OCT Angiography in Noninfectious Uveitis: A Description of Five Cases and Clinical Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results
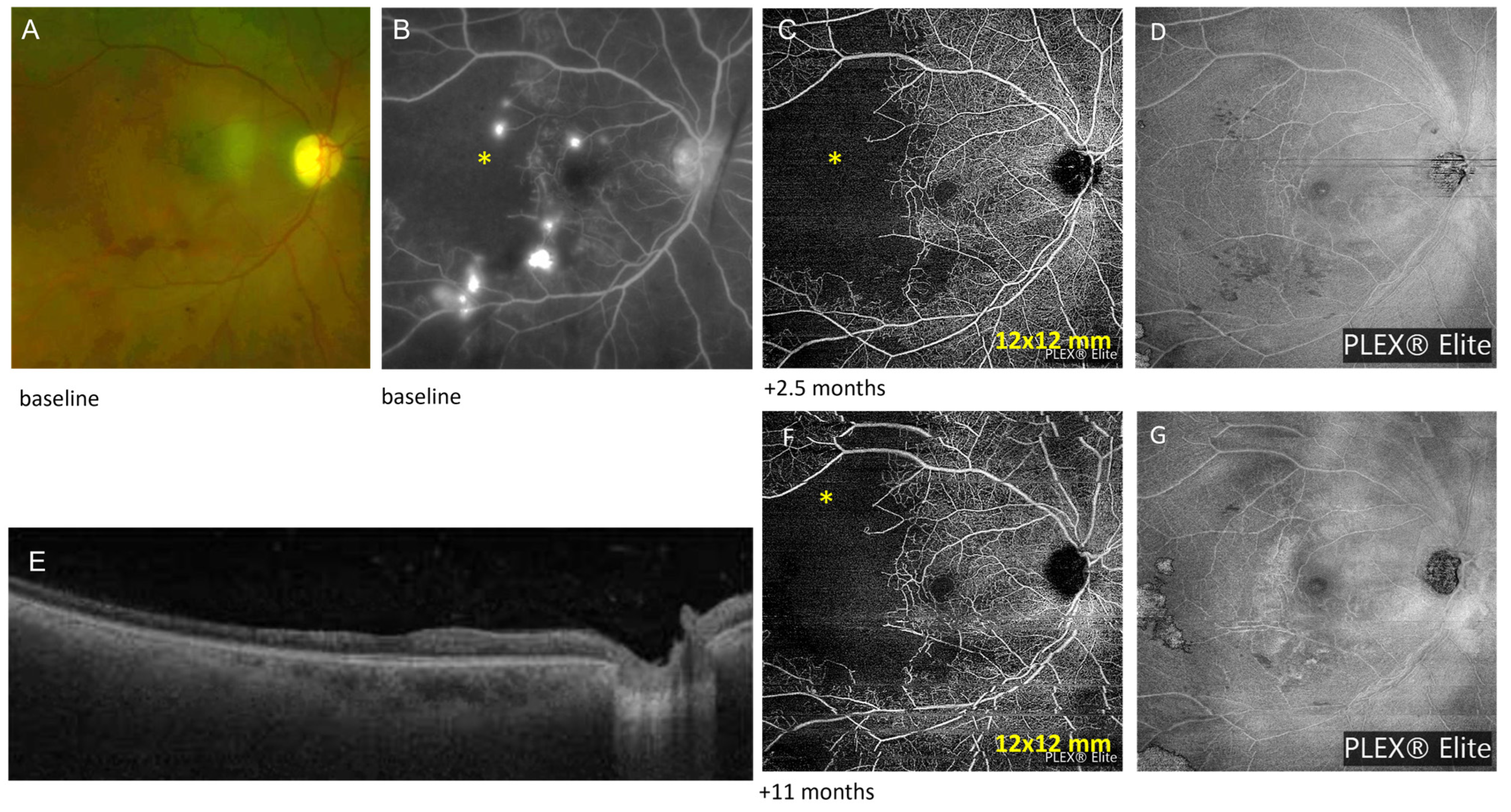
3.1. Case Number 1
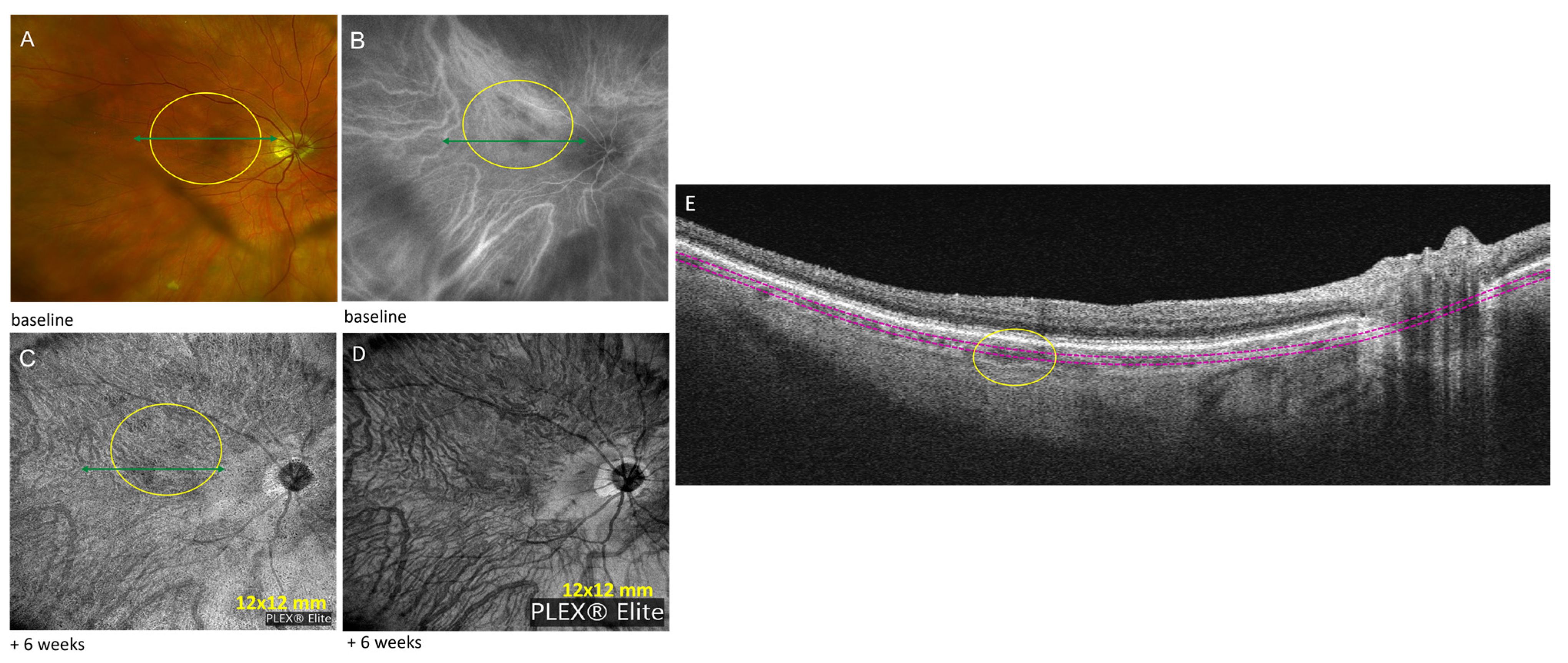
3.2. Case Number 2
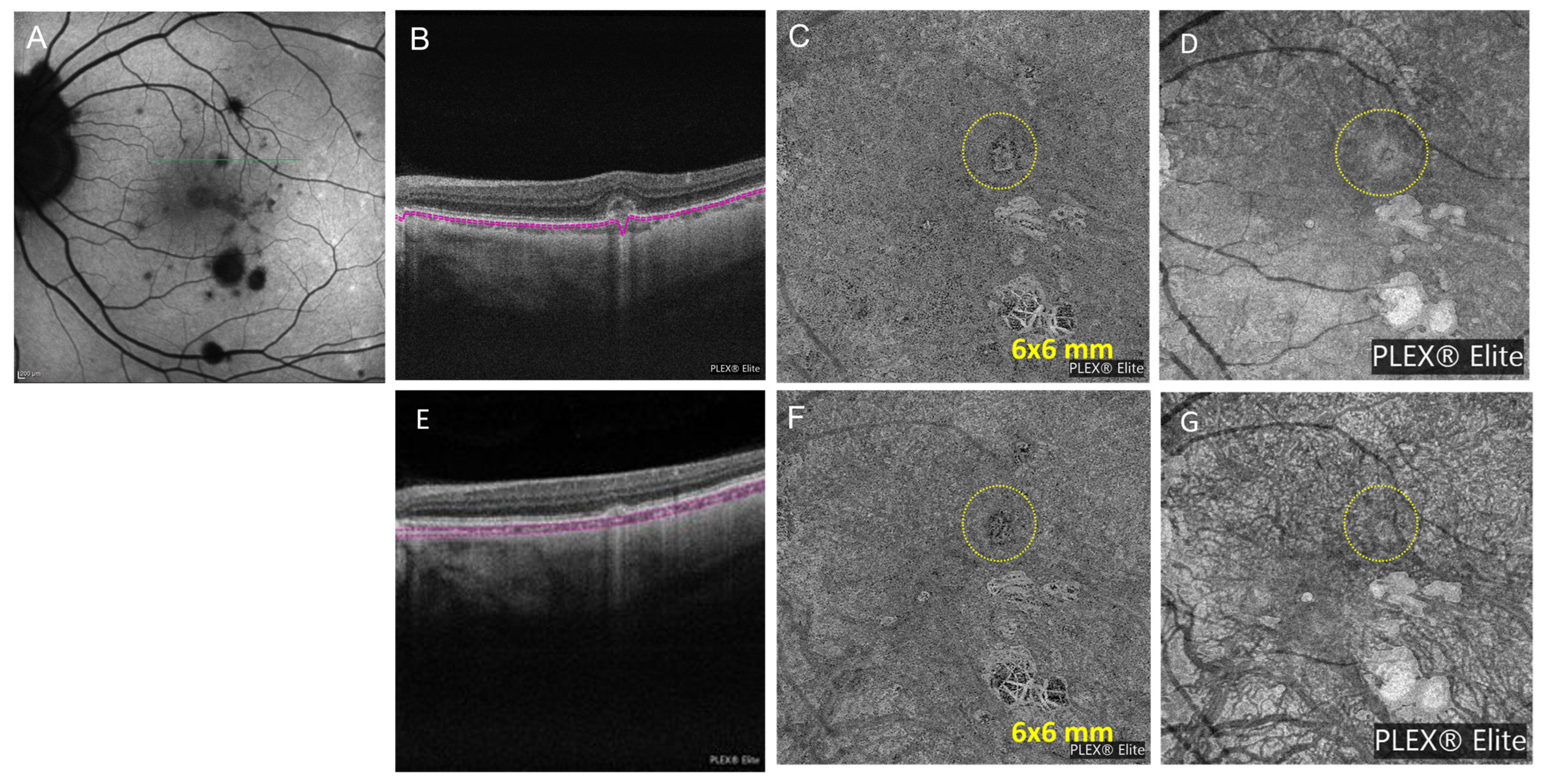
3.3. Case Number 3
3.4. Case Number 4
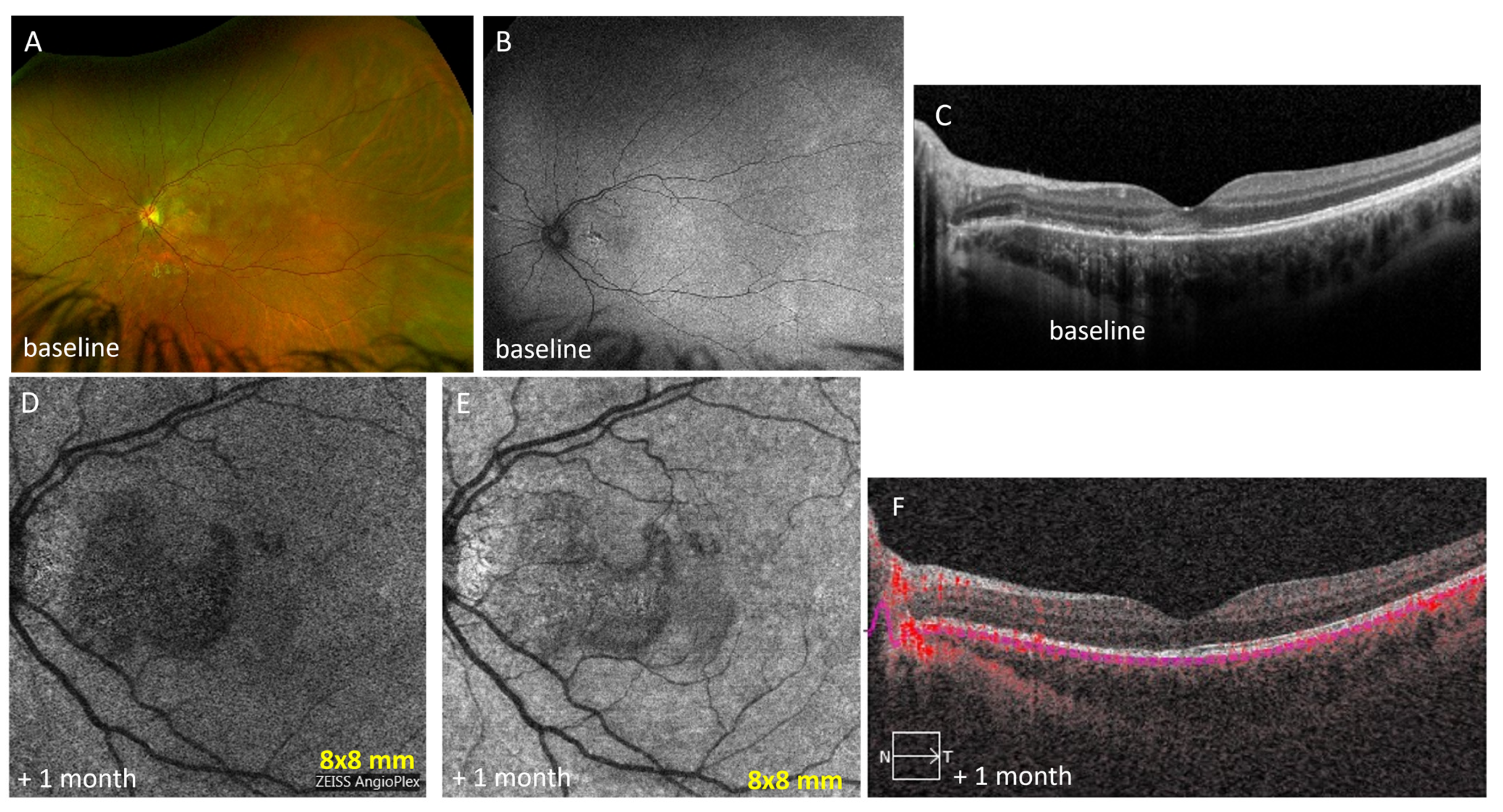
3.5. Case Number 5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dingerkus, V.L.S.; Munk, M.R.; Brinkmann, M.P.; Freiberg, F.J.; Heussen, F.M.A.; Kinzl, S.; Lortz, S.; Orgül, S.; Becker, M. Optical coherence tomography angiography (OCTA) as a new diagnostic tool in uveitis. J. Ophthalmic Inflamm. Infect. 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Pi, S.; Guo, Y.; Jian, Y.; Jia, Y. High dynamic range optical coherence tomography angiography (HDR-OCTA). Biomed. Opt. Express 2019, 10, 3560–3571. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Invernizzi, A. The Role of Optical Coherence Tomography and Optical Coherence Tomography Angiography in the Differential Diagnosis of Posterior Uveitis. Ocul. Immunol. Inflamm. 2022, 30, 682–689. [Google Scholar] [CrossRef]
- Pichi, F.; Chexal, S.; Lembo, A.; Lima, L.H.; Neri, P.; Saitta, A.; Chhablani, J.; Albini, T.A.; Nucci, P.; Freund, K.B.; et al. En face optical coherence tomography and optical coherence tomography angiography of multiple evanescent white dot syndrome: New insights into pathogenesis. Retina 2016, 36, S178–S188. [Google Scholar] [CrossRef]
- Kongwattananon, W.; Lin, H.; Oyeniran, E.; Sen, H.N.; Kodati, S. Role of optical coherence tomography angiography in detecting and monitoring inflammatory choroidal neovascularization. Retina 2022, 42, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Mebsout-Pallado, C.; Terrada, C.; Dansingani, K.K.; Chhablani, J.; Eller, A.W.; Martel, J.N.; Anetakis, A.; Harwick, J.C.; Waxman, E.L.; Gallagher, D.S.; et al. Review of the Current Literature and Our Experience on the Value of OCT-angiography in White Dot Syndromes. Ocul. Immunol. Inflamm. 2022, 30, 364–378. [Google Scholar] [CrossRef]
- Pichi, F.; Smith, S.D.; Neri, P.; Woodstock, E.; Hay, S.; Parrulli, S.; Corvi, F.; Mapelli, C.; Invernizzi, A. Choroidal granulomas visualized by swept-source optical coherence tomography angiography. Retina 2021, 41, 602–609. [Google Scholar] [CrossRef]
- Barteselli, G.; Weinreb, R.N.; Camacho, N.; Nezgoda, J.T.; Marvasti, A.H.; Freeman, W.R. Real-Time Full-Depth Visualization of Posterior Ocular Structures: Comparison between Full-Depth Imaging Spectral Domain Optical Coherence Tomography and Swept-Source Optical Coherence Tomography. Retina 2016, 36, 1153–1161. [Google Scholar] [CrossRef]
- Chu, Z.; Wang, R.K.; Pepple, K. Quantitative Analysis of the Choriocapillaris in Uveitis Using En Face Swept-Source Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2020, 218, 217–227. [Google Scholar] [CrossRef]
- Pichi, F.; Neri, P. The broad spectrum of application of optical coherence tomography angiography to the anterior segment of the eye in inflammatory conditions: A review of the literature. J. Ophthalmic Inflamm. Infect. 2019, 9, 18. [Google Scholar] [CrossRef]
- Pichi, F.; Carreño, E.; Pavesio, C.; Denniston, A.K.; Grewal, D.S.; Deak, G.; Khairallah, M.; Ruiz-Cruz, M.; de Oliveira Dias, J.R.; Adan, A.; et al. Consensus-based recommendations for optical coherence tomography angiography reporting in uveitis. Br. J. Ophthalmol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A.; Rosenbaum, J.T. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509. [Google Scholar] [PubMed]
- Standardization of Uveitis Nomenclature (SUN) Working Group. Development of Classification Criteria for the Uveitides. Am. J. Ophthalmol. 2021, 228, 96–105. [Google Scholar] [CrossRef]
- Invernizzi, A.; Staurenghi, G. Optical coherence tomography and optical coherence tomography angiography in uveitis: A review. Clin. Exp. Ophthalmol. 2019, 47, 357–371. [Google Scholar] [CrossRef]
- Zarranz-Ventura, J.; Keane, P.A.; Patel, P.; Westcott, M.C.; Lee, R.W.; Tufail, A.; Pavesio, C.E. Characterization of punctate inner choroidopathy using enhanced depth imaging optical coherence tomography. Ophthalmology 2014, 121, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Neri, P.; Fortuna, C.; Manoni, M.; Giovannini, A. Inflammatory choroidal neovascularization. Middle East Afr. J. Ophthalmol. 2009, 16, 245–251. [Google Scholar]
- Brown, J.; Reddy, C.V.; Kimura, A.E. Visual prognosis of multifocal choroiditis, punctate inner choroidopathy, and the diffuse subretinal fibrosis syndrome. Ophthalmology 1996, 103, 1100–1105. [Google Scholar] [CrossRef]
- Karti, O.; Ates, Y.; Saatci, A.O. Inflammatory Choroidal Neovascular Membranes in Patients with Noninfectious Uveitis: The Place of Intravitreal Anti-VEGF Therapy. Med. Hypothesis Discov. Innov. Ophthalmol. 2020, 9, 118–126. [Google Scholar]
- Cohen, S.Y.; Nghiem-Buffet, S.; Fajnkuchen, F.; Souied, E.H.; Mrejen, S. Clinical applications of optical coherence tomography angiography: What we have learnt in the first 3 years. Eur. J. Ophthalmol. 2018, 28, 491–502. [Google Scholar] [CrossRef]
- De Carlo, T.E.; Adhi, M.; Duker, J.S. Retinal and choroidal vasculature in birdshot chorioretinopathy analyzed using spectral domain optical coherence tomography angiography. Retina 2015, 35, 2392–2399. [Google Scholar] [CrossRef]
- Sadiq, M.A.; Afridi, R. Posterior segment inflammatory outcomes assessed using fluorescein angiography in the STOP-UVEITIS study. Int. J. Retin. Vitr. 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, T.E.; Waheed, N.K.; Duker, J.S. A review of optical coherence tomography angiography. Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef]
- Spaide, R.F.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Su, Y.; Shen, M.; Peng, Y.; Wen, F. OCTA versus dye angiography for the diagnosis and evaluation of neovascularization in punctate inner choroidopathy. Br. J. Ophthalmol. 2022, 106, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, D.; Joussen, A.M.; Winterhalter, S. Optical coherence tomography angiography in comparison with other multimodal imaging techniques in punctate inner choroidopathy. Br. J. Ophthalmol. 2019, 103, 60–66. [Google Scholar] [CrossRef]
- Ahnood, D.; Tsaloumas, M.D.; Waheed, N.K.; Keane, P.A.; Denniston, A.K. Punctate inner choroidopathy: A review. Surv. Ophthalmol. 2017, 62, 113–126. [Google Scholar] [CrossRef]
- Invernizzi, A.; Agarwal, A.; Mapelli, C.; Nguyen, Q.D.; Staurenghi, G.; Viola, F. Longitudinal Follow-up of Choroidal Granulomas Using Enhanced Depth Imaging Optical Coherence Tomography. Retina 2017, 37, 144–153. [Google Scholar] [CrossRef]
- Gaudric, A.; Mrejen, S. Why the Dots Are Black Only in the late Phase of the Indocyanine Green Angiography in Multiple Evanescent White Dot Syndrome. Retin. Cases Brief Rep. 2017, 11, S81–S85. [Google Scholar] [CrossRef]
- Burke, T.R.; Salvatore, S.; Bailey, C.; Dick, A.D.; Lee, R.W.J. Application of OCT-angiography to characterize the evolution of chorioretinal lesions in acute posterior multifocal placoid pigment epitheliopathy. Eye 2017, 31, 1399–1408. [Google Scholar] [CrossRef]
- Werner, J.U.; Lang, G.K.; Lang, G.E. Multi-Modal Imaging Including Optical Coherence Tomography Angiography in Patients With Posterior Multifocal Placoid Pigment Epitheliopathy. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 727–733. [Google Scholar] [CrossRef]
- Pichi, F.; Arepall, S.; Lowder, C.Y.; Cunningham, E.T.; Neri, P.; Albini, T.A.; Gupta, V.; Baynes, K.; Srivastava, S.K. The application of optical coherence tomography angiography in uveitis and inflammatory eye diseases. Prog. Retin. Eye Res. 2017, 59, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.C.; Cui, Y.; Zhu, Y.; Zeng, R.; Lu, E.S.; Katz, R.; Husain, D.; Vavvas, D.G.; Kim, L.A.; et al. A quantitative comparison of four optical coherence tomography angiography devices in healthy eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Filali Ansary, M.; Crincoli, E.; Semoun, O.; Uzzan, J.; Amoroso, F.; Jung, C.; Miere, A.; Souied, E. Undetectable Macular Neovascularization on OCT Angiography in Age Related Macular Degeneration: Comparison between Different Devices. Medicina 2022, 58, 1246. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aggarwal, K.; Mandadi, S.; Kumar, A.; Grewal, D.; Invernizzi, A.; Bansal, R.; Sharma, A.; Sharma, K.; Gupta, V. Longitudinal Follow-up of Tubercular Serpiginous-Like Choroiditis Using Optical Coherence Tomography Angiography. Retina 2023, 41, 793–803. [Google Scholar] [CrossRef] [PubMed]

| Cases | Diagnosis | OCT | OCTA | Choroidal Thickness (µm) | ||
|---|---|---|---|---|---|---|
| 1 | Lupus retinopathy | Foveal thinning in the right eye, normal anatomy in the left eye | Zeiss PLEX® Elite 9000 OCTA (12 mm × 12 mm): area of flow deficit or decreased signal in the SCP corresponding to retinal ischemia in the temporal area | baseline: 335 +11 months: 283 +21 months: 271 +32 months: 304 | ||
| 2 | Sarcoid choroidal granulomas | Hyper-reflective choroidal material: choroidal granulomas without subretinal or intraretinal fluid | Zeiss PLEX® Elite 9000 OCTA (12 mm × 12 mm): non-detectable flow signal in corresponding choroid; One month post-injection: size decrease Eleven months post-injection: no changes | Baseline: 220 | ||
| 3 | Punctate inner choroiditis and inflammatory choroidal neovascular membranes | RPE elevation with loss of ellipsoid zone without IRF in the left eye | Heidelberg Spectralis OCTA (6 mm × 6 mm): dense neovascular network capillary branching resembling a fine-net pattern occurring at the level of choriocapillaris | baseline: 144 +1 month: 151 +3 months: 152 +7 months: 137 | ||
| 4 | Multifocal choroiditis | Multi-inflammatory RPE and choriocapillaris lesions in left eye | Zeiss PLEX® Elite 9000 OCTA (12 mm × 12 mm): non-detectable flow signal at posterior pole in choriocapillaris and choroid in left eye | baseline: 398 +3 months: 401 +4 month: 390 +6 months: 415 +7 months: 339 | ||
| 5 | Acute posterior multifocal placoid pigment epitheliopathy | Outer retinal reflectivity with ellipsoid zone disruption at the macula with corresponding hyperreflective lesions in the choriocapillaris in left eye | Zeiss PLEX® Elite 9000 OCTA 9 mm × 9 mm): non-detectable flow signals at the macula | baseline: 371 | ||
| Cases | Age/Sex | Diagnosis | Uveitis Laterality | Treatment Decision | BCVA | Current Tx |
| 1 | 52 F | Lupus retinopathy | mono | Intravitreal anti-VEGF Subtenon triamcinolone injections | 20/25 | Prednisolone acetate drop to right eye daily |
| 2 | 74 F | Sarcoid choroidal granulomas | mono | Subtenon triamcinolone injection; adalimumab 40 mg subQ every two weeks | 20/20 | Hydroxyquinoline 200 mg daily |
| Intraocular immunomodulatory therapy discontinued | ||||||
| 3 | 41 F | PIC and inflammatory CNVM | mono | Methylprednisolone 500 mg IV for 3 days; prednisone starting dose: 40 mg daily after IV steroid course; Mycophenolate mofetil 250 mg twice a day; Bevacizumab injections in left eye | 20/25 | Mycophenolate mofetil 500 mg twice a day |
| 4 | 78 F | Multifocal choroiditis | mono | Increased prednisone dose to 15 mg daily; brimonidine eye drops in left eye three times a day; adalimumab 40 mg subQ every two weeks; intravitreal dexamethasone injection in left eye | 20/60 | Methotrexate 15 mg weekly, oral prednisone 10 mg daily, adalimumab 40 mg weekly |
| 5 | 17 F | APMPPE | mono | Prednisone 0.75 mg/kg/day with 3 weeks of slow steroid taper | 20/30 | No current treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melachuri, S.; Dansingani, K.K.; Wesalo, J.; Paez-Escamilla, M.; Gagrani, M.; Atta, S.; Indermill, C.; Sahel, J.-A.; Nischal, K.K.; Chhablani, J.; et al. OCT Angiography in Noninfectious Uveitis: A Description of Five Cases and Clinical Applications. Diagnostics 2023, 13, 1296. https://doi.org/10.3390/diagnostics13071296
Melachuri S, Dansingani KK, Wesalo J, Paez-Escamilla M, Gagrani M, Atta S, Indermill C, Sahel J-A, Nischal KK, Chhablani J, et al. OCT Angiography in Noninfectious Uveitis: A Description of Five Cases and Clinical Applications. Diagnostics. 2023; 13(7):1296. https://doi.org/10.3390/diagnostics13071296
Chicago/Turabian StyleMelachuri, Samyuktha, Kunal K. Dansingani, Joshua Wesalo, Manuel Paez-Escamilla, Meghal Gagrani, Sarah Atta, Chad Indermill, José-Alain Sahel, Ken K. Nischal, Jay Chhablani, and et al. 2023. "OCT Angiography in Noninfectious Uveitis: A Description of Five Cases and Clinical Applications" Diagnostics 13, no. 7: 1296. https://doi.org/10.3390/diagnostics13071296
APA StyleMelachuri, S., Dansingani, K. K., Wesalo, J., Paez-Escamilla, M., Gagrani, M., Atta, S., Indermill, C., Sahel, J.-A., Nischal, K. K., Chhablani, J., & Errera, M.-H. (2023). OCT Angiography in Noninfectious Uveitis: A Description of Five Cases and Clinical Applications. Diagnostics, 13(7), 1296. https://doi.org/10.3390/diagnostics13071296








