Pitfalls in the Diagnosis and Management of Hypercortisolism (Cushing Syndrome) in Humans; A Review of the Laboratory Medicine Perspective
Abstract
1. Introduction
2. Clinical Syndrome and Steroid Metabolism
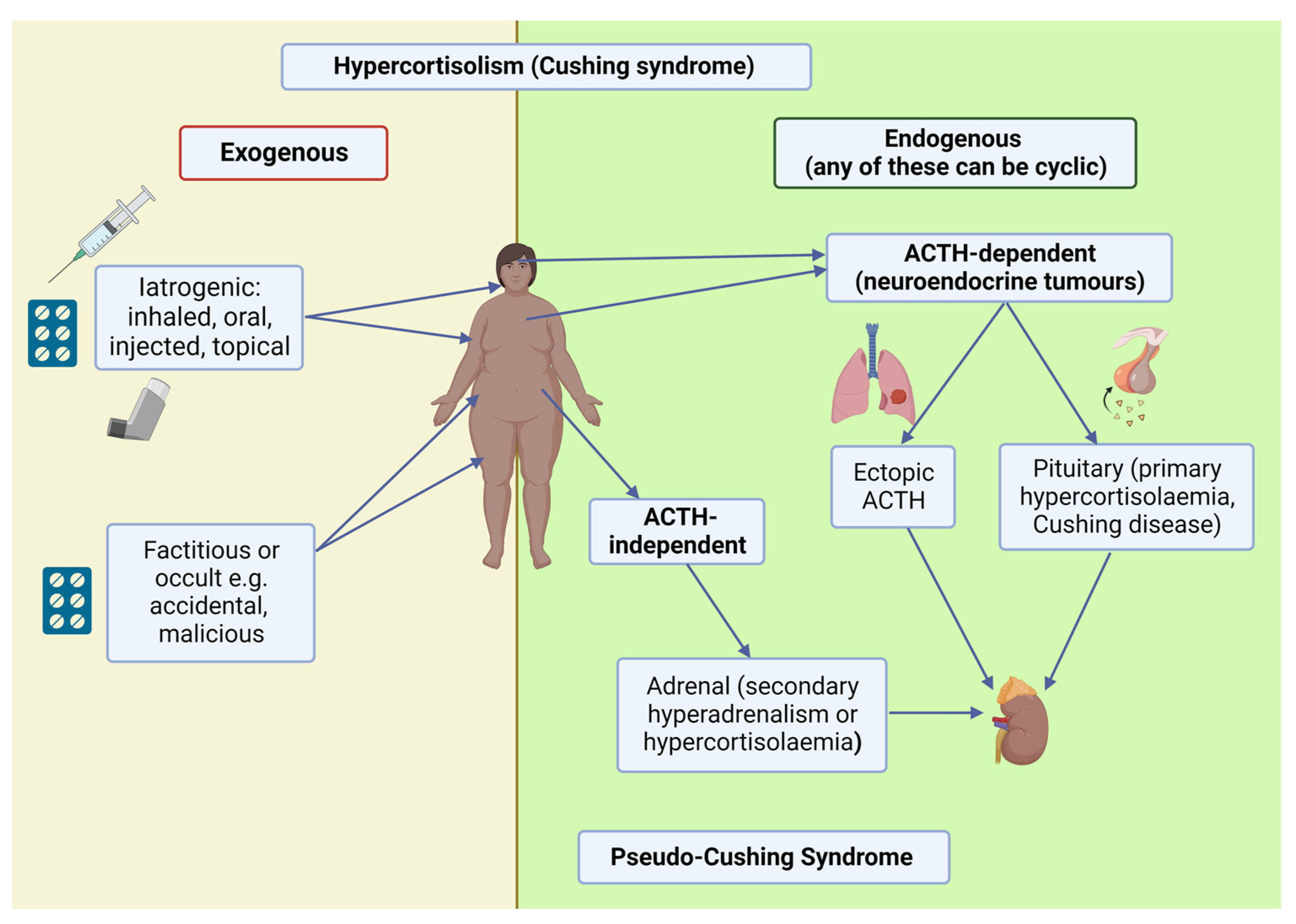
2.1. Clinical Syndrome
2.2. Steroid Metabolism
2.2.1. Homeostasis and Diurnal Variation
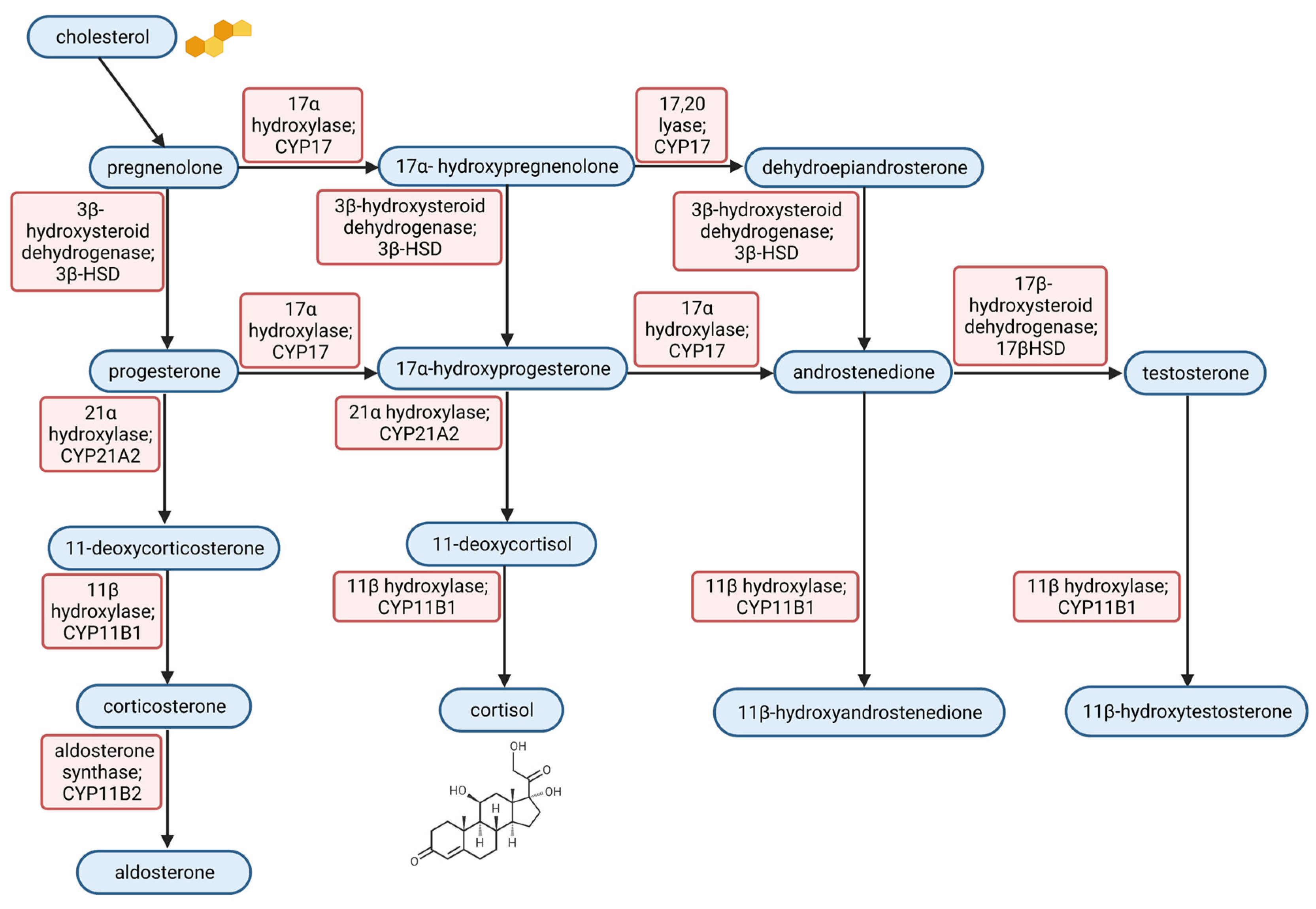
2.2.2. Steroid Synthesis and Interference
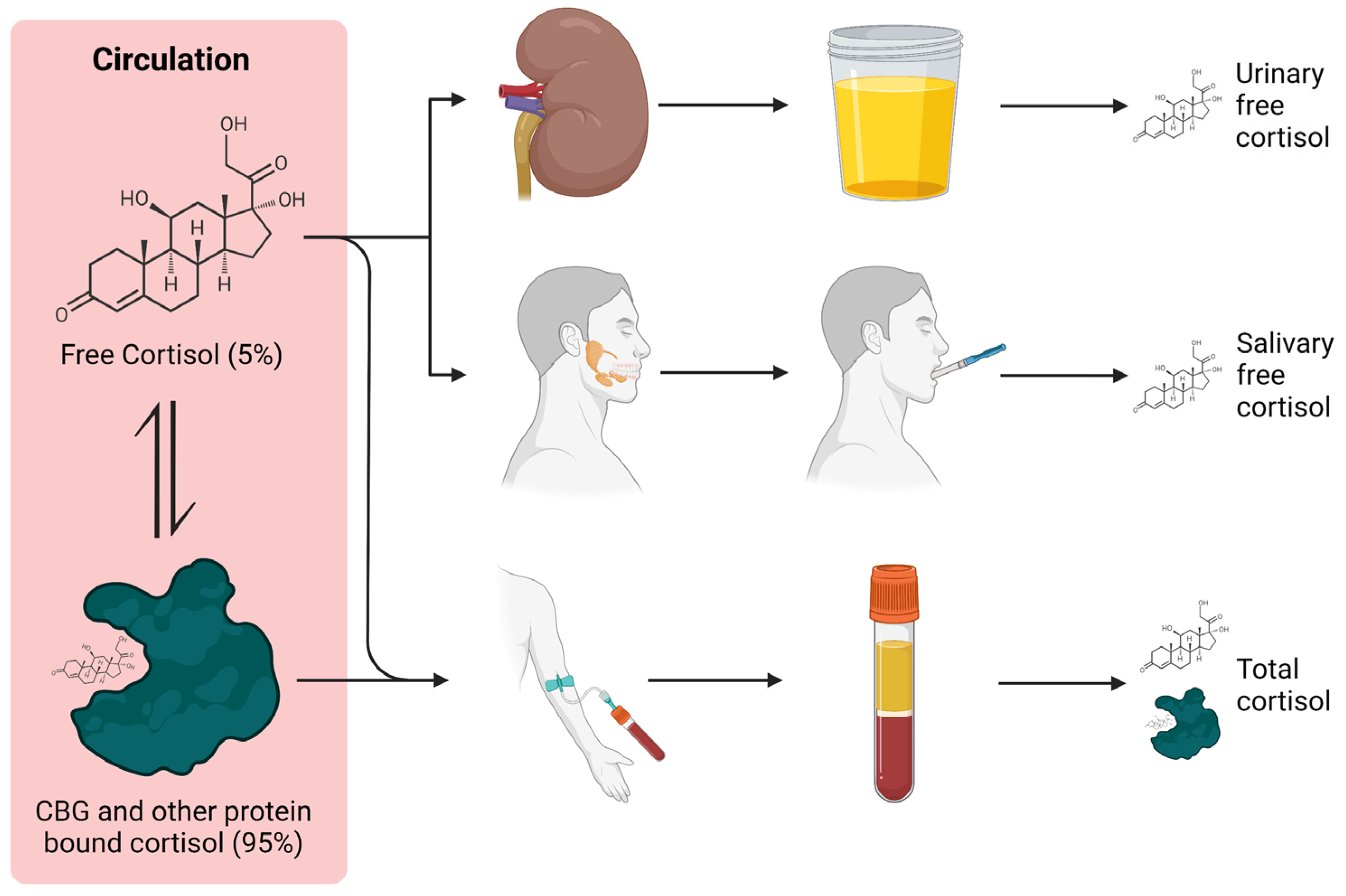
2.2.3. Cortisol Binding Globulin and Interference
2.2.4. Steroid Metabolism and Interference
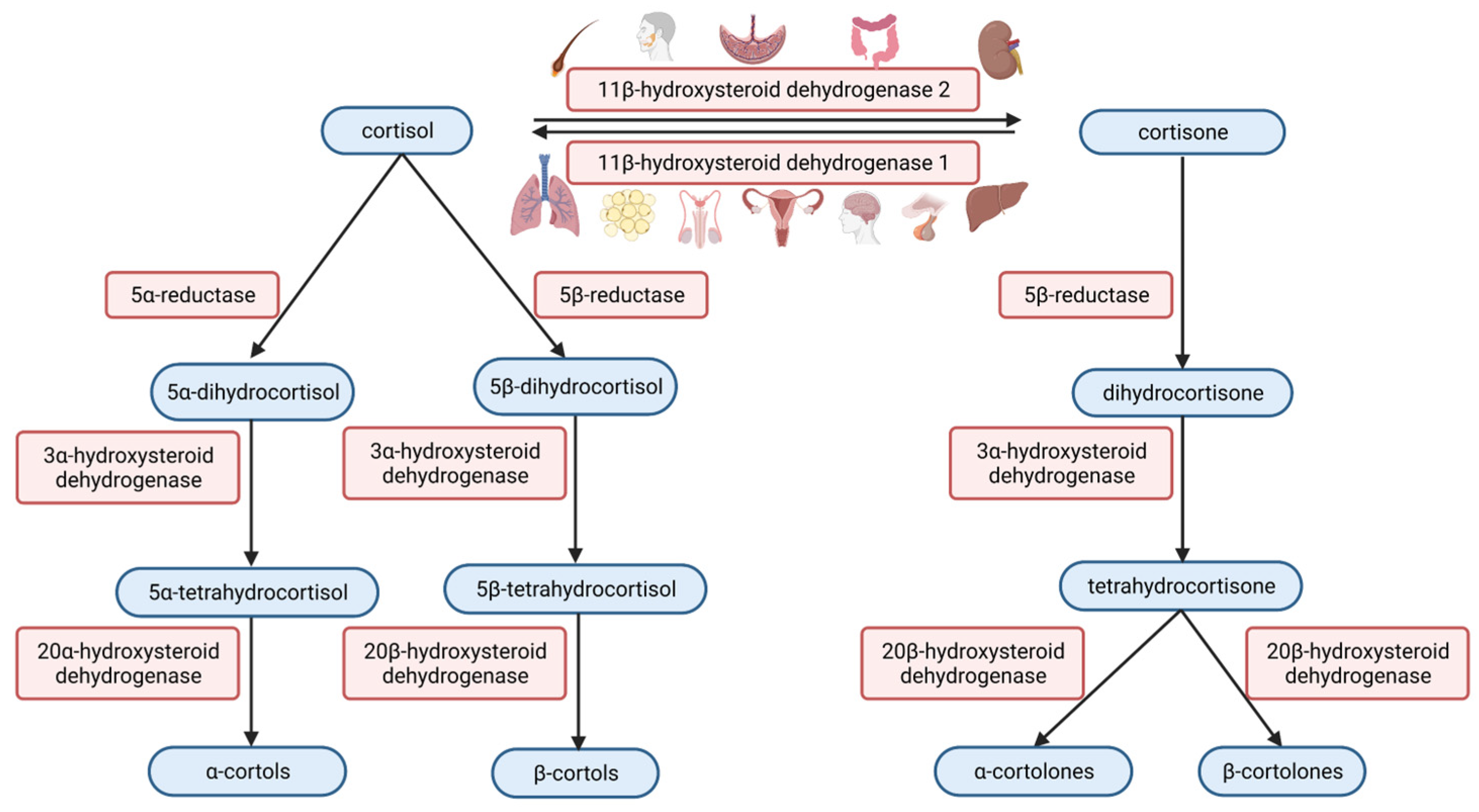
3. Cortisol Analysis
3.1. Immunoassay
3.2. Structural Assays: Mass Spectrometry and High-Performance Liquid Chromatography
3.2.1. Mass Spectrometry
3.2.2. HPLC
3.3. Specimen Type and Timing
3.3.1. Plasma/Serum (Midnight)
3.3.2. Saliva (Midnight)
3.3.3. Urine (24 h)
3.3.4. Plasma/Serum (Early Morning)
3.3.5. Plasma/Serum (Free)
3.3.6. Hair
4. ACTH
4.1. ACTH Analysis
4.1.1. Techniques
4.1.2. Performance
5. Other Steroid Hormones
5.1. Adrenal Androgens in Women
5.1.1. Dehydroepiandrosterone-Sulfate
5.1.2. Other Female Androgens
5.2. Steroid Profile
5.3. Salivary Cortisone
6. Dynamic Function Tests
6.1. Dexamethasone Tests
6.1.1. 1 mg Overnight Dexamethasone Suppression Test
6.1.2. 2 mg/Day (Low Dose) 48 h Dexamethasone Suppression Test
6.1.3. High Dose Dexamethasone Suppression Test
6.1.4. Dexamethasone-CRH Test
6.1.5. Dexamethasone Analysis in DST Protocol
6.2. CRH Stimulation with ACTH and Cortisol Quantification
6.3. Desmopressin (DDAVP) Test
6.4. Anatomical
7. Other Tests
7.1. Neuroendocrine Markers
7.1.1. Chromogranin A
7.1.2. Serotonin (5-Hydroxyindole Acetic Acid)
7.1.3. Calcitonin
7.1.4. Copeptin
7.2. Electrolytes, Acid Base, Physiological
7.2.1. Hypokalaemic Alkalosis
7.2.2. Metabolome
7.3. Molecular Genetic Techniques
7.4. Others
Anti-Müllerian Hormone and PCOS
8. Testing in Medically Treated Cushing Syndrome
8.1. Steroid Synthesis Inhibition
8.2. Central Inhibition
8.3. Glucocorticoid Receptor Antagonism
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. The Diagnosis of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef]
- Parikh, R.; Mathai, A.; Parikh, S.; Chandra Sekhar, G.; Thomas, R. Understanding and using sensitivity, specificity and predictive values. Indian J. Ophthalmol. 2008, 56, 45–50. [Google Scholar] [CrossRef]
- Meinardi, J.R.; Wolffenbuttel, B.H.R.; Dullaart, R.P.F. Cyclic Cushing’s syndrome: A clinical challenge. Eur. J. Endocrinol. 2007, 157, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Sherf, S.; Lai, N.B.; Yu, R. Exogenous Cushing Syndrome Caused by a “Herbal” Supplement. AACE Clin. Case Rep. 2022, 8, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, H.M.; Goddard, W.; Gill, S.; Moss, C. Herbal creams used for atopic eczema in Birmingham, UK illegally contain potent corticosteroids. Arch. Dis. Child. 2003, 88, 1056–1057. [Google Scholar] [CrossRef] [PubMed]
- Maneli, M.H.; Wiesner, L.; Tinguely, C.; Davids, L.M.; Spengane, Z.; Smith, P.; van Wyk, J.C.; Jardine, A.; Khumalo, N.P. Combinations of potent topical steroids, mercury and hydroquinone are common in internationally manufactured skin-lightening products: A spectroscopic study. Clin. Exp. Dermatol. 2016, 41, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, S.W.; Klijn, J.G.; de Jong, F.H.; Birkenhäger, J.C. Hormone secretion in alcohol-induced pseudo-Cushing’s syndrome. Differential diagnosis with Cushing disease. JAMA 1979, 242, 1640–1643. [Google Scholar] [CrossRef]
- Scaroni, C.; Albiger, N.M.; Palmieri, S.; Iacuaniello, D.; Graziadio, C.; Damiani, L.; Zilio, M.; Stigliano, A.; Colao, A.; Pivonello, R. Approach to patients with pseudo-Cushing’s states. Endocr. Connect. 2020, 9, R1–R13. [Google Scholar] [CrossRef]
- Krieger, D.T.; Allen, W.; Rizzo, F.; Krieger, H.P. Characterization of the normal temporal pattern of plasma corticosteroid levels. J. Clin. Endocrinol. Metab. 1971, 32, 266–284. [Google Scholar] [CrossRef]
- Faiman, C.; Winter, J.S. Diurnal cycles in plasma FSH, testosterone and cortisol in men. J. Clin. Endocrinol. Metab. 1971, 33, 186–192. [Google Scholar] [CrossRef]
- Isidori, A.; Fraiolo, F.; Rotolo, A.; Piro, C.; Conte, D.; Muratorio, A.; Murri, L.; Cerone, G. Poly-hormonal evaluationof pulsatile adenohypophysis incretory activity during sleep in normal, experimental and pathologic conditions. Chronobiologia 1976, 3, 39–50. [Google Scholar] [PubMed]
- de Weerth, C.; Zijl, R.H.; Buitelaar, J.K. Development of cortisol circadian rhythm in infancy. Early Hum. Dev. 2003, 73, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Yesiladali, M.; Yazici, M.G.K.; Attar, E.; Kelestimur, F. Differentiating Polycystic Ovary Syndrome from Adrenal Disorders. Diagnostics 2022, 12, 2045. [Google Scholar] [CrossRef] [PubMed]
- Kotłowska, A.; Puzyn, T.; Sworczak, K.; Stepnowski, P.; Szefer, P. Metabolomic Biomarkers in Urine of Cushing’s Syndrome Patients. Int. J. Mol. Sci. 2017, 18, 294. [Google Scholar] [CrossRef]
- Davio, A.; Woolcock, H.; Nanba, A.T.; Rege, J.; O’Day, P.; Ren, J.; Zhao, L.; Ebina, H.; Auchus, R.; Rainey, W.E.; et al. Sex Differences in 11-Oxygenated Androgen Patterns Across Adulthood. J. Clin. Endocrinol. Metab. 2020, 105, 2921. [Google Scholar] [CrossRef]
- Perogamvros, I.; Ray, D.W.; Trainer, P.J. Regulation of cortisol bioavailability—Effects on hormone measurement and action. Nat. Rev. Endocrinol. 2012, 8, 717–727. [Google Scholar] [CrossRef]
- Lewis, J.G.; Bagley, C.J.; Elder, P.A.; Bachmann, A.W.; Torpy, D.J. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin. Chim. Acta 2005, 359, 189–194. [Google Scholar] [CrossRef]
- Hawley, J.M.; Owen, L.J.; Lockhart, S.J.; Monaghan, P.J.; Armston, A.; Chadwick, C.A.; Wilshaw, H.; Freire, M.; Perry, L.; Keevil, B.G. Serum Cortisol: An Up-To-Date Assessment of Routine Assay Performance. Clin. Chem. 2016, 62, 1220–1229. [Google Scholar] [CrossRef]
- Qureshi, A.C.; Bahri, A.; Breen, L.A.; Barnes, S.C.; Powrie, J.K.; Thomas, S.M.; Carroll, P.V. The influence of the route of oestrogen administration on serum levels of cortisol-binding globulin and total cortisol. Clin. Endocrinol. 2007, 66, 632–635. [Google Scholar] [CrossRef]
- Klose, M.; Lange, M.; Rasmussen, A.K.; Skakkebaek, N.E.; Hilsted, L.; Haug, E.; Andersen, M.; Feldt-Rasmussen, U. Factors influencing the adrenocorticotropin test: Role of contemporary cortisol assays, body composition, and oral contraceptive agents. J. Clin. Endocrinol. Metab. 2007, 92, 1326–1333. [Google Scholar] [CrossRef]
- Nickelsen, T.; Lissner, W.; Schöffling, K. The dexamethasone suppression test and long-term contraceptive treatment: Measurement of ACTH or salivary cortisol does not improve the reliability of the test. Exp. Clin. Endocrinol. 1989, 94, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hamrahian, A.H.; Oseni, T.S.; Arafah, B.M. Measurements of serum free cortisol in critically ill patients. N. Engl. J. Med. 2004, 350, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Salvagno, G.L.; Guzzo, A.; Scurati, S.; Fava, C.; Lippi, G. Urinary free cortisol assessment by liquid chromatography tandem mass spectrometry: A case study of ion suppression due to unacquainted administration of piperacillin. Biochem. Med. 2017, 27, 031001. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, K.; Brabant, S.; Prie, D.; Piketty, M. Hormone Immunoassay Interference: A 2021 Update. Ann. Lab. Med. 2022, 42, 3–23. [Google Scholar] [CrossRef]
- Monaghan, P.J.; Owen, L.J.; Trainer, P.J.; Brabant, G.; Keevil, B.G.; Darby, D. Comparison of serum cortisol measurement by immunoassay and liquid chromatography-tandem mass spectrometry in patients receiving the 11β-hydroxylase inhibitor metyrapone. Ann. Clin. Biochem. 2011, 48, 441–446. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Thynne, T.; White, G.H.; Burt, M.G. Factitious Cushing’s syndrome masquerading as Cushing’s disease. Clin. Endocrinol. 2014, 80, 328–332. [Google Scholar] [CrossRef]
- Flockhart, D.A.; Thacker, D.; McDonald, C.; Desta, Z. The Flockhart Cytochrome P450 Drug-Drug Interaction Table. Available online: https://drug-interactions.medicine.iu.edu/ (accessed on 11 January 2023).
- Nieman, L. Pitfalls in the diagnosis and differential diagnosis of Cushing’s syndrome. Clin. Endocrinol. 2014, 80, 333–334. [Google Scholar] [CrossRef]
- Raff, H.; Auchus, R.J.; Findling, J.W.; Nieman, L.K. Urine free cortisol in the diagnosis of Cushing’s syndrome: Is it worth doing and, if so, how? J. Clin. Endocrinol. Metab. 2015, 100, 395–397. [Google Scholar] [CrossRef]
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H.L. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504. [Google Scholar] [CrossRef]
- Smith, R.E.; Maguire, J.A.; Stein-Oakley, A.N.; Sasano, H.; Takahashi, K.; Fukushima, K.; Krozowski, Z.S. Localization of 11 beta-hydroxysteroid dehydrogenase type II in human epithelial tissues. J. Clin. Endocrinol. Metab. 1996, 81, 3244–3248. [Google Scholar] [CrossRef] [PubMed]
- Bäcklund, N.; Brattsand, G.; Israelsson, M.; Ragnarsson, O.; Burman, P.; Edén Engström, B.; Høybye, C.; Berinder, K.; Wahlberg, J.; Olsson, T.; et al. Reference intervals of salivary cortisol and cortisone and their diagnostic accuracy in Cushing’s syndrome. Eur. J. Endocrinol. 2020, 182, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.; Adaway, J.; Keevil, B.; Ross, R. Salivary cortisol and cortisone in the clinical setting. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.; Harrison, R.F.; Whitaker, M.J.; Eckland, D.; Arlt, W.; Keevil, B.G.; Ross, R.J. Salivary Cortisone Reflects Cortisol Exposure Under Physiological Conditions and After Hydrocortisone. J. Clin. Endocrinol. Metab. 2016, 101, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, B.; Mensinga, T.; Sips, A.; Deerenberg, C.; Meulenbelt, J.; DeJongh, J. A population physiologically based pharmacokinetic/pharmacodynamic model for the inhibition of 11-beta-hydroxysteroid dehydrogenase activity by glycyrrhetic acid. Toxicol. Appl. Pharmacol. 2001, 170, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Imamovic, M.; Bäcklund, N.; Lundstedt, S.; Brattsand, G.; Aardal, E.; Olsson, T.; Dahlqvist, P. Confounding effects of liquorice, hydrocortisone, and blood contamination on salivary cortisol but not cortisone. Endocr. Connect. 2023, 12, e220324. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lian, Q.; Dong, Q.; Ge, R. Environmental inhibitors of 11β-hydroxysteroid dehydrogenase type 2. Toxicology 2011, 285, 83–89. [Google Scholar] [CrossRef]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and mechanisms of phthalates’ action on neurological processes and neural health: A literature review. Pharmacol. Rep. 2021, 73, 386–404. [Google Scholar] [CrossRef]
- Draper, N.; Stewart, P.M. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J. Endocrinol. 2005, 186, 251–271. [Google Scholar] [CrossRef]
- Tai, S.S.; Welch, M.J. Development and evaluation of a candidate reference method for the determination of total cortisol in human serum using isotope dilution liquid chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. Anal. Chem. 2004, 76, 1008–1014. [Google Scholar] [CrossRef]
- Friedman, T.C.; Ghods, D.E.; Shahinian, H.K.; Zachery, L.; Shayesteh, N.; Seasholtz, S.; Zuckerbraun, E.; Lee, M.L.; McCutcheon, I.E. High prevalence of normal tests assessing hypercortisolism in subjects with mild and episodic Cushing’s syndrome suggests that the paradigm for diagnosis and exclusion of Cushing’s syndrome requires multiple testing. Horm. Metab. Res. 2010, 42, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, S.; Raff, H.; Findling, J.W. Limitations of nocturnal salivary cortisol and urine free cortisol in the diagnosis of mild Cushing’s syndrome. Eur. J. Endocrinol. 2007, 157, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Raff, H. Cushing’s syndrome: Diagnosis and surveillance using salivary cortisol. Pituitary 2012, 15, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Baid, S.K.; Sinaii, N.; Wade, M.; Rubino, D.; Nieman, L.K. Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: A comparison of assays to establish hypercortisolism. J. Clin. Endocrinol. Metab. 2007, 92, 3102–3107. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Gaudl, A.; Jaeger, S.; Stadelmann, S.; Hiemisch, A.; Kiess, W.; Willenberg, A.; Schaab, M.; von Klitzing, K.; Thiery, J.; et al. Immunoassay or LC-MS/MS for the measurement of salivary cortisol in children? Clin. Chem. Lab. Med. 2016, 54, 811–822. [Google Scholar] [CrossRef]
- Ceccato, F.; Marcelli, G.; Martino, M.; Concettoni, C.; Brugia, M.; Trementino, L.; Michetti, G.; Arnaldi, G. The diagnostic accuracy of increased late night salivary cortisol for Cushing’s syndrome: A real-life prospective study. J. Endocrinol. Investig. 2019, 42, 327–335. [Google Scholar] [CrossRef]
- Monaghan, P.J.; Keevil, B.G.; Trainer, P.J. The use of mass spectrometry to improve the diagnosis and the management of the HPA axis. Rev. Endocr. Metab. Disord. 2013, 14, 143–157. [Google Scholar] [CrossRef]
- Keevil, B.G. Novel liquid chromatography tandem mass spectrometry (LC-MS/MS) methods for measuring steroids. Best. Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 663–674. [Google Scholar] [CrossRef]
- Methlie, P.; Hustad, S.S.; Kellmann, R.; Almås, B.; Erichsen, M.M.; Husebye, E.; Løvås, K. Multisteroid LC-MS/MS assay for glucocorticoids and androgens, and its application in Addison’s disease. Endocr. Connect. 2013, 2, 125–136. [Google Scholar] [CrossRef]
- Meikle, A.W.; Findling, J.; Kushnir, M.M.; Rockwood, A.L.; Nelson, G.J.; Terry, A.H. Pseudo-Cushing syndrome caused by fenofibrate interference with urinary cortisol assayed by high-performance liquid chromatography. J. Clin. Endocrinol. Metab. 2003, 88, 3521–3524. [Google Scholar] [CrossRef]
- Findling, J.W.; Pinkstaff, S.M.; Shaker, J.L.; Raff, H.; Nelson, J.C. Pseudohypercortisoluria: Spurious Elevation of Urinary Cortisol due to Carbamazepine. Endocrinologist 1998, 8, 51–54. [Google Scholar] [CrossRef]
- Glass, A.R.; Zavadil, A.P.; Halberg, F.; Cornelissen, G.; Schaaf, M. Circadian rhythm of serum cortisol in Cushing’s disease. J. Clin. Endocrinol. Metab. 1984, 59, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Ironside, N.; Chatain, G.; Asuzu, D.; Benzo, S.; Lodish, M.; Sharma, S.; Nieman, L.; Stratakis, C.A.; Lonser, R.R.; Chittiboina, P. Earlier post-operative hypocortisolemia may predict durable remission from Cushing’s disease. Eur. J. Endocrinol. 2018, 178, 255–263. [Google Scholar] [CrossRef]
- Lentjes, E.G.; Romijn, F.; Maassen, R.J.; de Graaf, L.; Gautier, P.; Moolenaar, A.J. Free cortisol in serum assayed by temperature-controlled ultrafiltration before fluorescence polarization immunoassay. Clin. Chem. 1993, 39, 2518–2521. [Google Scholar] [CrossRef] [PubMed]
- Brossaud, J.; Gatta, B.; Tabarin, A.; Corcuff, J. Different methods to estimate serum free cortisol: A comparison during cortisol tetracosactide testing. Clin. Chem. Lab. Med. 2015, 53, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.W.; Besser, G.M. Pituitary-adrenal function in severe depressive illness. Lancet 1968, 1, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Nieman, L.K. Cushing’s syndrome: Update on signs, symptoms and biochemical screening. Eur. J. Endocrinol. 2015, 173, 33. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; García Cano, A.; Jiménez Mendiguchía, L.; Escobar-Morreale, H.F.; Valderrábano, P. Diagnostic accuracy of the different hormonal tests used for the diagnosis of autonomous cortisol secretion. Sci. Rep. 2021, 11, 20539. [Google Scholar] [CrossRef]
- Lopes, L.M.L.; Francisco, R.P.V.; Galletta, M.A.K.; Bronstein, M.D. Determination of nighttime salivary cortisol during pregnancy: Comparison with values in non-pregnancy and Cushing’s disease. Pituitary 2016, 19, 30–38. [Google Scholar] [CrossRef]
- Tritos, N.A.; Fazeli, P.K.; McCormack, A.; Mallea-Gil, S.M.; Pineyro, M.M.; Christ-Crain, M.; Frara, S.; Labadzhyan, A.; Ioachimescu, A.G.; Shimon, I.; et al. Pituitary Society Delphi Survey: An international perspective on endocrine management of patients undergoing transsphenoidal surgery for pituitary adenomas. Pituitary 2022, 25, 64–73. [Google Scholar] [CrossRef]
- Eulate-Beramendi, S.; Casajús, A.; Ollero, L.; Niemann, L.K.; Fernández-Miranda, J.C.; Bruneau, M.; Berhouma, M.; Cavallo, L.M.; Cornelius, J.F.; Daniel, R.T.; et al. Update in Cushing disease: What the neurosurgeon has to KNOW, on behalf of the EANS skull base section. Brain Spine 2022, 2, 100917. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.A.; García, M.; Goycoolea, M.; Cerda, J.; Bertherat, J.; Padilla, O.; Meza, D.; Wohllk, N.; Quiroga, T. Reproducibility and performance of one or two samples of salivary cortisol in the diagnosis of Cushing’s syndrome using an automated immunoassay system. Endocrine 2012, 41, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Belaya, Z.E.; Iljin, A.V.; Melnichenko, G.A.; Rozhinskaya, L.Y.; Dragunova, N.V.; Dzeranova, L.K.; Butrova, S.A.; Troshina, E.A.; Dedov, I.I. Diagnostic performance of late-night salivary cortisol measured by automated electrochemiluminescence immunoassay in obese and overweight patients referred to exclude Cushing’s syndrome. Endocrine 2012, 41, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Poll, E.; Kreitschmann-Andermahr, I.; Langejuergen, Y.; Stanzel, S.; Gilsbach, J.M.; Gressner, A.; Yagmur, E. Saliva collection method affects predictability of serum cortisol. Clin. Chim. Acta 2007, 382, 15–19. [Google Scholar] [CrossRef]
- Inder, W.J.; Dimeski, G.; Russell, A. Measurement of salivary cortisol in 2012—Laboratory techniques and clinical indications. Clin. Endocrinol. 2012, 77, 645–651. [Google Scholar] [CrossRef]
- Garde, A.H.; Hansen, A.M. Long-term stability of salivary cortisol. Scand. J. Clin. Lab. Investig. 2005, 65, 433–436. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, M.; Tomlinson, B. Effects of grapefruit juice on cortisol metabolism in healthy male Chinese subjects. Food Chem. Toxicol. 2014, 74, 85–90. [Google Scholar] [CrossRef]
- Badrick, E.; Kirschbaum, C.; Kumari, M. The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. Metab. 2007, 92, 819–824. [Google Scholar] [CrossRef]
- Miller, R.; Plessow, F.; Rauh, M.; Gröschl, M.; Kirschbaum, C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology 2013, 38, 50–57. [Google Scholar] [CrossRef]
- Liu, H.; Bravata, D.M.; Cabaccan, J.; Raff, H.; Ryzen, E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin. Endocrinol. 2005, 63, 642–649. [Google Scholar] [CrossRef]
- Elias, P.C.L.; Martinez, E.Z.; Barone, B.F.C.; Mermejo, L.M.; Castro, M.; Moreira, A.C. Late-night salivary cortisol has a better performance than urinary free cortisol in the diagnosis of Cushing’s syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Guarnotta, V.; Amato, M.C.; Pivonello, R.; Arnaldi, G.; Ciresi, A.; Trementino, L.; Citarrella, R.; Iacuaniello, D.; Michetti, G.; Simeoli, C.; et al. The degree of urinary hypercortisolism is not correlated with the severity of cushing’s syndrome. Endocrine 2017, 55, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.; Ducroq, D.H.; Fraser, H.L.; Gillingwater, S.; Evans, C.; Pickett, A.J.; Rees, D.W.; John, R.; Turkes, A. Measurement of urinary free cortisol by tandem mass spectrometry and comparison with results obtained by gas chromatography-mass spectrometry and two commercial immunoassays. Ann. Clin. Biochem. 2008, 45, 380–388. [Google Scholar] [CrossRef]
- Aranda, G.; Careaga, M.; Hanzu, F.A.; Patrascioiu, I.; Ríos, P.; Mora, M.; Morales-Romero, B.; Jiménez, W.; Halperin, I.; Casals, G. Accuracy of immunoassay and mass spectrometry urinary free cortisol in the diagnosis of Cushing’s syndrome. Pituitary 2016, 19, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Petersenn, S.; Newell-Price, J.; Findling, J.W.; Gu, F.; Maldonado, M.; Sen, K.; Salgado, L.R.; Colao, A.; Biller, B.M.K. High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin. Endocrinol. 2014, 80, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Kulle, A.; Wolters, B.; Knop, C.; Lass, N.; Welzel, M.; Holterhus, P. Relationships between 24-hour urinary free cortisol concentrations and metabolic syndrome in obese children. J. Clin. Endocrinol. Metab. 2014, 99, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Mericq, M.V.; Cutler, G.B. High fluid intake increases urine free cortisol excretion in normal subjects. J. Clin. Endocrinol. Metab. 1998, 83, 682–684. [Google Scholar] [CrossRef]
- Chen, A.X.; Haas, A.V.; Williams, G.H.; Vaidya, A. Dietary sodium intake and cortisol measurements. Clin. Endocrinol. 2020, 93, 539–545. [Google Scholar] [CrossRef]
- Chan, K.C.A.; Lit, L.C.W.; Law, E.L.K.; Tai, M.H.L.; Yung, C.U.; Chan, M.H.M.; Lam, C.W.K. Diminished urinary free cortisol excretion in patients with moderate and severe renal impairment. Clin. Chem. 2004, 50, 757–759. [Google Scholar] [CrossRef]
- Issa, B.G.; Page, M.D.; Read, G.; John, R.; Douglas-Jones, A.; Scanlon, M.F. Undetectable urinary free cortisol concentrations in a case of Cushing’s disease. Eur. J. Endocrinol. 1999, 140, 148–151. [Google Scholar] [CrossRef]
- Côté, A.; Firoz, T.; Mattman, A.; Lam, E.M.; von Dadelszen, P.; Magee, L.A. The 24-hour urine collection: Gold standard or historical practice? Am. J. Obstet. Gynecol. 2008, 199, 625.e1–625.6. [Google Scholar] [CrossRef] [PubMed]
- Carnes, K.; Howe, A.; Feustel, P.J.; Listman, J.A.; White, M.; Kogan, B.A. 24-Hour urine collection for first time pediatric stone formers: Is it worth it? J. Pediatr. Urol. 2021, 17, 387.e1–387.e7. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.; Curran, D.A.; Mayfield, B.P. Dried urine and salivary profiling for complete assessment of cortisol and cortisol metabolites. J. Clin. Transl. Endocrinol. 2020, 22, 100243. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.A.R.; Clark, J.; Russell, A.W. Concordance of the late night salivary cortisol in patients with Cushing’s syndrome and elevated urine-free cortisol. Endocrine 2013, 43, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Lodish, M.B.; Papadakis, G.Z.; Lyssikatos, C.; Belyavskaya, E.; Stratakis, C.A. Diurnal Plasma Cortisol Measurements Utility in Differentiating Various Etiologies of Endogenous Cushing Syndrome. Horm. Metab. Res. 2016, 48, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Manzano, A.J. Detection of recurrent Cushing’s disease: Proposal for standardized patient monitoring following transsphenoidal surgery. J. Neurooncol. 2014, 119, 235–242. [Google Scholar] [CrossRef]
- Stroud, A.; Dhaliwal, P.; Alvarado, R.; Winder, M.J.; Jonker, B.P.; Grayson, J.W.; Hamizan, A.; Harvey, R.J.; McCormack, A. Outcomes of pituitary surgery for Cushing’s disease: A systematic review and meta-analysis. Pituitary 2020, 23, 595–609. [Google Scholar] [CrossRef]
- Choi, M.H. Clinical and Technical Aspects in Free Cortisol Measurement. Endocrinol. Metab. 2022, 37, 599–607. [Google Scholar] [CrossRef]
- Thomson, S.; Koren, G.; Fraser, L.-A.; Rieder, M.; Friedman, T.C.; Van Uum, S.H.M. Hair analysis provides a historical record of cortisol levels in Cushing’s syndrome. Exp. Clin. Endocrinol. Diabetes 2010, 118, 133–138. [Google Scholar] [CrossRef]
- Wester, V.L.; van Rossum, E.F.C. Clinical applications of cortisol measurements in hair. Eur. J. Endocrinol. 2015, 173, 1. [Google Scholar] [CrossRef]
- Hodes, A.; Meyer, J.; Lodish, M.B.; Stratakis, C.A.; Zilbermint, M. Mini-review of hair cortisol concentration for evaluation of Cushing syndrome. Expert Rev. Endocrinol. Metab. 2018, 13, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Savas, M.; Mehta, S.; Agrawal, N.; van Rossum, E.F.C.; Feelders, R.A. Approach to the Patient: Diagnosis of Cushing Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, 3162–3174. [Google Scholar] [CrossRef] [PubMed]
- Brossaud, J.; Charret, L.; De Angeli, D.; Haissaguerre, M.; Ferriere, A.; Puerto, M.; Gatta-Cherifi, B.; Corcuff, J.; Tabarin, A. Hair cortisol and cortisone measurements for the diagnosis of overt and mild Cushing’s syndrome. Eur. J. Endocrinol. 2021, 184, 445–454. [Google Scholar] [CrossRef] [PubMed]
- López, F.J.; Negro-Vilar, A. Estimation of endogenous adrenocorticotropin half-life using pulsatility patterns: A physiological approach to the evaluation of secretory episodes. Endocrinology 1988, 123, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, H. Preanalytical stability of adrenocorticotropic hormone depends on both time to centrifugation and temperature. J. Clin. Lab. Anal. 2017, 31, e22081. [Google Scholar] [CrossRef] [PubMed]
- Livesey, J.H.; Dolamore, B. Stability of plasma adrenocorticotrophic hormone (ACTH): Influence of hemolysis, rapid chilling, time, and the addition of a maleimide. Clin. Biochem. 2010, 43, 1478–1480. [Google Scholar] [CrossRef]
- Toprak, B.; Yalcin, H.; Arı, E.; Colak, A. EDTA interference in electrochemiluminescence ACTH assay. Ann. Clin. Biochem. 2016, 53, 699–701. [Google Scholar] [CrossRef]
- Donegan, D.M.; Algeciras-Schimnich, A.; Hamidi, O.; Young, W.F.; Nippoldt, T.; Bancos, I.; Erickson, D. Corticotropin hormone assay interference: A case series. Clin. Biochem. 2019, 63, 143–147. [Google Scholar] [CrossRef]
- Jex, R.K.; van Heerden, J.A.; Carpenter, P.C.; Grant, C.S. Ectopic ACTH syndrome. Diagnostic and therapeutic aspects. Am. J. Surg. 1985, 149, 276–282. [Google Scholar] [CrossRef]
- Yener, S.; Yilmaz, H.; Demir, T.; Secil, M.; Comlekci, A. DHEAS for the prediction of subclinical Cushing’s syndrome: Perplexing or advantageous? Endocrine 2015, 48, 669–676. [Google Scholar] [CrossRef]
- Carafone, L.E.; Zhang, C.D.; Li, D.; Lazik, N.; Hamidi, O.; Hurtado, M.D.; Young, W.F.; Thomas, M.A.; Dy, B.M.; Lyden, M.L.; et al. Diagnostic Accuracy of Dehydroepiandrosterone Sulfate and Corticotropin in Autonomous Cortisol Secretion. Biomedicines 2021, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Velikanova, L.I.; Shafigullina, Z.R.; Lisitsin, A.A.; Vorokhobina, N.V.; Grigoryan, K.; Kukhianidze, E.A.; Strelnikova, E.G.; Krivokhizhina, N.S.; Krasnov, L.M.; Fedorov, E.A.; et al. Different Types of Urinary Steroid Profiling Obtained by High-Performance Liquid Chromatography and Gas Chromatography-Mass Spectrometry in Patients with Adrenocortical Carcinoma. Horm. Cancer 2016, 7, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Ghataore, L.; Couchman, L.; Vincent, R.P.; Whitelaw, B.; Lewis, D.; Diaz-Cano, S.; Galata, G.; Schulte, K.; Aylwin, S.; et al. A 13-Steroid Serum Panel Based on LC-MS/MS: Use in Detection of Adrenocortical Carcinoma. Clin. Chem. 2017, 63, 1836–1846. [Google Scholar] [CrossRef]
- Vogg, N.; Müller, T.; Floren, A.; Dandekar, T.; Scherf-Clavel, O.; Fassnacht, M.; Kroiss, M.; Kurlbaum, M. Targeted metabolic profiling of urinary steroids with a focus on analytical accuracy and sample stability. J. Mass Spectrom. Adv. Clin. Lab 2022, 25, 44–52. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; Valderrábano, P.; Escobar-Morreale, H.F.; Hanzu, F.A.; Casals, G. Urine steroid profile as a new promising tool for the evaluation of adrenal tumors. Literature review. Endocrine 2021, 72, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Arioli, F.; Gamberini, M.C.; Pavlovic, R.; Di Cesare, F.; Draghi, S.; Bussei, G.; Mungiguerra, F.; Casati, A.; Fidani, M. Quantification of cortisol and its metabolites in human urine by LC-MSn: Applications in clinical diagnosis and anti-doping control. Anal. Bioanal. Chem. 2022, 414, 6841–6853. [Google Scholar] [CrossRef]
- Athimulam, S.; Grebe, S.; Bancos, I. Steroid profiling in the diagnosis of mild and overt Cushing’s syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101488. [Google Scholar] [CrossRef]
- Mohamed, R.S.; Abuelgasim, B.; Barker, S.; Prabhudev, H.; Martin, N.M.; Meeran, K.; Williams, E.L.; Darch, S.; Matthew, W.; Tan, T.; et al. Late-night salivary cortisol and cortisone should be the initial screening test for Cushing’s syndrome. Endocr. Connect. 2022, 11, e220050. [Google Scholar] [CrossRef]
- Kannankeril, J.; Carroll, T.; Findling, J.W.; Javorsky, B.; Gunsolus, I.L.; Phillips, J.; Raff, H. Prospective Evaluation of Late-Night Salivary Cortisol and Cortisone by EIA and LC-MS/MS in Suspected Cushing Syndrome. J. Endocr. Soc. 2020, 4, bvaa107. [Google Scholar] [CrossRef]
- Lindholm, J. Cushing’s disease, pseudo-Cushing states and the dexamethasone test: A historical and critical review. Pituitary 2014, 17, 374–380. [Google Scholar] [CrossRef]
- Kyriazopoulou, V.; Vagenakis, A.G. Abnormal overnight dexamethasone suppression test in subjects receiving rifampicin therapy. J. Clin. Endocrinol. Metab. 1992, 75, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Valassi, E.; Swearingen, B.; Lee, H.; Nachtigall, L.B.; Donoho, D.A.; Klibanski, A.; Biller, B.M.K. Concomitant Medication Use Can Confound Interpretation of the Combined Dexamethasone-Corticotropin Releasing Hormone Test in Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 4851–4859. [Google Scholar] [CrossRef] [PubMed]
- Meikle, A.W. Dexamethasone suppression tests: Usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clin. Endocrinol. 1982, 16, 401–408. [Google Scholar] [CrossRef]
- Asvold, B.O.; Grill, V.; Thorstensen, K.; Bjørgaas, M.R. Association between posttest dexamethasone and cortisol concentrations in the 1 mg overnight dexamethasone suppression test. Endocr. Connect. 2012, 1, 62–67. [Google Scholar] [CrossRef]
- Carton, T.; Mathieu, E.; Wolff, F.; Bouziotis, J.; Corvilain, B.; Driessens, N. Two-day low-dose dexamethasone suppression test more accurate than overnight 1-mg in women taking oral contraceptives. Endocrinol. Diabetes Metab. 2021, 4, e00255. [Google Scholar] [CrossRef] [PubMed]
- al-Saadi, N.; Diederich, S.; Oelkers, W. A very high dose dexamethasone suppression test for differential diagnosis of Cushing’s syndrome. Clin. Endocrinol. 1998, 48, 45–51. [Google Scholar] [CrossRef]
- Barbot, M.; Trementino, L.; Zilio, M.; Ceccato, F.; Albiger, N.; Daniele, A.; Frigo, A.C.; Mardari, R.; Rolma, G.; Boscaro, M.; et al. Second-line tests in the differential diagnosis of ACTH-dependent Cushing’s syndrome. Pituitary 2016, 19, 488–495. [Google Scholar] [CrossRef]
- Young, J.; Haissaguerre, M.; Viera-Pinto, O.; Chabre, O.; Baudin, E.; Tabarin, A. MANAGEMENT OF ENDOCRINE DISEASE: Cushing’s syndrome due to ectopic ACTH secretion: An expert operational opinion. Eur. J. Endocrinol. 2020, 182, R29–R58. [Google Scholar] [CrossRef]
- Aron, D.C.; Raff, H.; Findling, J.W. Effectiveness versus efficacy: The limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 1997, 82, 1780–1785. [Google Scholar] [CrossRef]
- Yanovski, J.A.; Cutler, G.B.; Chrousos, G.P.; Nieman, L.K. Corticotropin-releasing hormone stimulation following low-dose dexamethasone administration. A new test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. JAMA 1993, 269, 2232–2238. [Google Scholar] [CrossRef]
- Yanovski, J.A.; Cutler, G.B.J.; Chrousos, G.P.; Nieman, L.K. The dexamethasone-suppressed corticotropin-releasing hormone stimulation test differentiates mild Cushing’s disease from normal physiology. J. Clin. Endocrinol. Metab. 1998, 83, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Detomas, M.; Ritzel, K.; Nasi-Kordhishti, I.; Wolfsberger, S.; Quinkler, M.; Losa, M.; Tröger, V.; Kroiss, M.; Fassnacht, M.; Vila, G.; et al. Outcome of CRH stimulation test and overnight 8 mg dexamethasone suppression test in 469 patients with ACTH-dependent Cushing’s syndrome. Front. Endocrinol. 2022, 13, 955945. [Google Scholar] [CrossRef] [PubMed]
- Nieman, L.K. Is it Time for a New Approach to the Differential Diagnosis of ACTH-Dependent Cushing Syndrome? J. Clin. Endocrinol. Metab. 2020, 105, e4964–e4966. [Google Scholar] [CrossRef]
- Tirabassi, G.; Papa, R.; Faloia, E.; Boscaro, M.; Arnaldi, G. Corticotrophin-releasing hormone and desmopressin tests in the differential diagnosis between Cushing’s disease and pseudo-Cushing state: A comparative study. Clin. Endocrinol. 2011, 75, 666–672. [Google Scholar] [CrossRef]
- Valizadeh, M.; Ahmadi, A.R.; Ebadinejad, A.; Rahmani, F.; Abiri, B. Diagnostic accuracy of bilateral inferior petrosal sinus sampling using desmopressin or corticotropic- releasing hormone in ACTH-dependent Cushing’s syndrome: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2022, 23, 881–892. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Wang, Z.; You, H.; Li, M.; Feng, F.; Jin, Z. High positive predictive value of the combined pituitary dynamic enhanced MRI and high-dose dexamethasone suppression tests in the diagnosis of Cushing’s disease bypassing bilateral inferior petrosal sinus sampling. Sci. Rep. 2020, 10, 14694. [Google Scholar] [CrossRef] [PubMed]
- Hasholzner, U.; Baumgartner, L.; Stieber, P.; Meier, W.; Reiter, W.; Pahl, H.; Fateh-Moghadam, A. Clinical significance of the tumour markers CA 125 II and CA 72-4 in ovarian carcinoma. Int. J. Cancer 1996, 69, 329–334. [Google Scholar] [CrossRef]
- Al Ojaimi, E.H. Cushing’s syndrome due to an ACTH-producing primary ovarian carcinoma. Hormones 2014, 13, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Landry, I.; Medina Mora, L.A.; Siddiqui, R.; Tumarinson, T.; Reich, D.M. Atypical Carcinoid Tumor of the Mediastinum Presenting as Cushing’s Syndrome in an Otherwise Healthy Young Male. Cureus 2021, 13, e14940. [Google Scholar] [CrossRef]
- Gkolfinopoulos, S.; Tsapakidis, K.; Papadimitriou, K.; Papamichael, D.; Kountourakis, P. Chromogranin A as a valid marker in oncology: Clinical application or false hopes? World J. Methodol. 2017, 7, 9–15. [Google Scholar] [CrossRef]
- Alvarez-Payares, J.C.; Bello-Simanca, J.D.; De La Peña-Arrieta, E.D.J.; Agamez-Gomez, J.E.; Garcia-Rueda, J.E.; Rodriguez-Arrieta, A.; Rodriguez-Arrieta, L.A. Common Pitfalls in the Interpretation of Endocrine Tests. Front. Endocrinol. 2021, 12, 727628. [Google Scholar] [CrossRef]
- Molina, R.; Alvarez, E.; Aniel-Quiroga, A.; Borque, M.; Candás, B.; Leon, A.; Poyatos, R.M.; Gelabert, M. Evaluation of chromogranin A determined by three different procedures in patients with benign diseases, neuroendocrine tumors and other malignancies. Tumour Biol. 2011, 32, 13–22. [Google Scholar] [CrossRef] [PubMed]
- van der Knaap, R.H.P.; Kwekkeboom, D.J.; Ramakers, C.R.B.; de Rijke, Y.B. Evaluation of a new immunoassay for chromogranin A measurement on the Kryptor system. Pract. Lab. Med. 2015, 1, 5–11. [Google Scholar] [CrossRef]
- Dittadi, R.; Bertoli, I. Evaluation of an ELISA method for the measurement of chromogranin A and comparison with an immunoradiometric method. Int. J. Biol. Mrk. 2013, 28, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Ershadi, R.; Vahedi, M.; Jahanbin, B.; Sarbazzadeh, J.; Rafieian, S. Cushing syndrome secondary to a mediastinal carcinoid tumor: A case report. Int. Cancer Conf. J. 2022, 11, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kema, I.P.; Schellings, A.M.; Meiborg, G.; Hoppenbrouwers, C.J.; Muskiet, F.A. Influence of a serotonin- and dopamine-rich diet on platelet serotonin content and urinary excretion of biogenic amines and their metabolites. Clin. Chem. 1992, 38, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Kema, I.P.; de Vries, E.G.; Schellings, A.M.; Postmus, P.E.; Muskiet, F.A. Improved diagnosis of carcinoid tumors by measurement of platelet serotonin. Clin. Chem. 1992, 38, 534–540. [Google Scholar] [CrossRef]
- Ilias, I.; Torpy, D.J.; Pacak, K.; Mullen, N.; Wesley, R.A.; Nieman, L.K. Cushing’s syndrome due to ectopic corticotropin secretion: Twenty years’ experience at the National Institutes of Health. J. Clin. Endocrinol. Metab. 2005, 90, 4955–4962. [Google Scholar] [CrossRef]
- Flippo, C.; Tatsi, C.; Sinaii, N.; Sierra, M.D.L.L.; Belyavskaya, E.; Lyssikatos, C.; Keil, M.; Spanakis, E.; Stratakis, C.A. Copeptin Levels Before and After Transsphenoidal Surgery for Cushing Disease: A Potential Early Marker of Remission. J. Endocr. Soc. 2022, 6, bvac053. [Google Scholar] [CrossRef]
- Jalleh, R.; Torpy, D.J. The Emerging Role of Copeptin. Clin. Biochem. Rev. 2021, 42, 17–25. [Google Scholar] [CrossRef]
- Timper, K.; Fenske, W.; Kühn, F.; Frech, N.; Arici, B.; Rutishauser, J.; Kopp, P.; Allolio, B.; Stettler, C.; Müller, B.; et al. Diagnostic Accuracy of Copeptin in the Differential Diagnosis of the Polyuria-polydipsia Syndrome: A Prospective Multicenter Study. J. Clin. Endocrinol. Metab. 2015, 100, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Sugimoto, K.; Miyajima, T.; Ide, T.; Minezaki, M.; Takeshita, K.; Takahara, S.; Nakagawa, M.; Fujimura, Y.; Kudo, T.; et al. Clinical Investigation of Adrenal Incidentalomas in Japanese Patients of the Fukuoka Region with Updated Diagnostic Criteria for Sub-clinical Cushing’s Syndrome. Intern. Med. 2018, 57, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Vega-Beyhart, A.; Laguna-Moreno, J.; Díaz-Catalán, D.; Boswell, L.; Mora, M.; Halperin, I.; Casals, G.; Hanzu, F.A. Ketoconazole- and Metyrapone-Induced Reductions on Urinary Steroid Metabolites Alter the Urinary Free Cortisol Immunoassay Reliability in Cushing Syndrome. Front. Endocrinol. 2022, 13, 833644. [Google Scholar] [CrossRef]
- Bancos, I.; Hatipoglu, B.A.; Yuen, K.C.J.; Chandramohan, L.; Chaudhari, S.; Moraitis, A.G. Evaluation of FKBP5 as a cortisol activity biomarker in patients with ACTH-dependent Cushing syndrome. J. Clin. Transl. Endocrinol. 2021, 24, 100256. [Google Scholar] [CrossRef] [PubMed]
- Armignacco, R.; Jouinot, A.; Bouys, L.; Septier, A.; Lartigue, T.; Neou, M.; Gaspar, C.; Perlemoine, K.; Braun, L.; Riester, A.; et al. Identification of glucocorticoid-related molecular signature by whole blood methylome analysis. Eur. J. Endocrinol. 2022, 186, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Armignacco, R.; Reel, P.S.; Reel, S.; Jouinot, A.; Septier, A.; Gaspar, C.; Perlemoine, K.; Larsen, C.K.; Bouys, L.; Braun, L.; et al. Whole blood methylome-derived features to discriminate endocrine hypertension. Clin. Epigenetics 2022, 14, 142. [Google Scholar] [CrossRef]
- Ji, D.; Zhong, R.; Fan, S. Prognosis and Therapeutic Efficacy Prediction of Adrenocortical Carcinoma Based on a Necroptosis-Associated Gene Signature. Biomed. Res. Int. 2022, 2022, 8740408. [Google Scholar] [CrossRef]
- Yoshida, K.; Fukuoka, H.; Odake, Y.; Nakajima, S.; Tachibana, M.; Ito, J.; Hosokawa, Y.; Yamada, T.; Miura, H.; Suematsu, N.; et al. Multiple Salivary Cortisol Measurements Are a Useful Tool to Optimize Metyrapone Treatment in Patients with Cushing’s Syndromes Treatment: Case Presentations. Front. Endocrinol. 2018, 8, 375. [Google Scholar] [CrossRef]
- Nader, N.; Raverot, G.; Emptoz-Bonneton, A.; Déchaud, H.; Bonnay, M.; Baudin, E.; Pugeat, M. Mitotane has an estrogenic effect on sex hormone-binding globulin and corticosteroid-binding globulin in humans. J. Clin. Endocrinol. Metab. 2006, 91, 2165–2170. [Google Scholar] [CrossRef]
- Castinetti, F.; Brue, T.; Conte-Devolx, B. The use of the glucocorticoid receptor antagonist mifepristone in Cushing’s syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 295–299. [Google Scholar] [CrossRef]

| False Positive | False Negative | ||
|---|---|---|---|
| Drug | Mechanism; Assay | Drug | Mechanism; Assay |
| Carbamazepine | Induction of CYP 3A4 *, accelerates metabolism of dexamethasone; any | Aprepitant | Inhibition of CYP 3A4 *, inhibition of dexamethasone metabolism; any |
| Ethosuximide | Cimetidine | ||
| Phenobarbital | Diltiazem | ||
| Phenytoin | Fosaprepitant | ||
| Pioglitazone | Fluoxetine | ||
| Primidone | Itraconazole | ||
| Rifampin | Ritonavir | ||
| Rifapentine | |||
| Estrogens | Increase CBG; plasma total | Biotin | Competes for binding; streptavidin-based IA |
| Mitotane | |||
| Carbamazepine | Overlaps cortisol peak; UFC by HPLC or single transition HPLC MS/MS | Piperacillin | Ion suppression; MS UFC |
| Fenofibrate | Interferes with HPLC peak; UFC HPLC | ||
| Synthetic glucocorticoids | Cross-reactivity; IA | ||
| Carbenoxolone | Inhibition of 11β-HSD2; UFC and salivary; any | ||
| Biotin | Competes for binding; streptavidin-based IA | ||
| Steroid synthesis inhibitors | Cross-reactivity of steroid precursors; any particularly IA | ||
| Mifespristone | Result correct but tissue activity blocked, cortisol analysis should not be performed; any | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flowers, K.C.; Shipman, K.E. Pitfalls in the Diagnosis and Management of Hypercortisolism (Cushing Syndrome) in Humans; A Review of the Laboratory Medicine Perspective. Diagnostics 2023, 13, 1415. https://doi.org/10.3390/diagnostics13081415
Flowers KC, Shipman KE. Pitfalls in the Diagnosis and Management of Hypercortisolism (Cushing Syndrome) in Humans; A Review of the Laboratory Medicine Perspective. Diagnostics. 2023; 13(8):1415. https://doi.org/10.3390/diagnostics13081415
Chicago/Turabian StyleFlowers, Kade C., and Kate E. Shipman. 2023. "Pitfalls in the Diagnosis and Management of Hypercortisolism (Cushing Syndrome) in Humans; A Review of the Laboratory Medicine Perspective" Diagnostics 13, no. 8: 1415. https://doi.org/10.3390/diagnostics13081415
APA StyleFlowers, K. C., & Shipman, K. E. (2023). Pitfalls in the Diagnosis and Management of Hypercortisolism (Cushing Syndrome) in Humans; A Review of the Laboratory Medicine Perspective. Diagnostics, 13(8), 1415. https://doi.org/10.3390/diagnostics13081415






