Investigating the Impact of COVID-19 Infection on Dry Eye Parameters
Abstract
1. Introduction
2. Methods
2.1. Study Subject
2.2. Ophthalmic Examinations
2.3. Statistical Analyses
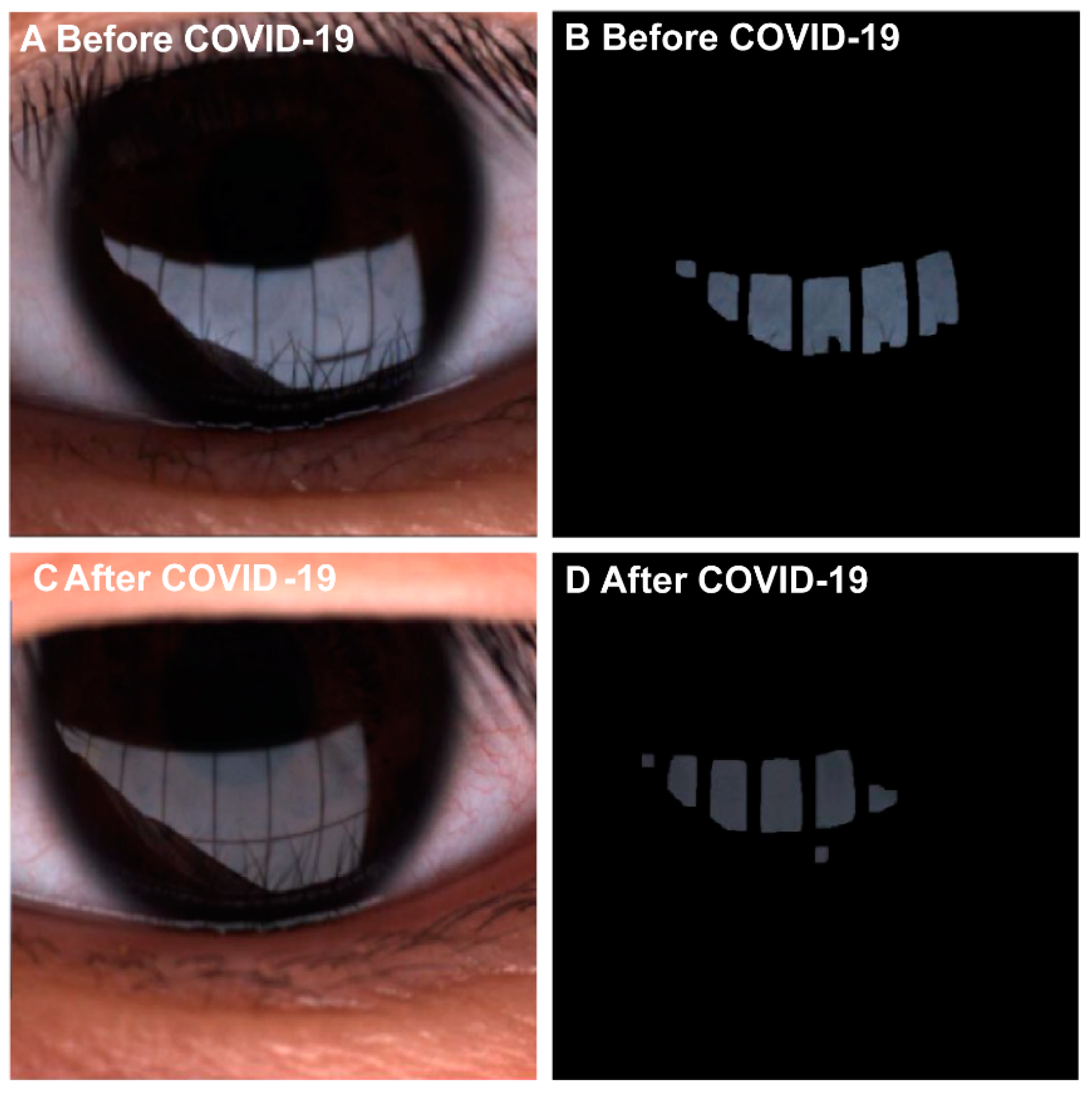
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrand, K.F.; Fridman, M.; Stillman, I.; Schaumberg, D.A. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Kolanu, S. Contact lens wear and dry eyes: Challenges and solutions. Clin. Optom. 2017, 9, 41–48. [Google Scholar] [CrossRef]
- Lin, F.; Cai, Y.; Fei, X.; Wang, Y.; Zhou, M.; Liu, Y. Prevalence of dry eye disease among Chinese high school students during the COVID-19 outbreak. BMC Ophthalmol. 2022, 22, 190. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.-M.; Tchah, H.-W.; Hyon, J.Y.; et al. A New Perspective on Dry Eye Classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 2020, 46 (Suppl. S1), S2–S13. [Google Scholar] [CrossRef]
- Uchino, M.; Nishiwaki, Y.; Michikawa, T.; Shirakawa, K.; Kuwahara, E.; Yamada, M.; Dogru, M.; Schaumberg, D.A.; Kawakita, T.; Takebayashi, T.; et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology 2011, 118, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Vaidya, A.; Kakizaki, H. Changes in Dry Eye Status after Steroid Pulse and Orbital Radiation Therapies in Active Thyroid Eye Disease. J. Clin. Med. 2022, 11, 3604. [Google Scholar] [CrossRef]
- Gandolfo, S.; Ciccia, F. JAK/STAT Pathway Targeting in Primary Sjögren Syndrome. Rheumatol. Immunol. Res. 2022, 3, 95–102. [Google Scholar] [CrossRef]
- Wan, K.H.; Lui, G.C.Y.; Poon, K.C.F.; Ng, S.S.S.; Young, A.L.; Hui, D.S.C.; Tham, C.C.Y.; Chan, P.K.S.; Pang, C.P.; Chong, K.K.L. Ocular surface disturbance in patients after acute COVID-19. Clin. Exp. Ophthalmol. 2022, 50, 398–406. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2, A comparative computational study of spike protein. J. Med. Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, R.K.; Chen, I.P.; Ma, T.; Syed, A.M.; Brazer, N.; Saldhi, P.; Simoneau, C.R.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature 2022, 607, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Li, N.; Deng, X.G.; He, M.F. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. Int. J. Ophthalmol. 2012, 5, 478–481. [Google Scholar]
- Fukushima, A.; Ohashi, Y.; Ebihara, N.; Uchio, E.; Okamoto, S.; Kumagai, N.; Shoji, J.; Takamura, E.; Nakagawa, Y.; Namba, K.; et al. Therapeutic effects of 0.1% tacrolimus eye drops for refractory allergic ocular diseases with proliferative lesion or corneal involvement. Br. J. Ophthalmol. 2014, 98, 1023–1027. [Google Scholar]
- Finis, D.; Pischel, N.; Schrader, S.; Geerling, G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea 2013, 32, 1549–1553. [Google Scholar] [CrossRef]
- Arita, R.; Itoh, K.; Inoue, K.; Amano, S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008, 115, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.G.; Potts, K.; Kim, J. Noninvasive tear breakup times and ocular surface disease. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2013, 90, 1086–1091. [Google Scholar] [CrossRef]
- Partridge, L.J.; Urwin, L.; Nicklin, M.J.H.; James, D.C.; Green, L.R.; Monk, P.N. ACE2-Independent Interaction of SARS-CoV-2 Spike Protein with Human Epithelial Cells Is Inhibited by Unfractionated Heparin. Cells 2021, 10, 1419. [Google Scholar] [PubMed]
- Raghuvamsi, P.V.; Tulsian, N.K.; Samsudin, F.; Qian, X.; Purushotorman, K.; Yue, G.; Kozma, M.M.; Hwa, W.Y.; Lescar, J.; Bond, P.J.; et al. SARS-CoV-2 S protein:ACE2 interaction reveals novel allosteric targets. eLife 2021, 10, e63646. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Shahraki, T.; Hassanpour, K.; Arabi, A.; Ansari, I.; Sadoughi, M.M. Corona virus disease 2019-associated Stevens-Johnson syndrome: A case report. BMC Ophthalmol. 2021, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Kapelushnik, N.; Benyosef, S.; Skaat, A.; Abdelkader, A.; Landau Prat, D.; Blum-Meirovitch, S.; Leshno, A. The Effect of Face Masks during COVID-19 Pandemic on Ocular Surface Temperature-A Clinical Thermographic Analysis. Diagnostics 2022, 12, 1431. [Google Scholar] [CrossRef]
- Mohammad Alrawashdeh, H.; Al Zubi, K.; Abdulmannan, D.M.; Al-Habahbeh, O.; Abu-Ismail, L. Conjunctivitis as the only sign and symptom of COVID-19, A case report and review of literature. Qatar Med. J. 2021, 2021, 31. [Google Scholar] [CrossRef]
- Tavakoli, A.; Markoulli, M.; Papas, E.; Flanagan, J. The Impact of Probiotics and Prebiotics on Dry Eye Disease Signs and Symptoms. J. Clin. Med. 2022, 11, 4889. [Google Scholar]
- Andersson, J.; Vogt, J.K.; Dalgaard, M.D.; Pedersen, O.; Holmgaard, K.; Heegaard, S. Ocular surface microbiota in patients with aqueous tear-deficient dry eye. Ocul. Surf. 2021, 19, 210–217. [Google Scholar] [CrossRef]
- Mak, J.W.Y.; Chan, F.K.L.; Ng, S.C. Probiotics and COVID-19, one size does not fit all. Lancet Gastroenterol. Hepatol. 2020, 5, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Abdulmannan, D.M.; Naser, A.Y.; Ibrahim, O.K.; Mahmood, A.S.; Alkrad, J.A.; Sweiss, K.; Alrawashdeh, H.M.; Kautsar, A.P. Visual health and prevalence of dry eye syndrome among university students in Iraq and Jordan. BMC Ophthalmol. 2022, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Allayed, R.; Ayed, A.; Fashafsheh, I. Prevalence and Risk Factors Associated with Symptomatic Dry Eye in Nurses in Palestine During the COVID-19 Pandemic. SAGE Open Nurs. 2022, 8, 23779608221127948. [Google Scholar] [CrossRef] [PubMed]
- Tangmonkongvoragul, C.; Chokesuwattanaskul, S.; Khankaeo, C.; Punyasevee, R.; Nakkara, L.; Moolsan, S.; Unruan, O. Prevalence of symptomatic dry eye disease with associated risk factors among medical students at Chiang Mai University due to increased screen time and stress during COVID-19 pandemic. PloS ONE 2022, 17, e0265733. [Google Scholar] [CrossRef] [PubMed]
- Uzun, S.L.; Topcu, H. The relationship of distance learning with ocular surface disorders in students in the COVID-19 pandemic. Int. Ophthalmol. 2022, 42, 3045–3051. [Google Scholar] [CrossRef]
- Acet, Y.; Çil, B.; Kabak, M.; Vural, E. Instability of Tear Film after Novel Coronavirus Disease: A Noninvasive and No Contact Method by a Scheimpflug-Placido Disc Topographer. Klin. Mon. Fur Augenheilkd. 2022, 239, 338–345. [Google Scholar] [CrossRef]
- Pardhan, S.; Islam, M.S.; López-Sánchez, G.F.; Upadhyaya, T.; Sapkota, R.P. Self-isolation negatively impacts self-management of diabetes during the coronavirus (COVID-19) pandemic. Diabetol. Metab. Syndr. 2021, 13, 123. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Zhang, Y.; Zhang, X.; Jiang, Y.; Wang, X.; Zheng, P.; Chen, Y. Symptoms of Dry Eye Disease in Hospitalized Patients with Coronavirus Disease 2019 (COVID-19). J. Ophthalmol. 2021, 2021, 2678706. [Google Scholar] [CrossRef]
- Bayer, I.S. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Inanc, N.; Kostov, B.; Priori, R.; Flores-Chavez, A.; Carubbi, F.; Szántó, A.; Valim, V.; Bootsma, H.; Praprotnik, S.; Trevisani, V.F.; et al. Safety and efficacy of SARS-CoV-2 vaccination in 1237 patients with primary Sjögren syndrome. Clin. Exp. Rheumatol. 2022, 40, 2290–2297. [Google Scholar] [CrossRef] [PubMed]

| Post-COVID-19 | Non-COVID-19 | p-Value | |
|---|---|---|---|
| Patient numbers | 22 | 22 | |
| Eye numbers | 44 | 44 | |
| Age (years) | 38.36 ± 14.99 | 42.32 ± 17.52 | 0.372 |
| Female: Male | 18:4 | 15:7 | 0.296 |
| Intervals times (months) | |||
| 1st Test to 2nd test | 16.92 ± 5.40 | 12.94 ± 4.11 | 0.113 |
| Infection to 2nd test | 4.61 ± 2.39 | NA | NA |
| COVID-vaccine (N%) # | 0.001 | ||
| 0 doses | 15 (68.18%) | 0 (0.00%) | |
| 2 doses | 3 (13.64%) | 1 (4.55%) | |
| 3 doses | 3 (13.64%) | 21 (95.45%) | |
| 4 doses | 1 (4.55%) | 0 (0.00%) |
| Post-COVID-19 | Non-COVID-19 | |||||
|---|---|---|---|---|---|---|
| Before | After | p-Value # | Baseline | Follow-Up | p-Value # | |
| Eye numbers | 44 | 44 | 44 | 44 | ||
| Visual acuity (Log MAR) | −0.01 ± 0.14 | 0.02 ± 0.15 | 0.236 | −0.02 ± 0.12 | 0.01 ± 0.15 | 0.130 |
| OSDI | 18.64 ± 18.15 | 14.68 ±12.99 | 0.133 | 20.68 ± 18.30 | 25.50 ± 19.32 | 0.063 |
| NIKBUT-first(s) | 9.74 ± 4.65 | 9.15 ± 5.75 | 0.612 | 9.97 ± 5.53 | 11.49 ± 6.22 | 0.133 |
| NIKBUT-average(s) | 14.26 ± 4.35 | 12.96 ± 4.82 | 0.164 | 14.30 ± 5.32 | 15.99 ± 4.79 | 0.059 |
| Aqueous Parameters | ||||||
| Schirmer’s Test (mm) | 13.30 ± 9.21 | 14.48 ± 10.62 | 0.473 | 12.09 ± 10.32 | 13.02 ± 9.95 | 0.438 |
| Tear Meniscus Height (mm) | 0.21 ± 0.07 | 0.22 ± 0.07 | 0.252 | 0.28 ± 0.18 | 0.30 ± 0.19 | 0.193 |
| Lipid Parameters | ||||||
| LLT-average(nm) | 63.00 ± 22.40 | 52.86 ± 18.00 | <0.001 | 69.25 ± 23.87 | 74.41 ± 22.46 | 0.090 |
| LLT-max(nm) | 78.48 ± 20.55 | 67.89 ± 20.81 | <0.001 | 80.73 ± 18.20 | 85.84 ± 18.41 | 0.077 |
| LLT-min(nm) | 46.41 ± 22.20 | 42.25 ± 15.06 | 0.140 | 57.82 ± 22.42 | 59.14 ± 22.51 | 0.718 |
| Meibosocre upper eyelid (0–3) | 1.55 ± 0.73 | 1.75 ± 0.84 | 0.011 | 1.39 ± 0.84 | 1.50 ± 0.73 | 0.133 |
| Meibosocre lower eyelid (0–3) | 1.30 ± 0.51 | 1.43 ± 0.73 | 0.001 | 1.23 ± 0.74 | 1.34 ± 0.61 | 0.133 |
| Conjunctiva | ||||||
| Papillae (0–3) | 0.16 ± 0.37 | 0.61 ± 0.69 | 0.001 | 0.14 ± 0.41 | 0.30 ± 0.46 | 0.070 |
| Follicle (0–3) | 0.07 ± 0.33 | 0.27 ± 0.69 | 0.071 | 0.11 ± 0.39 | 0.16 ± 0.48 | 0.643 |
| Post-COVID-19 | Non-COVID-19 | ||
|---|---|---|---|
| Difference | Difference | p-Value # | |
| Eye numbers | 44 | 44 | |
| Visual acuity (Log MAR) | 0.03 ± 0.12 | 0.04 ± 0.15 | 0.734 |
| OSDI | −0.86 ± 17.55 | 4.82 ± 16.71 | 0.124 |
| NIKBUT-first(s) | −0.59 ± 7.67 | 1.52 ± 6.57 | 0.170 |
| NIKBUT-average(s) | −1.30 ± 6.11 | 1.69 ± 5.78 | 0.020 |
| Aqueous Parameters | |||
| Schirmer’s Test (mm) | 1.18 ± 10.83 | 0.93 ± 7.89 | 0.902 |
| Tear Meniscus Height (mm) | 0.02 ± 0.09 | 0.03 ± 0.14 | 0.619 |
| Lipid Parameters | |||
| LLT-average(nm) | −10.14 ± 16.29 | 5.16 ± 19.73 | <0.001 |
| LLT-max(nm) | −10.59 ± 15.48 | 5.11 ± 18.72 | <0.001 |
| LLT-min(nm) | −4.16 ± 18.36 | 1.32 ± 24.03 | 0.233 |
| Meibosocre upper eyelid (0–3) | 0.20 ± 0.51 | 0.11 ± 0.49 | 0.397 |
| Meibosocre lower eyelid (0–3) | 0.14 ± 0.59 | 0.11 ± 0.49 | 0.846 |
| Conjunctiva | |||
| Papillae (0–3) | 0.45 ± 0.82 | 0.20 ± 0.59 | 0.105 |
| Follicle (0–3) | 0.20 ± 0.73 | 0.05 ± 0.65 | 0.283 |
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| β | 95%CI | p-Value | β | 95%CI | p-Value | |
| Visual acuity (Log MAR) | −0.01 | −0.07, 0.05 | 0.765 | −0.02 | −0.08, 0.05 | 0.605 |
| OSDI | −5.68 | −15.69,4.33 | 0.266 | −4.80 | 14.69, 5.09 | 0.342 |
| NIKBUT-first(s) | −2.11 | −5.21, 1.00 | 0.184 | −1.98 | −4.89, 0.92 | 0.181 |
| NIKBUT-average(s) | −3.00 | −5.78, −0.21 | 0.035 | −2.98 | −5.82, −0.15 | 0.039 |
| Aqueous Parameters | ||||||
| Schirmer’s Test (mm) | 0.25 | −3.94, 4.44 | 0.907 | 0.74 | −3.31, 4.79 | 0.720 |
| Tear Meniscus Height (mm) | −0.01 | −0.07, 0.05 | 0.686 | 0.00 | −0.06, 0.06 | 0.933 |
| Lipid Parameters | ||||||
| LLT-average (nm) | −15.30 | −24.72, −5.87 | 0.002 | −14.12 | −22.66, −5.59 | 0.001 |
| LLT-max (nm) | −15.70 | −23.89, −7.52 | <0.001 | −15.65 | −23.09, −8.20 | <0.001 |
| LLT-min (nm) | −5.48 | −16.38, 5.42 | 0.325 | −3.74 | −14.39, 6.91 | 0.491 |
| Meibosocre upper eyelid (0–3) | 0.09 | −0.17, 0.36 | 0.500 | 0.08 | −0.19, 0.35 | 0.554 |
| Meibosocre lower eyelid (0–3) | 0.02 | −0.25, 0.29 | 0.868 | 0.07 | −0.18, 0.33 | 0.574 |
| Conjunctiva | ||||||
| Papillae (0–3) | 0.25 | −0.14, 0.64 | 0.210 | 0.22 | −0.16, 0.60 | 0.257 |
| Follicle (0–3) | 0.16 | −0.23, 0.55 | 0.425 | 0.18 | −0.22, 0.58 | 0.370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; Wong, A.C.C.; Wong, J.O.Y.; Jia, R.; Chen, W.; Wong, H.Y.M.; Aljufairi, F.M.A.A.; Lai, K.K.H.; Hu, Z.; Wei, Y.; et al. Investigating the Impact of COVID-19 Infection on Dry Eye Parameters. Diagnostics 2023, 13, 1524. https://doi.org/10.3390/diagnostics13091524
Liao X, Wong ACC, Wong JOY, Jia R, Chen W, Wong HYM, Aljufairi FMAA, Lai KKH, Hu Z, Wei Y, et al. Investigating the Impact of COVID-19 Infection on Dry Eye Parameters. Diagnostics. 2023; 13(9):1524. https://doi.org/10.3390/diagnostics13091524
Chicago/Turabian StyleLiao, Xulin, Arthur Chun Chi Wong, June Oi Yau Wong, Ruofan Jia, Wanxue Chen, Hanson Yiu Man Wong, Fatema Mohamed Ali Abdulla Aljufairi, Kenneth Ka Hei Lai, Zhichao Hu, Yingying Wei, and et al. 2023. "Investigating the Impact of COVID-19 Infection on Dry Eye Parameters" Diagnostics 13, no. 9: 1524. https://doi.org/10.3390/diagnostics13091524
APA StyleLiao, X., Wong, A. C. C., Wong, J. O. Y., Jia, R., Chen, W., Wong, H. Y. M., Aljufairi, F. M. A. A., Lai, K. K. H., Hu, Z., Wei, Y., Tham, C. C. Y., Pang, C. P., & Chong, K. K. L. (2023). Investigating the Impact of COVID-19 Infection on Dry Eye Parameters. Diagnostics, 13(9), 1524. https://doi.org/10.3390/diagnostics13091524








