Comparison of a Presbyopia-Correcting Supplementary Intraocular Lens Combination and a Capsular-Bag Lens: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
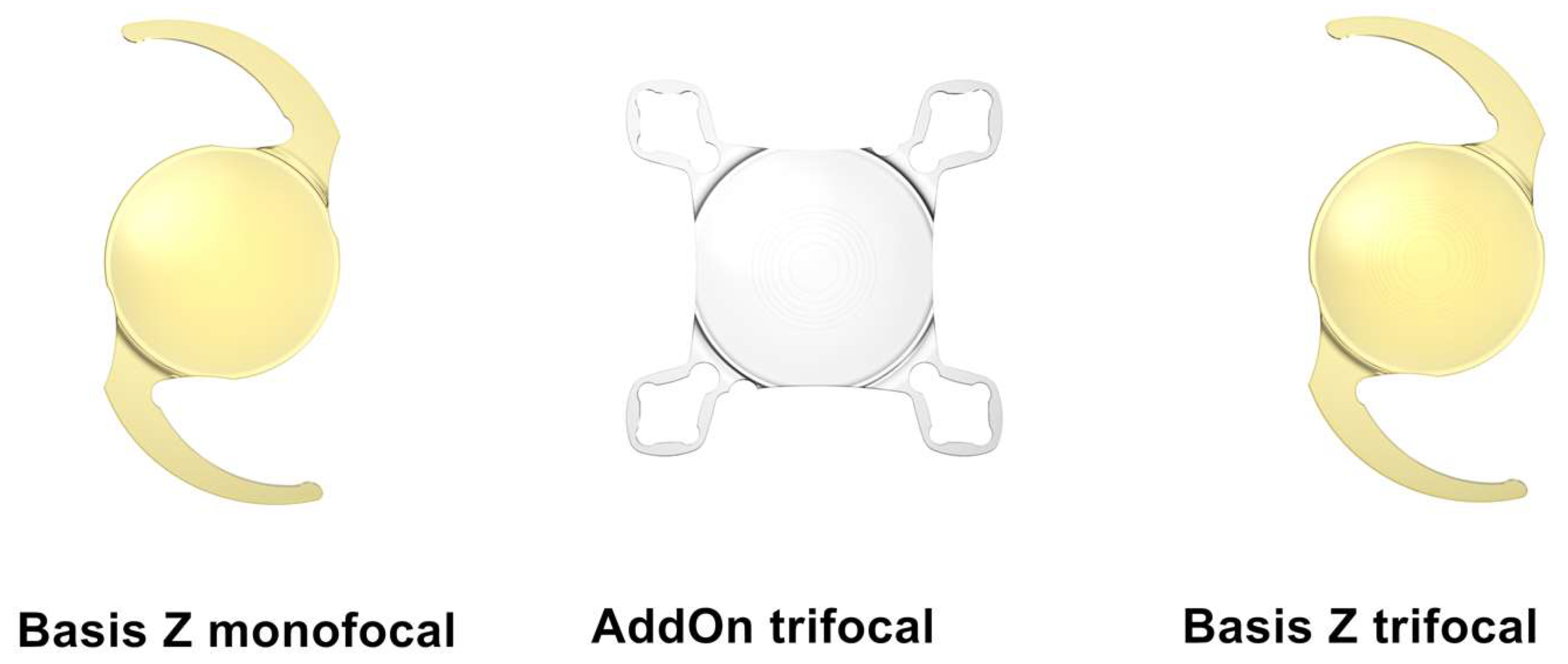
2.1. Intraocular Lenses
- monofocal Basis Z B1AWY0 with a dioptric power of +20.0;
- Basis Z Trifocal B1EWYN with a dioptric power of +20.0 and near addition of +3.5 diopters for capsular bag fixation;
- AddOn Trifocal A4DW0M with a dioptric power of 0.0 and near addition of +3.0 diopters for sulcus implantation.
2.2. Optical Metrology
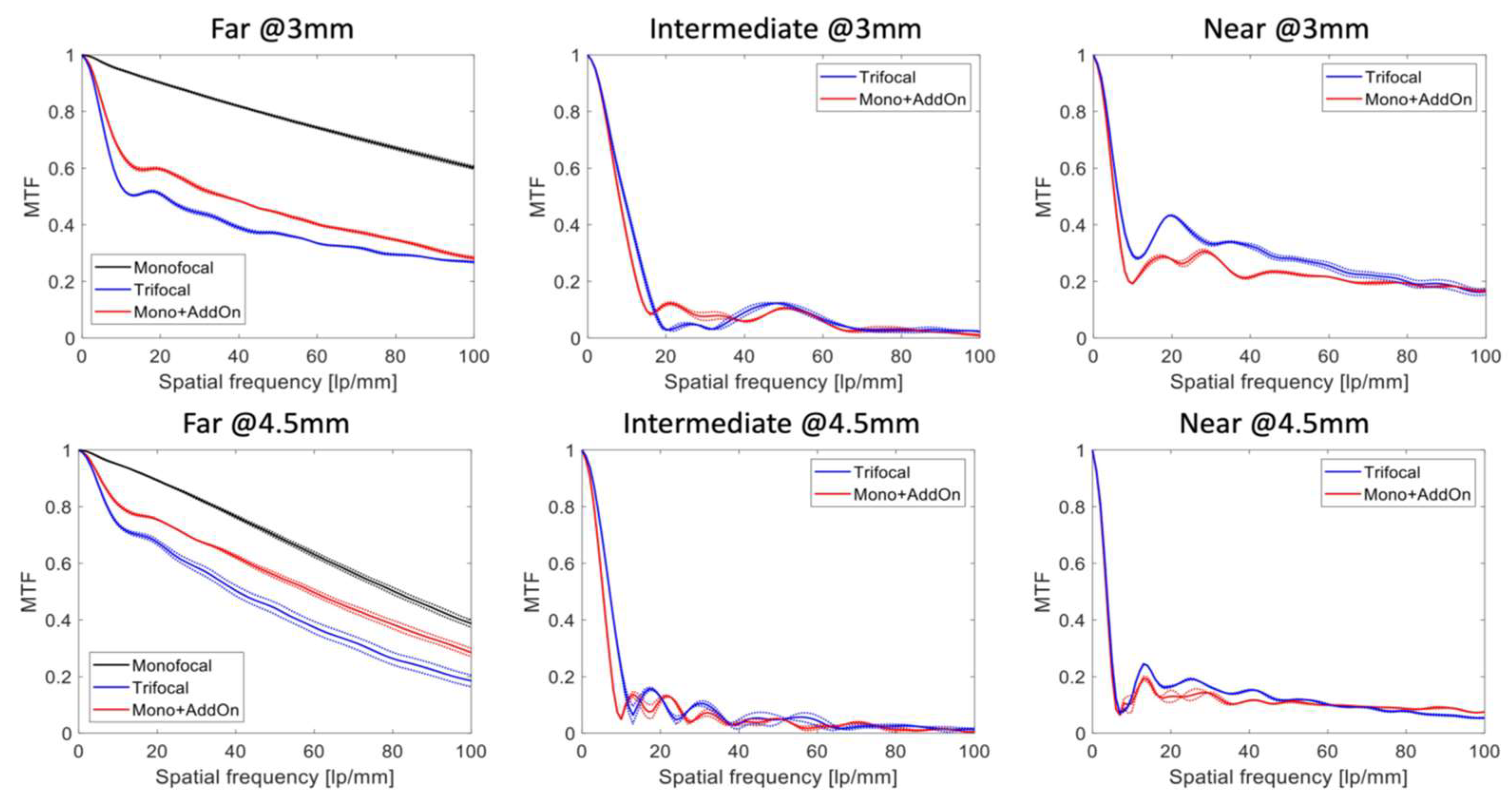
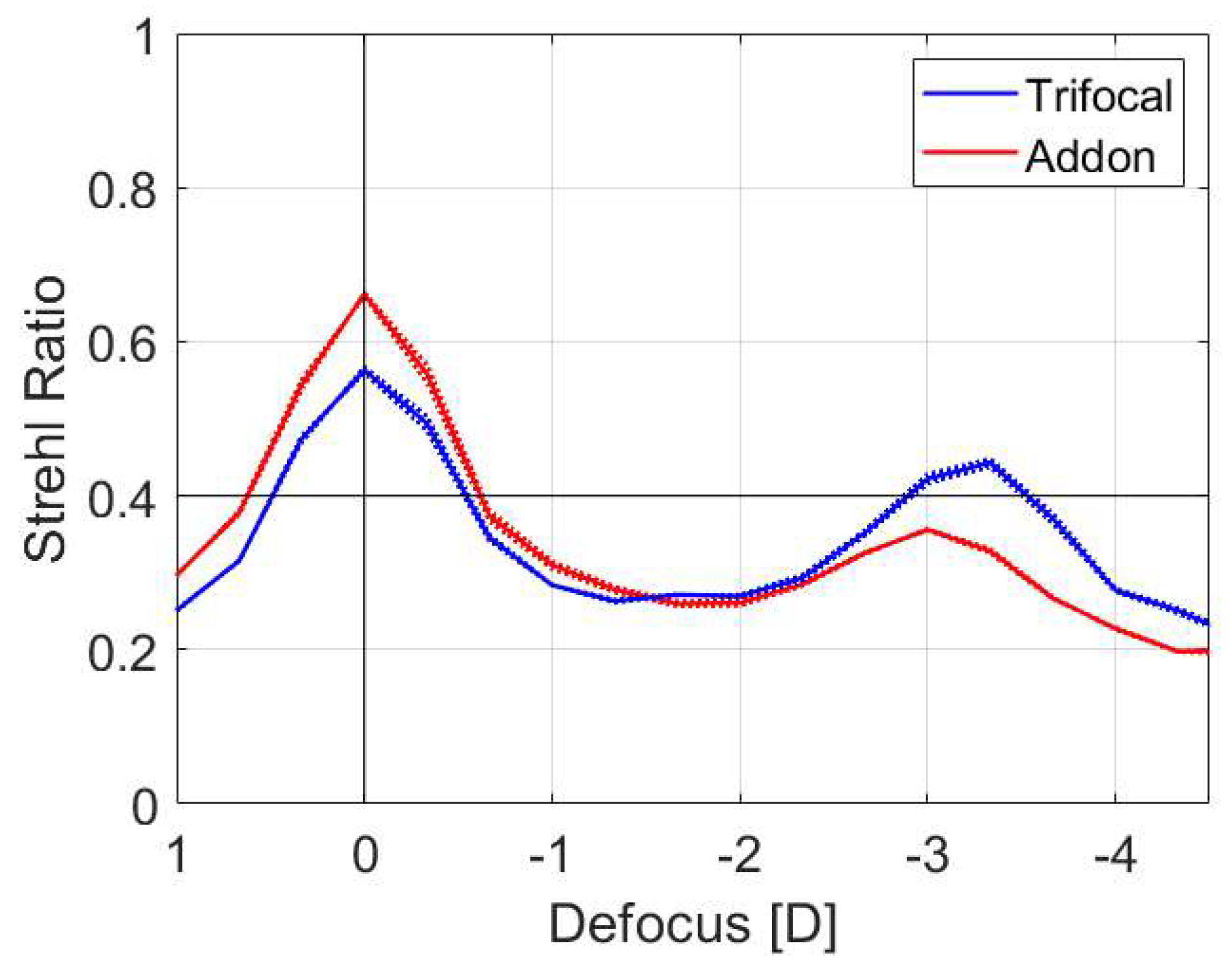
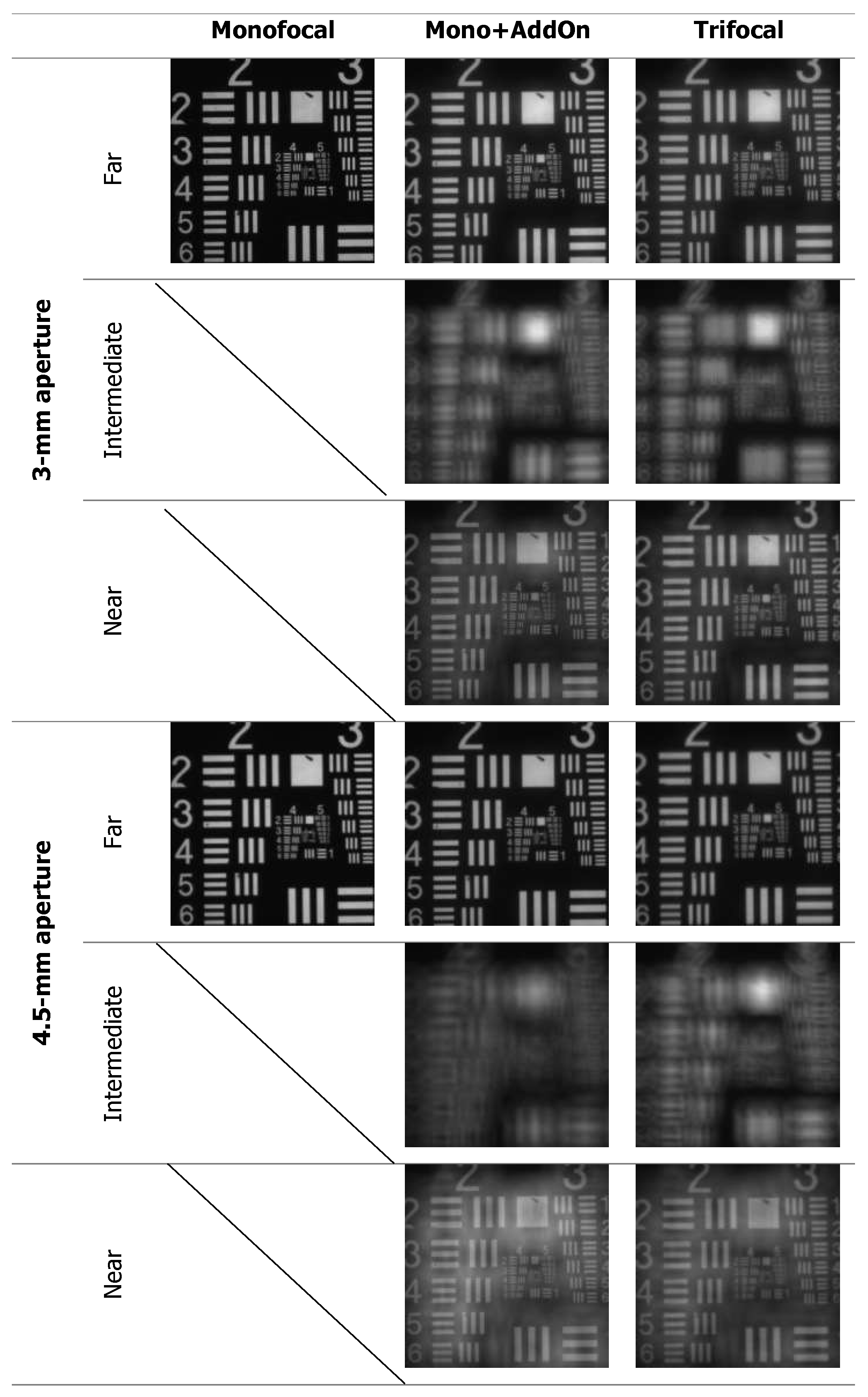
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breyer, D.R.; Kaymak, H.; Ax, T.; Kretz, F.T.; Auffarth, G.U.; Hagen, P.R. Multifocal intraocular lenses and extended depth of focus intraocular lenses. Asia-Pac. J. Ophthalmol. 2017, 6, 339–349. [Google Scholar]
- Lundström, M.; Barry, P.; Henry, Y.; Rosen, P.; Stenevi, U. Evidence-based guidelines for cataract surgery: Guidelines based on data in the European Registry of Quality Outcomes for Cataract and Refractive Surgery database. J. Cataract Refract. Surg. 2012, 38, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Khoramnia, R.; Yildirim, T.M.; Son, H.S.; Łabuz, G.; Mayer, C.S.; Auffarth, G.U. Duet procedure to achieve reversible trifocality. Der Ophthalmologe 2020, 117, 999–1004. Correction in Der Ophthalmol. Z. Dtsch. Ophthalmol. Ges. 2020, 117, 1005. [Google Scholar]
- Gayton, J.L.; Apple, D.J.; Peng, Q.; Visessook, N.; Sanders, V.; Werner, L.; Pandey, S.K.; Escobar-Gomez, M.; Hoddinott, D.S.; Van Der Karr, M. Interlenticular opacification: Clinicopathological correlation of a complication of posterior chamber piggyback intraocular lenses. J. Cataract Refract. Surg. 2000, 26, 330–336. [Google Scholar] [CrossRef]
- Werner, L.; Apple, D.J.; Pandey, S.K.; Solomon, K.D.; Snyder, M.E.; Brint, S.F.; Gayton, J.L.; Shugar, J.K.; Trivedi, R.H.; Izak, A.M. Analysis of elements of interlenticular opacification. Am. J. Ophthalmol. 2002, 133, 320–326. [Google Scholar] [CrossRef]
- Hesse, R.J. Refractive changes produced by capsule contraction after piggyback acrylic intraocular lens implantation. J. Cataract Refract. Surg. 2002, 28, 2229–2230. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.F.; Masket, S.; Miller, K.M.; Braga-Mele, R.; Little, B.C.; Mamalis, N.; Oetting, T.A.; Packer, M. ASCRS Cataract Clinical Committee. Complications of sulcus placement of single-piece acrylic intraocular lenses: Recommendations for backup IOL implantation following posterior capsule rupture. J. Cataract Refract. Surg. 2009, 35, 1445–1458. [Google Scholar] [CrossRef]
- Gundersen, K.G.; Gjerdrum, B.; Potvin, R. Efficacy of a Secondary Trifocal Sulcus IOL in Providing Near and Intermediate Vision in Patients with Prior Myopic Laser Vision Correction and Cataract Surgery. Clin. Ophthalmol. 2022, 16, 2219. [Google Scholar] [CrossRef]
- Baur, I.D.; Auffarth, G.U.; Łabuz, G.; Khoramnia, R. Clinical outcomes in patients after duet procedure for reversible trifocality using a supplementary trifocal IOL. Am. J. Ophthalmol. 2022, 241, 217–226. [Google Scholar] [CrossRef]
- Baur, I.D.; Auffarth, G.U.; Yildirim, T.M.; Mayer, C.S.; Khoramnia, R. Reversibility of the duet procedure: Bilateral exchange of a supplementary trifocal sulcus-fixated intraocular lens for correction of a postoperative refractive error. Am. J. Ophthalmol. Case Rep. 2020, 20, 100957. [Google Scholar] [CrossRef] [PubMed]
- Khoramnia, R.; Yildirim, T.M.; Baur, I.; Auffarth, G.U. Duet procedure to achieve reversible trifocality in a young patient with hereditary hyperferritinemia-cataract syndrome. Am. J. Ophthalmol. Case Rep. 2021, 21, 101026. [Google Scholar] [CrossRef]
- Palomino-Bautista, C.; Sánchez-Jean, R.; Gonzalez, D.C.; Domínguez, M.R.; Gómez, A.C. Spectacle independence for pseudophakic patients–experience with a trifocal supplementary add-on intraocular lens. Clin. Ophthalmol. 2020, 14, 1043. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, S.; Comba, Ö.B.; Karakaya, M. Visual performance and patient satisfaction following the implantation of a novel trifocal supplementary intraocular lens. Eur. J. Ophthalmol. 2021, 31, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Harrisberg, B.P.; Chua, A.W.; Chua, M.J.; Taher, A. Comparison of Primary Duet Lens Procedures: In-The-Bag Monofocal with Sulcus Multifocal, and Standard Single Multifocal Lens for Cataract Surgery. Clin. Ophthalmol. 2023, 17, 273–282. [Google Scholar] [CrossRef] [PubMed]
- ISO 11979-2; Ophthalmic Implants—Intraocular Lenses—Part 2: Optical Properties and Test Methods. Standardization ISO: Geneva, Switzerland, 2014.
- Gatinel, D.; Pagnoulle, C.; Houbrechts, Y.; Gobin, L. Design and qualification of a diffractive trifocal optical profile for intraocular lenses. J. Cataract Refract. Surg. 2011, 37, 2060–2067. [Google Scholar] [CrossRef]
- Rawer, R.; Stork, W.; Spraul, C.W.; Lingenfelder, C. Imaging quality of intraocular lenses. J. Cataract Refract. Surg. 2005, 31, 1618–1631. [Google Scholar] [CrossRef]
- Gerten, G.; Kermani, O.; Schmiedt, K.; Farvili, E.; Foerster, A.; Oberheide, U. Dual intraocular lens implantation: Monofocal lens in the bag and additional diffractive multifocal lens in the sulcus. J. Cataract Refract. Surg. 2009, 35, 2136–2143. [Google Scholar] [CrossRef]
- Erickson, P. Effects of intraocular lens position errors on postoperative refractive error. J. Cataract Refract. Surg. 1990, 16, 305–311. [Google Scholar] [CrossRef]
- Łabuz, G.; Auffarth, G.U.; Knorz, M.C.; Son, H.-S.; Yildirim, T.M.; Khoramnia, R. Trifocality achieved through polypseudophakia: Optical quality and light loss compared with a Single trifocal intraocular lens. J. Refract. Surg. 2020, 36, 570–577. [Google Scholar] [CrossRef]
- Son, H.-S.; Łabuz, G.; Khoramnia, R.; Yildirim, T.M.; Auffarth, G.U. Laboratory analysis and ray visualization of diffractive optics with enhanced intermediate vision. BMC Ophthalmol. 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Sirohi, R.S. Introduction to Optical Metrology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Schrecker, J.; Zoric, K.; Meßner, A.; Eppig, T. Effect of interface reflection in pseudophakic eyes with an additional refractive intraocular lens. J. Cataract Refract. Surg. 2012, 38, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Erie, J.C.; Bandhauer, M.H.; McLaren, J.W. Analysis of postoperative glare and intraocular lens design. J. Cataract Refract. Surg. 2001, 27, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Cornsweet, T.N.; Pinsker, H.M. Luminance discrimination of brief flashes under various conditions of adaptation. J. Physiol. 1965, 176, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.; Xu, Z.; Alexander, E.; Choi, M.; Zhao, Z.; Hong, X. Optical bench performance of 3 trifocal intraocular lenses. J. Cataract Refract. Surg. 2016, 42, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Valvecchia, G.; Cervantes-Coste, G.; Asis, O.; Pereyra, F.; Garza-León, M.; Gomez Caride, G.; Ferlini, L.; Gonzalez-Salinas, R. Clinical outcomes of the piggyback secondary implantation of a novel multifocal lens in the ciliary sulcus: A case series. Eur. J. Ophthalmol. 2021. [CrossRef]
- Gundersen, K.G.; Potvin, R. Refractive and visual outcomes after implantation of a secondary toric sulcus intraocular lenses. Clin. Ophthalmol. 2020, 14, 1337. [Google Scholar] [CrossRef] [PubMed]
- McLintock, C.A.; McKelvie, J.; Niyazmand, H.; Apel, A.J. Outcomes of a toric monofocal piggyback intraocular lens for residual astigmatic refractive error in pseudophakic eyes. Curr. Eye Res. 2022, 47, 443–449. [Google Scholar] [CrossRef]
- Meyer, J.J.; Kim, B.Z.; Ziaei, M.; McGhee, C.N. Postoperative rotation of supplementary sulcus-supported toric intraocular lenses. J. Cataract Refract. Surg. 2017, 43, 285–288. [Google Scholar] [CrossRef]
- Gundersen, K.G.; Potvin, R. A review of results after implantation of a secondary intraocular lens to correct residual refractive error after cataract surgery. Clin. Ophthalmol. 2017, 11, 1791. [Google Scholar] [CrossRef]
- Dekaris, I.; Gabrić, I.; Gabrić, D. Surgical Correction of Ametropia with AddOn™ Intraocular Lens in Post-Penetrating Keratoplasty Pseudophakia. In Refractive Surgery-Types of Procedures, Risks, and Benefits; IntechOpen: London, UK, 2022. [Google Scholar]
- Łabuz, G.; Auffarth, G.U.; Yan, W.; Yildirim, T.M.; Khoramnia, R. Simulations of Decentration and Tilt of a Supplementary Sulcus-Fixated Intraocular Lens in a Polypseudophakic Combination Using Ray-Tracing Software. Photonics 2021, 8, 309. [Google Scholar] [CrossRef]
- Madrid-Costa, D.; Ruiz-Alcocer, J.; Ferrer-Blasco, T.; García-Lázaro, S.; Montés-Micó, R. Optical quality differences between three multifocal intraocular lenses: Bifocal low add, bifocal moderate add, and trifocal. J. Refract. Surg. 2013, 29, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Łabuz, G.; Khoramnia, R.; Auffarth, G.U. Trifocal intraocular lens selection: Predicting visual function from optical quality measurements. J. Refract. Surg. 2023, 39, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Montés-Micó, R.; Madrid-Costa, D.; Ruiz-Alcocer, J.; Ferrer-Blasco, T.; Pons, Á.M. In vitro optical quality differences between multifocal apodized diffractive intraocular lenses. J. Cataract Refract. Surg. 2013, 39, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Tandogan, T.; Auffarth, G.U.; Liebing, S.; Choi, C.Y.; Khoramnia, R. Impact of Near Addition on the Optical Quality of Diffractive Multifocal Intraocular Lenses-a Laboratory Study Using an Optical Bench. Klin. Mon. Fur Augenheilkd. 2017, 234, 1533–1539. [Google Scholar]
- Domínguez-Vicent, A.; Esteve-Taboada, J.J.; Del Águila-Carrasco, A.J.; Monsálvez-Romin, D.; Montés-Micó, R. In vitro optical quality comparison of 2 trifocal intraocular lenses and 1 progressive multifocal intraocular lens. J. Cataract Refract. Surg. 2016, 42, 138–147. [Google Scholar] [CrossRef]

| Aperture | Focus | Monofocal | Mono-AddOn | Trifocal |
|---|---|---|---|---|
| 3 mm | Far | 0.97 ± 0.00 | 0.65 ± 0.01 | 0.56 ± 0.01 |
| Intermediate | 0.26 ± 0.00 | 0.27 ± 0.01 | ||
| Near | 0.35 ± 0.00 | 0.44 ± 0.00 | ||
| 4.5 mm | Far | 0.91 ± 0.00 | 0.78 ± 0.00 | 0.69 ± 0.02 |
| Intermediate | 0.18 ± 0.00 | 0.22 ± 0.01 | ||
| Near | 0.20 ± 0.00 | 0.23 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoramnia, R.; Baur, I.D.; Yan, W.; Łabuz, G.; Auffarth, G.U. Comparison of a Presbyopia-Correcting Supplementary Intraocular Lens Combination and a Capsular-Bag Lens: An In Vitro Study. Diagnostics 2023, 13, 1482. https://doi.org/10.3390/diagnostics13081482
Khoramnia R, Baur ID, Yan W, Łabuz G, Auffarth GU. Comparison of a Presbyopia-Correcting Supplementary Intraocular Lens Combination and a Capsular-Bag Lens: An In Vitro Study. Diagnostics. 2023; 13(8):1482. https://doi.org/10.3390/diagnostics13081482
Chicago/Turabian StyleKhoramnia, Ramin, Isabella Diana Baur, Weijia Yan, Grzegorz Łabuz, and Gerd Uwe Auffarth. 2023. "Comparison of a Presbyopia-Correcting Supplementary Intraocular Lens Combination and a Capsular-Bag Lens: An In Vitro Study" Diagnostics 13, no. 8: 1482. https://doi.org/10.3390/diagnostics13081482
APA StyleKhoramnia, R., Baur, I. D., Yan, W., Łabuz, G., & Auffarth, G. U. (2023). Comparison of a Presbyopia-Correcting Supplementary Intraocular Lens Combination and a Capsular-Bag Lens: An In Vitro Study. Diagnostics, 13(8), 1482. https://doi.org/10.3390/diagnostics13081482









