-Length Product 1 Purpose: Methods: Results: Conclusion. Radiology 2010, 257, 158–166. [Google Scholar] [CrossRef]
- Yao, Y.; Ng, J.M.; Megibow, A.; Pelc, N.J. Image quality comparison between single energy and dual energy CT protocols for hepatic imaging. Med. Phys. 2016, 43, 4877–4890. [Google Scholar] [CrossRef]
- Purysko, A.; Primak, A.; Baker, M.; Obuchowski, N.; Remer, E.; John, B.; Herts, B. Comparison of radiation dose and image quality from single-energy and dual-energy CT examinations in the same patients screened for hepatocellular carcinoma. Clin. Radiol. 2014, 69, e538–e544. [Google Scholar] [CrossRef]
- Walter, S.S.; Schneeweiß, S.; Maurer, M.; Kraus, M.S.; Wichmann, J.L.; Bongers, M.N.; Lescan, M.; Bamberg, F.; Othman, A.E. Virtual non-enhanced dual-energy CT reconstruction may replace true non-enhanced CT scans in the setting of suspected active hemorrhage. Eur. J. Radiol. 2018, 109, 218–222. [Google Scholar] [CrossRef]
- Graser, A.; Becker, C.R.; Staehler, M.; Clevert, D.A.; Macari, M.; Arndt, N.; Nikolaou, K.; Sommer, W.; Stief, C.; Reiser, M.F.; et al. Single-Phase Dual-Energy CT Allows for Characterization of Renal Masses as Benign or Malignant. Investig. Radiol. 2010, 45, 399–405. [Google Scholar] [CrossRef]
- Graser, A.; Johnson, T.R.C.; Hecht, E.M.; Becker, C.R.; Leidecker, C.; Staehler, M.; Stief, C.G.; Hildebrandt, H.; Godoy, M.C.B.; Finn, M.E.; et al. Dual-Energy CT in Patients Suspected of Having Renal Masses: Can Virtual Nonenhanced Images Replace True Nonenhanced Images? Radiology 2009, 252, 433–440. [Google Scholar] [CrossRef]
- Baffour, F.I.; Ferrero, A.; Aird, G.A.; Powell, G.M.; Adkins, M.C.; Bekele, D.I.; Johnson, M.P.; Fletcher, J.G.; Glazebrook, K.N. Evolving Role of Dual-Energy CT in the Clinical Workup of Gout: A Retrospective Study. Am. J. Roentgenol. 2022, 218, 1041–1050. [Google Scholar] [CrossRef]
- Choi, S.J.; Ahn, S.J.; Park, S.H.; Park, S.H.; Pak, S.Y.; Choi, J.W.; Shim, Y.S.; Jeong, Y.M.; Kim, B. Dual-source abdominopelvic computed tomography: Comparison of image quality and radiation dose of 80 kVp and 80/150 kVp with tin filter. PLoS ONE 2020, 15, e0231431. [Google Scholar] [CrossRef]

| Single-Energy CT | Dual-Energy CT | |
|---|---|---|
| Scanner | SOMATOM Force; Siemens Healthineers | SOMATOM Force; Siemens Healthineers |
| Phase | Arterial phase (+true non-contrast phase) | Arterial phase (+virtual non-contrast phase) |
| Automated dose modulation | CARE Dose 4D, CARE kV | CARE Dose 4D |
| Tube voltage [kVp] | 80–150 | 100/Sn 150 |
| Reference tube current [mAs] | 120 | 200/100 |
| Pitch | 0.9 | 0.6 |
| Rotation time [s] | 0.5 | 0.5 |
| Collimation [mm] | 192 × 0.6 mm | 128 × 0.6 mm |
| Iterative reconstruction | ADMIRE, strength level 3 | ADMIRE, strength level 3 |
| Convolution kernel [1/cm] | Br36 (ρ50 = 3.4/cm; ρ10 = 5.4) | Br36 (ρ50 = 3.4/cm; ρ10 = 5.4) |
| Contrast agent | Weight-adapted; iodine amount 350 mg/mL | Weight-adapted; iodine amount 350 mg/mL |
| All Patients | No Obesity | Pre-Obesity | Obesity | |
|---|---|---|---|---|
| n = 253 | n = 110 | n = 73 | n = 70 | |
| BMI < 25.0 kg/m2 | BMI 25.0–30.0 kg/m2 | BMI > 30.0 kg/m2 | ||
| Patient characteristics | ||||
| Sex [women/men] | 153/100 (60.5%/39.5%) | 80/30 (72.7%/27.3%) | 28/45 (38.4%/61.6%) | 45/25 (64.3%/35.7%) |
| Age [years] | 64.5 ± 16.2 | 63.8 ± 17.7 | 66.6 ± 12.2 | 63.6 ± 17.3 |
| Weight [kg] | 77.1 ± 16.8 | 62.8 ± 9.5 | 81.2 ± 9.1 | 95.2 ± 11.3 |
| Height [cm] | 170 ± 9 | 168 ± 9 | 173 ± 9 | 169 ± 8 |
| Body mass index [kg/m2] | 26.6 ± 5.1 | 22.2 ± 2.2 | 27.0 ± 1.3 | 33.4 ± 2.9 |
| Oncologic disease | ||||
| Melanoma | 110 (43.5%) | 32 (29.1%) | 38 (52.1%) | 40 (57.1%) |
| Breast carcinoma | 61 (24.1%) | 32 (29.1%) | 14 (19.2%) | 15 (21.4%) |
| Pancreatic carcinoma | 22 (8.7%) | 13 (11.8%) | 4 (5.5%) | 5 (7.1%) |
| Renal carcinoma | 19 (7.5%) | 9 (8.2%) | 8 (11.0%) | 2 (2.9%) |
| Liver carcinoma | 12 (4.7%) | 6 (5.5%) | 5 (6.8%) | 1 (1.4%) |
| Adrenal carcinoma | 10 (3.9%) | 4 (1.6%) | 1 (1.4%) | 5 (7.1%) |
| Miscellaneous | 19 (7.5%) | 14 (12.7%) | 3 (4.1%) | 2 (2.9%) |
| All Patients | No Obesity | Pre-Obesity | Obesity | |
|---|---|---|---|---|
| Single-energy CT | ||||
| Image noise in abdominal fat tissue [HU] | 17.8 (15.7–20.1) | 16.9 (15.2–19.6) | 18.4 (16.6–20.5) | 18.4 (15.9–20.9) |
| CTDIvol of true non-contrast phase [mGy] | 8.3 (6.2–11.0) | 6.0 (5.0–7.2) | 8.7 (7.5–10.1) | 12.8 (10.8–16.7) |
| CTDIvol of arterial phase [mGy] | 8.1 (6.1–10.9) | 6.0 (4.8–7.2) | 8.6 (7.3–10.2) | 12.9 (10.6–16.3) |
| DLP of true non-contrast phase [mGy·cm] | 240.1 (180.3–333.1) | 170.8 (135.5–214.1) | 258.7 (224.7–301.2) | 380.3 (319.4–514.3) |
| DLP of arterial phase [mGy·cm] | 228.7 (161.1–307.8) | 156.8 (120.6–194.2) | 247.0 (190.8–281.8) | 354.9 (293.6–482.0) |
| DLP of true non-contrast + arterial phase [mGy·cm] | 475.3 (343.4–648.9) | 325.6 (252.6–409.5) | 498.2 (426.2–575.6) | 729.9 (619.6–1026.2) |
| Dual-energy CT | ||||
| Image noise in abdominal fat tissue [HU] | 11.9 (10.6–13.6) | 11.2 (10.1–12.5) | 12.7 (11.0–13.6) | 12.9 (11.6–13.9) |
| CTDIvol of arterial phase [mGy] | 11.1 (8.5–15.5) | 8.4 (6.9–9.5) | 12.4 (10.7–14.5) | 17.8 (16.0–20.8) |
| DLP of arterial phase [mGy·cm] | 312.6 (231.5–450.6) | 224.6 (165.4–253.9) | 356.5 (287.1–414.8) | 536.5 (450.3–610.7) |
| Single-energy CT versus dual-energy CT | ||||
| Absolute noise difference [HU] | 5.7 (4.0–7.6) | 5.8 (3.9–7.8) | 5.7 (4.1–7.3) | 5.9 (4.1–7.2) |
| DLP difference [mGy·cm] | 134.6 (78.9–249.9) | 104.0.6 (68.3–165.0) | 134.5 (85.4–215.2) | 248.2 (121.5–377.6) |
| All Patients | No Obesity | Pre-Obesity | Obesity | |
|---|---|---|---|---|
| Full coverage of liver parenchyma in the DE-FOV | 238 (94.1%) | 110 (100%) | 70 (95.9%) | 58 (82.9%) |
| <1 cm of liver parenchyma outside of the DE-FOV | 5 (2.0%) | 0 (0%) | 0 (0%) | 5 (7.1%) |
| 1–2 cm of liver parenchyma outside of the DE-FOV | 5 (2.0%) | 0 (0%) | 3 (4.1%) | 2 (2.9%) |
| >2 cm of liver parenchyma outside of the DE-FOV | 5 (2.0%) | 0 (0%) | 0 (0%) | 5 (7.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huflage, H.; Kunz, A.S.; Hendel, R.; Kraft, J.; Weick, S.; Razinskas, G.; Sauer, S.T.; Pennig, L.; Bley, T.A.; Grunz, J.-P. Obesity-Related Pitfalls of Virtual versus True Non-Contrast Imaging—An Intraindividual Comparison in 253 Oncologic Patients. Diagnostics 2023, 13, 1558. https://doi.org/10.3390/diagnostics13091558
Huflage H, Kunz AS, Hendel R, Kraft J, Weick S, Razinskas G, Sauer ST, Pennig L, Bley TA, Grunz J-P. Obesity-Related Pitfalls of Virtual versus True Non-Contrast Imaging—An Intraindividual Comparison in 253 Oncologic Patients. Diagnostics. 2023; 13(9):1558. https://doi.org/10.3390/diagnostics13091558
Chicago/Turabian StyleHuflage, Henner, Andreas Steven Kunz, Robin Hendel, Johannes Kraft, Stefan Weick, Gary Razinskas, Stephanie Tina Sauer, Lenhard Pennig, Thorsten Alexander Bley, and Jan-Peter Grunz. 2023. "Obesity-Related Pitfalls of Virtual versus True Non-Contrast Imaging—An Intraindividual Comparison in 253 Oncologic Patients" Diagnostics 13, no. 9: 1558. https://doi.org/10.3390/diagnostics13091558
APA StyleHuflage, H., Kunz, A. S., Hendel, R., Kraft, J., Weick, S., Razinskas, G., Sauer, S. T., Pennig, L., Bley, T. A., & Grunz, J.-P. (2023). Obesity-Related Pitfalls of Virtual versus True Non-Contrast Imaging—An Intraindividual Comparison in 253 Oncologic Patients. Diagnostics, 13(9), 1558. https://doi.org/10.3390/diagnostics13091558







