Review on the Advancements of Stethoscope Types in Chest Auscultation
Abstract
1. Introduction
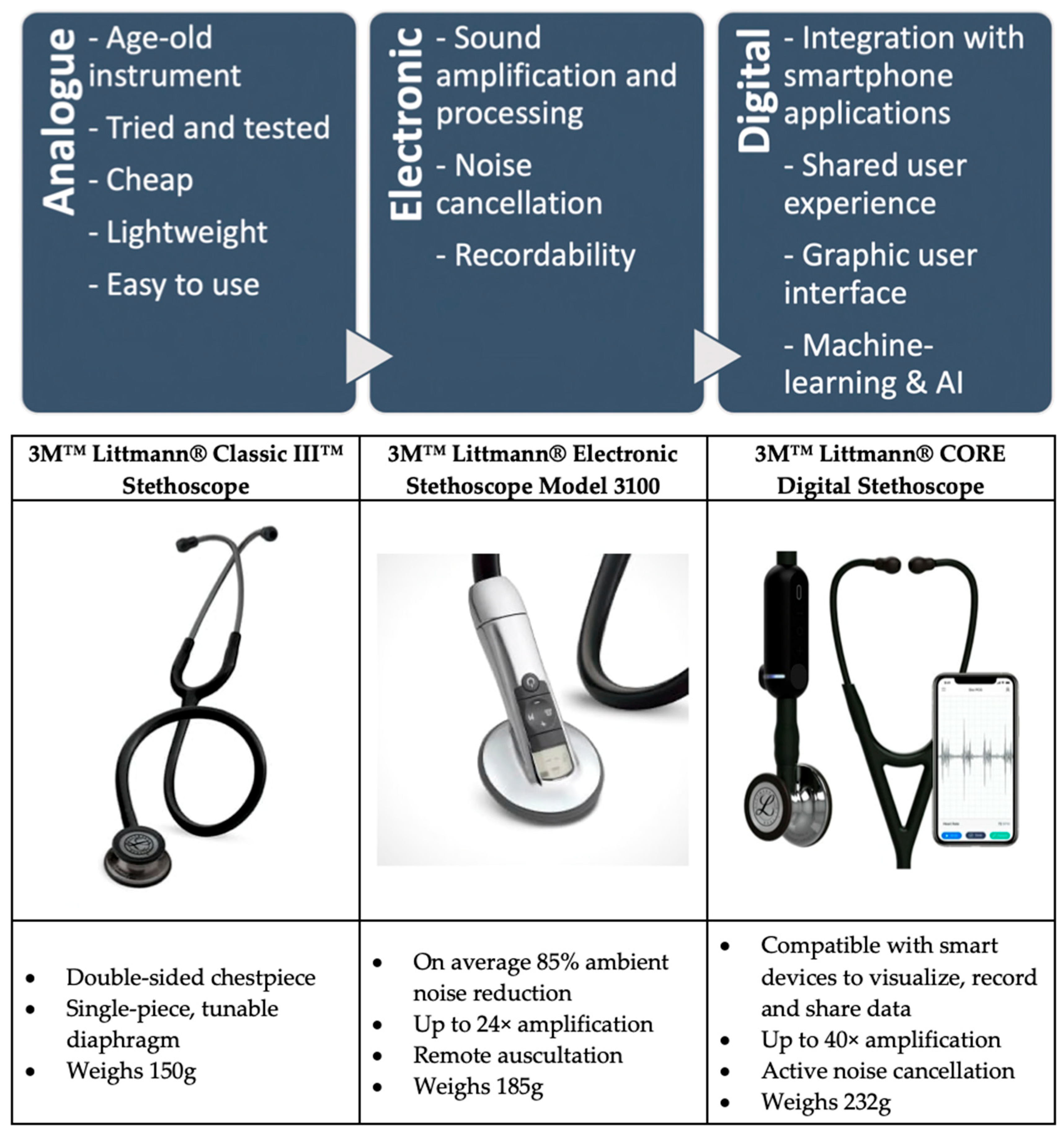
1.1. Analogue Stethoscope
1.2. Electronic Stethoscope
1.3. Digital Stethoscope
1.4. Potential Barriers to Implementation
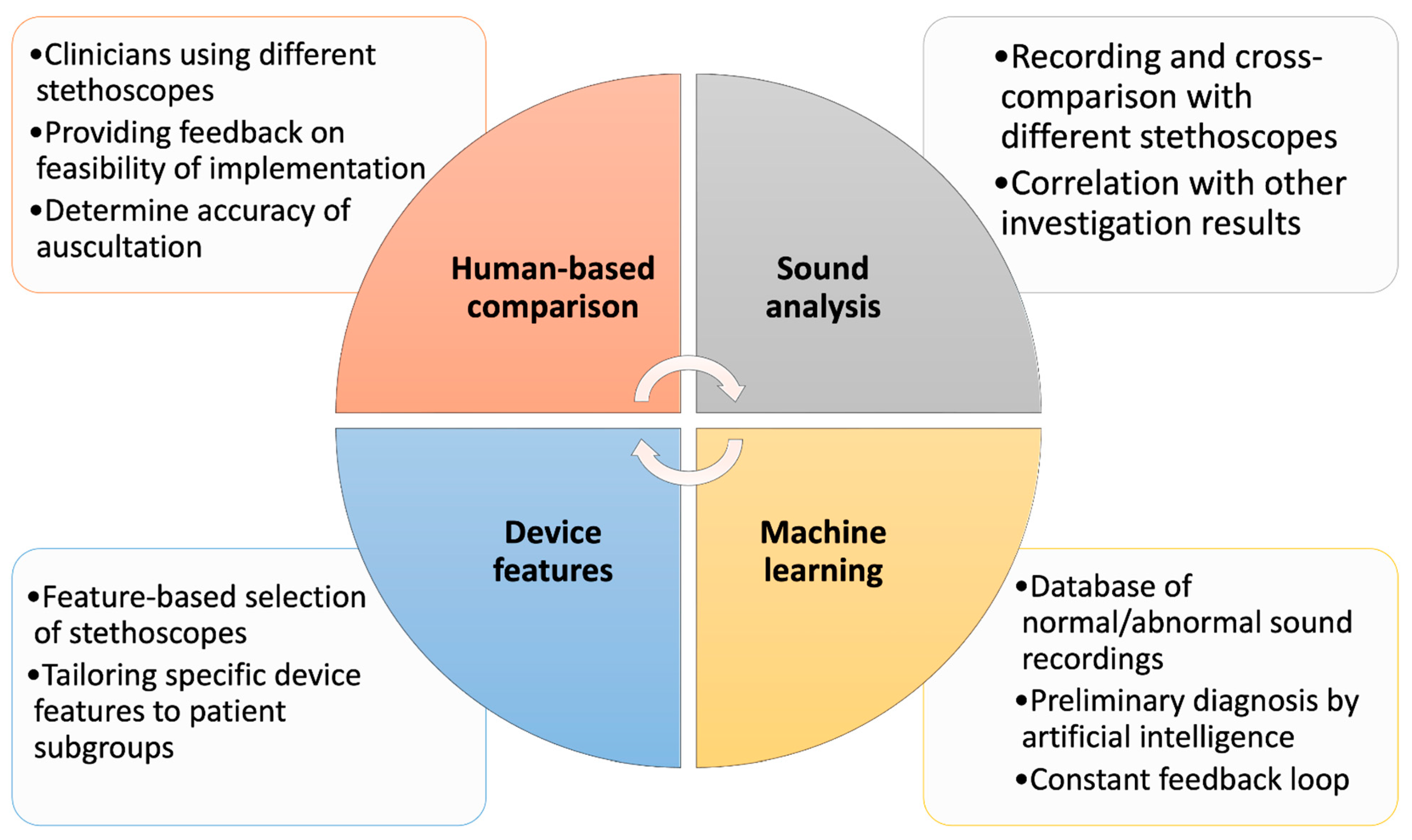
2. Benchmarking Methods
2.1. Human-Based Comparison
2.2. Audio Recording Data Comparison
2.3. Feature-Based Benchmarking
2.4. AI and Audio Data Comparison Analysis
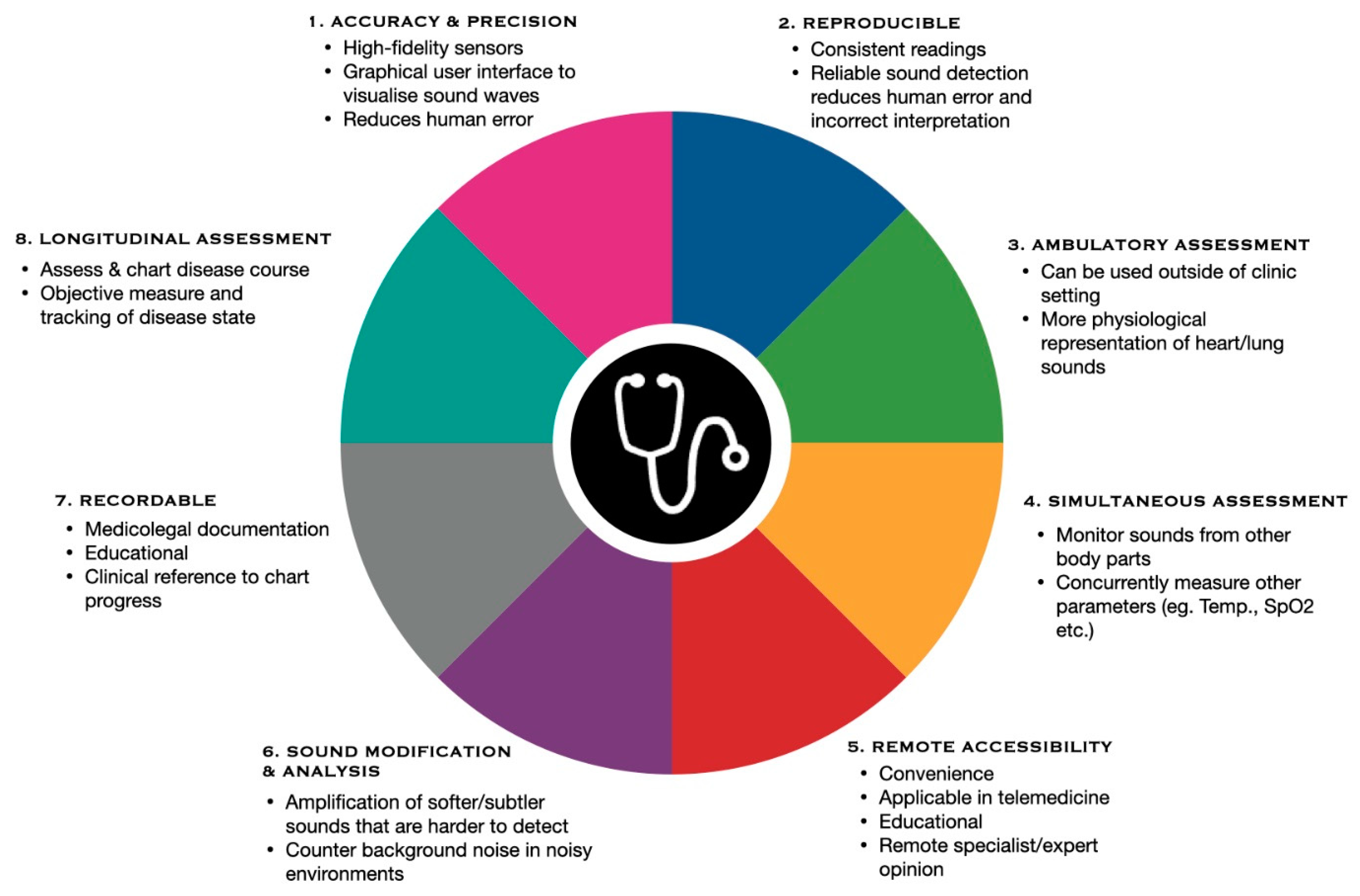
3. Potential Applications and Implementation of Digital Stethoscopes
3.1. Notable Advancements in Telemedicine
3.1.1. Benefits
3.1.2. Limitations
3.2. Potential Advancement in Wearable Devices Paired with Digital Stethoscopes
3.3. Contributions to Innovation of Smart Hospitals
3.4. Preventive Diagnosis and Monitoring
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beňačka, R. Auscultation of Lung Sounds and Murmurs. 1 January 2022. Available online: http://patfyz.medic.upjs.sk/simulatorvzorky/Respiratory+auscultation.htm (accessed on 8 December 2022).
- Legget, M.E.; Toh, M.; Meintjes, A.; Fitzsimons, S.; Gamble, G.; Doughty, R.N. Digital devices for teaching cardiac auscultation—A randomized pilot study. Med. Educ. Online 2018, 23, 1524688. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, H.; Kraman, S.S.; Wodicka, G.R. Respiratory Sounds. Am. J. Respir. Crit. Care Med. 1997, 156, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Thimbleby, H. Technology and the Future of Healthcare. J. Public Health Res. 2013, 2, e28. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, Y.-S.; Yeo, M.-K.; Mahmood, M.; Zavanelli, N.; Chung, C.; Heo, J.Y.; Kim, Y.; Jung, S.-S.; Yeo, W.-H. Fully portable continuous real-time auscultation with a soft wearable stethoscope designed for automated disease diagnosis. Sci. Adv. 2022, 8, eabo5867. [Google Scholar] [CrossRef]
- Michard, F. A sneak peek into digital innovations and wearable sensors for cardiac monitoring. J. Clin. Monit. Comput. 2016, 31, 253–259. [Google Scholar] [CrossRef]
- Nowak, L.J.; Nowak, K.M. Sound differences between electronic and acoustic stethoscopes. Biomed. Eng. Online 2018, 17, 104. [Google Scholar] [CrossRef]
- Littmann, D. An Approach to the Ideal Stethoscope. JAMA 1961, 178, 504–505. [Google Scholar] [CrossRef]
- 3M™. 3M™ Littmann® Classic III™ Stethoscope. 3M™ Littmann® Stethoscopes. 2023. Available online: https://www.littmann.3m.com.sg/3M/en_SG/littmann-stethoscopes-sg/products/~/3M-Littmann-Classic-III-Stethoscope-5811-Smoke-Finish-Chestpiece-Black-Tube-27-in-3-Each-Case/?N=5932256+8711017+3288984187+3294226472&preselect=8707414+8727096+3293786499&rt=rud (accessed on 15 April 2023).
- 3M™. Littmann® 3100 and 3200 Brochure. 3M™ Littmann® Stethoscopes. 2023. Available online: https://pdf.medicalexpo.com/pdf/3m-littmann-stethoscopes/littmann-3100-3200-brochure/70648-98443-_4.html (accessed on 15 April 2023).
- 3M™. 3M™ Littmann® CORE Digital Stethoscope. 3M™ Littmann® Stethoscopes. 2023. Available online: https://www.littmann.com/3M/en_US/littmann-stethoscopes/products/~/3M-Littmann-CORE-Digital-Stethoscope-8890-Mirror-Chestpiece-Black-Tube-Stem-and-Headset-27-inch/?N=5932256+8711017+3288508928+3294857497&preselect=8779523+3293786499&rt=rud (accessed on 15 April 2023).
- Bishop, P.J. Evolution of the Stethoscope. J. R. Soc. Med. 1980, 73, 448–456. [Google Scholar] [CrossRef]
- Ferns, T. Respiratory auscultation: How to use a stethoscope. Nurs. Times 2007, 103, 28–29. [Google Scholar]
- Landge, K.; Kidambi, B.R.; Singal, A.; Basha, A. Electronic stethoscopes: Brief review of clinical utility, evidence, and future implications. J. Pract. Cardiovasc. Sci. 2018, 4, 65. [Google Scholar] [CrossRef]
- Grenier, M.-C.; Gagnon, K.; Genest, J.; Durand, J.; Durand, L.-G. Clinical Comparison of Acoustic and Electronic Stethoscopes and Design of a New Electronic Stethoscope. Am. J. Cardiol. 1998, 81, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, M.; Agarwal, A. Stethoscope acoustics. J. Sound Vib. 2022, 539, 117194. [Google Scholar] [CrossRef]
- Takashina, T.; Shimizu, M.; Muratake, T.; Mayuzumi, S. New Stethoscope with Extensible Diaphragm. Circ. J. 2016, 80, 2047–2049. [Google Scholar] [CrossRef] [PubMed]
- Tavel, M.E. Cardiac Auscultation. Circulation 2006, 113, 1255–1259. [Google Scholar] [CrossRef]
- Leng, S.; Tan, R.S.; Chai, K.T.C.; Wang, C.; Ghista, D.; Zhong, L. The electronic stethoscope. Biomed. Eng. Online 2015, 14, 66. [Google Scholar] [CrossRef]
- Reinhart, R.A. “The report of my death…”. Chest 2022, 162, 872–877. [Google Scholar] [CrossRef]
- Luo, H.; Lamata, P.; Bazin, S.; Bautista, T.; Barclay, N.; Shahmohammadi, M.; Lubrecht, J.M.; Delhaas, T.; Prinzen, F.W. Smartphone as an electronic stethoscope: Factors influencing heart sound quality. Eur. Heart J.-Digit. Health 2022, 3, 473–480. [Google Scholar] [CrossRef]
- Perera, H. Advancements of Electronic Stethoscope: A Review. Available online: http://ir.kdu.ac.lk/handle/345/4811 (accessed on 23 February 2023).
- Swarup, S.; Makaryus, A.N. Digital stethoscope: Technology update. Med. Devices Evid. Res. 2018, 11, 29–36. [Google Scholar] [CrossRef]
- Bakshi, N.K.; Gupta, M. Wireless Electronic Stethoscope. Int. J. Eng. Res. Technol. 2014, 3. [Google Scholar]
- Silverman, B.; Balk, M. Digital Stethoscope—Improved Auscultation at the Bedside. Am. J. Cardiol. 2019, 123, 984–985. [Google Scholar] [CrossRef]
- Myint, W.W.; Dillard, B. An electronic stethoscope with diagnosis capability. In Proceedings of the 33rd Southeastern Symposium on System Theory (Cat. No.01EX460), Athens, OH, USA, 20 March 2001. [Google Scholar] [CrossRef]
- Cain, P.A.; Ahroon, W.A.; Greenburg, D. An Assessment of Acoustic and Electronic Stethoscope Performance in the UH-60 Noise Environment; Army Aeromedical Research Laboratory: Fort Novosel, AL, USA, 2002. [Google Scholar]
- Tourtier, J.P.; Fontaine, E.; Coste, S.; Ramsang, S.; Schiano, P.; Viaggi, M.; Libert, N.; Durand, X.; Chargari, C.; Borne, M. In flight auscultation: Comparison of electronic and conventional stethoscopes. Am. J. Emerg. Med. 2011, 29, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.R.; Ramakrishna, P.; Marjorie, S.R. Review of A Low Cost Digital Stethoscope. Indian J. Public Health Res. Dev. 2017, 8, 1259–1261. [Google Scholar] [CrossRef]
- Kevat, A.C.; Marzbanrad, F.; Roseby, R. Making digital auscultation accessible and accurate. Pediatr. Pulmonol. 2020, 56, 352–353. [Google Scholar] [CrossRef] [PubMed]
- Rennoll, V.; McLane, I.; Emmanouilidou, D.; West, J.; Elhilali, M. Electronic Stethoscope Filtering Mimics the Perceived Sound Characteristics of Acoustic Stethoscope. IEEE J. Biomed. Health Inform. 2020, 25, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Makino, H.; Sanjo, Y.; Nakai, T.; Mochizuki, K.; Shiraishi, Y.; Katoh, T.; Sato, S. A visual stethoscope for pediatric patient. Pediatr. Anesth. 2008, 18, 339. [Google Scholar] [CrossRef]
- Chowdhury, M.E.; Khandakar, A.; Alzoubi, K.; Mansoor, S.; Tahir, A.M.; Reaz, M.B.I.; Al-Emadi, N. Real-Time Smart-Digital Stethoscope System for Heart Diseases Monitoring. Sensors 2019, 19, 2781. [Google Scholar] [CrossRef] [PubMed]
- Andrès, E.; Reichert, S.; Brandt, C.; Hill, N.; Gass, R. Development and experimentation of a new digital communicating and intelligent stethoscope. Eur. Res. Telemed. 2016, 5, 145–155. [Google Scholar] [CrossRef]
- Alqudah, A.M.; Qazan, S.; Obeidat, Y.M. Deep learning models for detecting respiratory pathologies from raw lung auscultation sounds. Soft Comput. 2022, 26, 13405–13429. [Google Scholar] [CrossRef]
- DeGroff, C.G.; Bhatikar, S.; Hertzberg, J.; Shandas, R.; Valdes-Cruz, L.; Mahajan, R.L. Artificial Neural Network–Based Method of Screening Heart Murmurs in Children. Circulation 2001, 103, 2711–2716. [Google Scholar] [CrossRef]
- Brunese, L.; Mercaldo, F.; Reginelli, A.; Santone, A. A Neural Network-Based Method for Respiratory Sound Analysis and Lung Disease Detection. Appl. Sci. 2022, 12, 3877. [Google Scholar] [CrossRef]
- Grønnesby, M.; Solis, J.C.A.; Holsbø, E.; Melbye, H.; Bongo, L.A. Feature Extraction for Machine Learning Based Crackle Detection in Lung Sounds from a Health Survey. arXiv 2017, arXiv:1706.00005. [Google Scholar]
- Ferreira-Cardoso, H.; Jácome, C.; Silva, S.; Amorim, A.; Redondo, M.T.; Fontoura-Matias, J.; Vicente-Ferreira, M.; Vieira-Marques, P.; Valente, J.; Almeida, R.; et al. Lung Auscultation Using the Smartphone—Feasibility Study in Real-World Clinical Practice. Sensors 2021, 21, 4931. [Google Scholar] [CrossRef]
- Hui, X.; Kan, E.C. Monitoring vital signs over multiplexed radio by near-field coherent sensing. Nat. Electron. 2017, 1, 74–78. [Google Scholar] [CrossRef]
- Schmidt, S.E.; Holst-Hansen, C.; Graff, C.; Toft, E.; Struijk, J. Segmentation of heart sound recordings by a duration-dependent hidden Markov model. Physiol. Meas. 2010, 31, 513–529. [Google Scholar] [CrossRef]
- Jain, A.; Sahu, R.; Jain, A.; Gaumnitz, T.; Sethi, P.; Lodha, R. Development and validation of a low-cost electronic stethoscope: DIY digital stethoscope. BMJ Innov. 2021, 7, 609–613. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.-S.; Zhou, H.-Y.; Dong, B.; Zhang, L.; Zhang, F.; Liu, S.-J.; Wu, Y.-F.; Yuan, S.-H.; Tang, M.-Y.; et al. Real-World Verification of Artificial Intelligence Algorithm-Assisted Auscultation of Breath Sounds in Children. Front. Pediatr. 2021, 9, 627337. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, P.; Druzgalski, C.; Morelos, R.L.; González, O.H.; Vidales, J. Acoustics based assessment of respiratory diseases using GMM classification. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010. [Google Scholar] [CrossRef]
- Celik, N.; Gagarin, R.; Huang, G.C.; Iskander, M.F.; Berg, B.W. Microwave Stethoscope: Development and Benchmarking of a Vital Signs Sensor Using Computer-Controlled Phantoms and Human Studies. IEEE Trans. Biomed. Eng. 2014, 61, 2341–2349. [Google Scholar] [CrossRef]
- Aviles-Solis, J.C.; Storvoll, I.; Vanbelle, S.; Melbye, H. use of spectrograms improves the classification of wheezes and crackles in an educational setting. Sci. Rep. 2020, 10, 8461. [Google Scholar] [CrossRef]
- Fraiwan, M.; Fraiwan, L.; Khassawneh, B.; Ibnian, A. A dataset of lung sounds recorded from the chest wall using an electronic stethoscope. Data Brief 2021, 35, 106913. [Google Scholar] [CrossRef]
- Rice, T. Learning to listen: Auscultation and the transmission of auditory knowledge. J. R. Anthr. Inst. 2010, 16, S41–S61. [Google Scholar] [CrossRef]
- Ferns, T.; West, S. The art of auscultation: Evaluating a patient’s respiratory pathology. Br. J. Nurs. 2008, 17, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Wei, Q.; Lee, S.; Lee, M. Novel Design of a Multimodal Technology-Based Smart Stethoscope for Personal Cardiovascular Health Monitoring. Sensors 2022, 22, 6465. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.; Silva, H.; Rocha, M. Design and development of a digital stethoscope encapsulation for simultaneous acquisition of phonocardiography and electrocardiography signals: The SmartHeart case study. J. Med. Eng. Technol. 2020, 44, 153–161. [Google Scholar] [CrossRef]
- Brown, C.; Chauhan, J.; Grammenos, A.; Han, J.; Hasthanasombat, A.; Spathis, D.; Xia, T.; Cicuta, P.; Mascolo, C. Exploring Automatic Diagnosis of COVID-19 from Crowdsourced Respiratory Sound Data. In Proceedings of the 26th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Virtual Event, CA, USA, 6–10 July 2020. [Google Scholar] [CrossRef]
- Vogel, L. Doctors need retraining to keep up with technological change. Can. Med. Assoc. J. 2018, 190, E920. [Google Scholar] [CrossRef]
- Innova Smart Technologies (Pvt.) Ltd.; Rehman Medical Institute—RMI (Directors). Comparison of uSteth to the Conventional Stethoscope for Auscultation of Heart and Lung Sounds; ClinicalTrial.gov: Bethesda, MD, USA, 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05252130 (accessed on 23 February 2022).
- Blass, K.A.; Schober, K.E.; Bonagura, J.D.; Scansen, B.A.; Visser, L.C.; Lu, J.; Smith, D.N.; Ward, J.L. Clinical evaluation of the 3M Littmann Electronic Stethoscope Model 3200 in 150 cats. J. Feline Med. Surg. 2013, 15, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, M.B.; Sprague, H.B. The effects of improper fitting of stethoscope to ears on auscultatory efficiency. Am. Heart J. 1952, 43, 713–715. [Google Scholar] [CrossRef]
- Liu, Y.; Norton, J.J.S.; Qazi, R.; Zou, Z.; Ammann, K.R.; Liu, H.; Yan, L.; Tran, P.L.; Jang, K.-I.; Lee, J.W.; et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2016, 2, e1601185. [Google Scholar] [CrossRef]
- Polat, H.; Güler, I. A Simple Computer-Based Measurement and Analysis System of Pulmonary Auscultation Sounds. J. Med. Syst. 2004, 28, 665–672. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Zhang, X.; Wang, Z.; Gao, Y.; Chen, G.; Xiong, H. Classifying respiratory sounds using electronic stethoscope. In Proceedings of the 2017 IEEE Smart World, Ubiquitous Intelligence & Computing, Advanced & Trusted Computed, Scalable Computing & Communications, Cloud & Big Data Computing, Internet of People and Smart City Innovation (SmartWorld/SCALCOM/UIC/ATC/CBDCom/IOP/SCI), San Francisco, CA, USA, 4–8 August 2017. [Google Scholar] [CrossRef]
- Abella, M.; Formolo, J.; Penney, D.G. Comparison of the acoustic properties of six popular stethoscopes. J. Acoust. Soc. Am. 1992, 91, 2224–2228. [Google Scholar] [CrossRef]
- Kanga, J.F.; Kraman, S.S. Comparison of the lung sound frequency spectra of infants and adults. Pediatr. Pulmonol. 1986, 2, 292–295. [Google Scholar] [CrossRef]
- Zhou, L.; Marzbanrad, F.; Ramanathan, A.; Fattahi, D.; Pharande, P.; Malhotra, A. Acoustic analysis of neonatal breath sounds using digital stethoscope technology. Pediatr. Pulmonol. 2020, 55, 624–630. [Google Scholar] [CrossRef]
- Kevat, A.C.; Bullen, D.V.R.; Davis, P.G.; Kamlin, C.O.F. A systematic review of novel technology for monitoring infant and newborn heart rate. Acta Paediatr. 2017, 106, 710–720. [Google Scholar] [CrossRef]
- Ramanathan, A.; Zhou, L.; Marzbanrad, F.; Roseby, R.; Tan, K.; Kevat, A.; Malhotra, A. Digital stethoscopes in paediatric medicine. Acta Paediatr. 2019, 108, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Anton, O.; Dore, H.; Rendon-Morales, E.; Aviles-Espinosa, R.; Seddon, P.; Wertheim, D.; Fernandez, R.; Rabe, H. Non-invasive sensor methods used in monitoring newborn babies after birth, a clinical perspective. Matern. Health Neonatol. Perinatol. 2022, 8, 9. [Google Scholar] [CrossRef]
- Belmont, J.M.; Mattioli, L.F. Accuracy of Analog Telephonic Stethoscopy for Pediatric Telecardiology. Pediatrics 2003, 112, 780–786. [Google Scholar] [CrossRef]
- Tiwari, H.K.; Harsola, A. Development of embedded stethoscope for Heart Sound. In Proceedings of the 2016 International Conference on Wireless Communications, Signal Processing and Networking (WiSPNET), Chennai, India, 23–25 March 2016. [Google Scholar] [CrossRef]
- Monika, V.; Bommi, R.; Prabu, L.H.; Murali, M.; Nirmala, S. Embedded Stethoscope for Real Time Diagnosis of Cardiovascular Diseases. In Proceedings of the 2017 IEEE International Conference on Computational Intelligence and Computing Research (ICCIC), Coimbatore, India, 14–16 December 2017. [Google Scholar] [CrossRef]
- Zuhlke, L.; Myer, L.; Mayosi, B. The promise of computer-assisted auscultation in screening for structural heart disease and clinical teaching. Cardiovasc. J. Afr. 2012, 23, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Han, C.-C.; Chang, C.-S.; Chang, F.-L.; Chen, S.-F.; Shieh, T.-Y.; Chen, H.-M.; Lin, J.-Y. Development of an Electronic Stethoscope and a Classification Algorithm for Cardiopulmonary Sounds. Sensors 2022, 22, 4263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, B.; Liu, Y.; Fan, M.; Ji, Y.; Xu, H.; Xu, M.; Chen, S.; Li, Q.; Zhang, Z. Lung Auscultation of Hospitalized Patients with SARS-CoV-2 Pneumonia via a Wireless Stethoscope. Int. J. Med. Sci. 2021, 18, 1415–1422. [Google Scholar] [CrossRef]
- Simeone, S.; Condit, D.; Nadler, E. Do Not Give Up Your Stethoscopes Yet—Telemedicine for Chronic Respiratory Diseases in the Era of COVID-19. Life 2022, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Lakhe, A.; Sodhi, I.; Warrier, J.; Sinha, V. Development of digital stethoscope for telemedicine. J. Med. Eng. Technol. 2016, 40, 20–24. [Google Scholar] [CrossRef] [PubMed]
- McMehan, C.; So, P.; Li, K.F.; Jasechko, G.; Poulin, M. Electronic Stethoscope for eHealth and Telemedicine. CMBES Proc. 2010, 33. [Google Scholar]
- Hailey, D. Some successes and limitations with telehealth in Canada. J. Telemed. Telecare 2001, 7 (Suppl. S2), 73–75. [Google Scholar] [CrossRef] [PubMed]
- Poon, Z.; Tan, N.C. A qualitative research study of primary care physicians’ views of telehealth in delivering postnatal care to women. BMC Prim. Care 2022, 23, 206. [Google Scholar] [CrossRef]
- Daruwalla, Z.J.; Wong, K.L.; Thambiah, J. Application of Telemedicine in Orthopedic Surgery in Singapore: A Pilot Study on a Secure, Mobile Telehealth Application and Messaging Platform. JMIR MHealth UHealth 2014, 2, e28. [Google Scholar] [CrossRef] [PubMed]
- Ying, N.L.; Kin, W.; Han, L.C.; Tiong, C.; Ali, K. Rapid Transition to a Telemedicine Service at Singapore Community Dialysis Centers During COVID-19. NEJM Catal. Innov. Care Deliv. 2020. [Google Scholar] [CrossRef]
- Portnoy, J.M.; Waller, M.; De Lurgio, S.; Dinakar, C. Telemedicine is as effective as in-person visits for patients with asthma. Ann. Allergy Asthma Immunol. 2016, 117, 241–245. [Google Scholar] [CrossRef]
- McConnochie, K.M.; Wood, N.E.; Kitzman, H.J.; Herendeen, N.E.; Roy, J.; Roghmann, K.J. Telemedicine Reduces Absence Resulting from Illness in Urban Child Care: Evaluation of an Innovation. Pediatrics 2005, 115, 1273–1282. [Google Scholar] [CrossRef]
- Young, T.L.; Ireson, C. Effectiveness of School-Based Telehealth Care in Urban and Rural Elementary Schools. Pediatrics 2003, 112, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Thakur, C.; Kumar, P.; Goyal, J.P.; Singh, K. Telemedicine for Asthma Follow-up in Children During COVID-19 Pandemic. Indian J. Pediatr. 2021, 88, 1050. [Google Scholar] [CrossRef]
- Litvak, M.; Miller, K.; Boyle, T.; Bedenbaugh, R.; Smith, C.; Meguerdichian, D.; Reisman, D.; Biddinger, P.; Licurse, A.; Goralnick, E. Telemedicine Use in Disasters: A Scoping Review. Disaster Med. Public Health Prep. 2021, 16, 791–800. [Google Scholar] [CrossRef]
- Doarn, C.R.; Merrell, R.C. Telemedicine and e-Health in Disaster Response. Telemed. E-Health 2014, 20, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, C. Ethical, Legal, and Social Challenges in the Development and Implementation of Disaster Telemedicine | Disaster Medicine and Public Health Preparedness. Camb. Core 2020, 15, 649–656. Available online: https://www.cambridge.org/core/journals/disaster-medicine-and-public-health-preparedness/article/ethical-legal-and-social-challenges-in-the-development-and-implementation-of-disaster-telemedicine/F521543AFCE0910D9645D51681054F73 (accessed on 20 December 2022).
- Pasipanodya, E.C.; Shem, K. Provision of care through telemedicine during a natural disaster: A case study. Spinal Cord Ser. Cases 2020, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Almathami, H.K.Y.; Win, K.T.; Vlahu-Gjorgievska, E. Barriers and Facilitators That Influence Telemedicine-Based, Real-Time, Online Consultation at Patients’ Homes: Systematic Literature Review. J. Med. Internet Res. 2020, 22, e16407. [Google Scholar] [CrossRef] [PubMed]
- Cordova, F.C.; Ciccolella, D.; Grabianowski, C.; Gaughan, J.; Brennan, K.; Goldstein, F.; Jacobs, M.R.; Criner, G.J. A Telemedicine-Based Intervention Reduces the Frequency and Severity of COPD Exacerbation Symptoms: A Randomized, Controlled Trial. Telemed. E-Health 2016, 22, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Falk, D.M.; Bonello, R.S.; Kahn, J.M.; Perencevich, E.; Cram, P. The Costs of Critical Care Telemedicine Programs. Chest 2013, 143, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Maeder, A.; Mars, M.; Hartvigsen, G.; Basu, A.; Abbott, P.; Gogia, S.B. Unintended Consequences of Tele Health and their Possible Solutions. Yearb. Med. Inform. 2016, 25, 41–46. [Google Scholar] [CrossRef]
- Troncoso, N.; Ortega, J.A.; Seepold, R.; Madrid, N.M. Non-invasive devices for respiratory sound monitoring. Procedia Comput. Sci. 2021, 192, 3040–3048. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, D.; Xue, L.; Chen, L. A Piezoelectric Heart Sound Sensor for Wearable Healthcare Monitoring Devices. In Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Springer International Publishing: New York, NY, USA, 2019; pp. 12–23. [Google Scholar] [CrossRef]
- Gupta, P.; Moghimi, M.J.; Jeong, Y.; Gupta, D.; Inan, O.T.; Ayazi, F. Precision wearable accelerometer contact microphones for longitudinal monitoring of mechano-acoustic cardiopulmonary signals. NPJ Digit. Med. 2020, 3, 19. [Google Scholar] [CrossRef]
- Yilmaz, G.; Rapin, M.; Pessoa, D.; Rocha, B.M.; de Sousa, A.M.; Rusconi, R.; Carvalho, P.; Wacker, J.; Paiva, R.P.; Chételat, O. A Wearable Stethoscope for Long-Term Ambulatory Respiratory Health Monitoring. Sensors 2020, 20, 5124. [Google Scholar] [CrossRef]
- Klum, M.; Leib, F.; Oberschelp, C.; Martens, D.; Pielmus, A.-G.; Tigges, T.; Penzel, T.; Orglmeister, R. Wearable Multimodal Stethoscope Patch for Wireless Biosignal Acquisition and Long-Term Auscultation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar] [CrossRef]
- Kwon, H.; An, S.; Lee, H.-Y.; Cha, W.C.; Kim, S.; Cho, M.; Kong, H.-J. Review of Smart Hospital Services in Real Healthcare Environments. Health Inform. Res. 2022, 28, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Kim, S.; Kim, E.; Jung, E.; Lee, K.-H.; Hwang, H. Real-time location system-based asset tracking in the healthcare field: Lessons learned from a feasibility study. BMC Med. Inform. Decis. Mak. 2018, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Medical 4.0 technologies for healthcare: Features, capabilities, and applications. Internet Things Cyber-Phys. Syst. 2022, 2, 12–30. [Google Scholar] [CrossRef]
- Naik, N.; Hameed, B.M.Z.; Sooriyaperakasam, N.; Vinayahalingam, S.; Patil, V.; Smriti, K.; Saxena, J.; Shah, M.; Ibrahim, S.; Singh, A.; et al. Transforming healthcare through a digital revolution: A review of digital healthcare technologies and solutions. Front. Digit. Health 2022, 4, 919985. Available online: https://www.frontiersin.org/articles/10.3389/fdgth.2022.919985/full (accessed on 23 February 2023). [CrossRef]
- Katarzyna, M. Publication—The Use of an Electronic Stethoscope with Dedicated Software for Cardiovascular Screening of Patients Prepared for Hip Replacement—Military University of Technology. Available online: https://repo.bg.wat.edu.pl/info/article/WAT3c94e78bbb334edc84b4ba223fa67545/ (accessed on 20 December 2022).
- Nileshwar, A.; Ahuja, V.; Kini, P. Evaluation of the electronic stethoscope (FONODOC) as a cardiac screening tool during the preoperative evaluation of children. Indian J. Anaesth. 2022, 66, 625. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Tai, C.-C.; Chou, W.; Tang, S.-T.; Lin, J.-H. Analyzing the audio signals of degenerative arthritis with an electronic stethoscope. Rev. Sci. Instrum. 2018, 89, 085111. [Google Scholar] [CrossRef]
- King, A.; Blank, D.; Bhatia, R.; Marzbanrad, F.; Malhotra, A. Tools to assess lung aeration in neonates with respiratory distress syndrome. Acta Paediatr. 2019, 109, 667–678. [Google Scholar] [CrossRef]
- Ghanayim, T.; Lupu, L.; Naveh, S.; Bachner-Hinenzon, N.; Adler, D.; Adawi, S.; Banai, S.; Shiran, A. Artificial Intelligence-Based Stethoscope for the Diagnosis of Aortic Stenosis. Am. J. Med. 2022, 135, 1124–1133. [Google Scholar] [CrossRef]
- Dudik, J.M.; Coyle, J.L.; Sejdic, E. Dysphagia Screening: Contributions of Cervical Auscultation Signals and Modern Signal-Processing Techniques. IEEE Trans. Hum.-Mach. Syst. 2015, 45, 465–477. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, X.; Yu, Q.; Zhang, Y.; Li, Y.; Zhao, J.; Wang, G. LungBRN: A Smart Digital Stethoscope for Detecting Respiratory Disease Using bi-ResNet Deep Learning Algorithm. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seah, J.J.; Zhao, J.; Wang, D.Y.; Lee, H.P. Review on the Advancements of Stethoscope Types in Chest Auscultation. Diagnostics 2023, 13, 1545. https://doi.org/10.3390/diagnostics13091545
Seah JJ, Zhao J, Wang DY, Lee HP. Review on the Advancements of Stethoscope Types in Chest Auscultation. Diagnostics. 2023; 13(9):1545. https://doi.org/10.3390/diagnostics13091545
Chicago/Turabian StyleSeah, Jun Jie, Jiale Zhao, De Yun Wang, and Heow Pueh Lee. 2023. "Review on the Advancements of Stethoscope Types in Chest Auscultation" Diagnostics 13, no. 9: 1545. https://doi.org/10.3390/diagnostics13091545
APA StyleSeah, J. J., Zhao, J., Wang, D. Y., & Lee, H. P. (2023). Review on the Advancements of Stethoscope Types in Chest Auscultation. Diagnostics, 13(9), 1545. https://doi.org/10.3390/diagnostics13091545







