COVID-19 and Vasa vasorum: New Atherogenic Factor? A Case Report and Autopsy Findings
Abstract
:1. Introduction
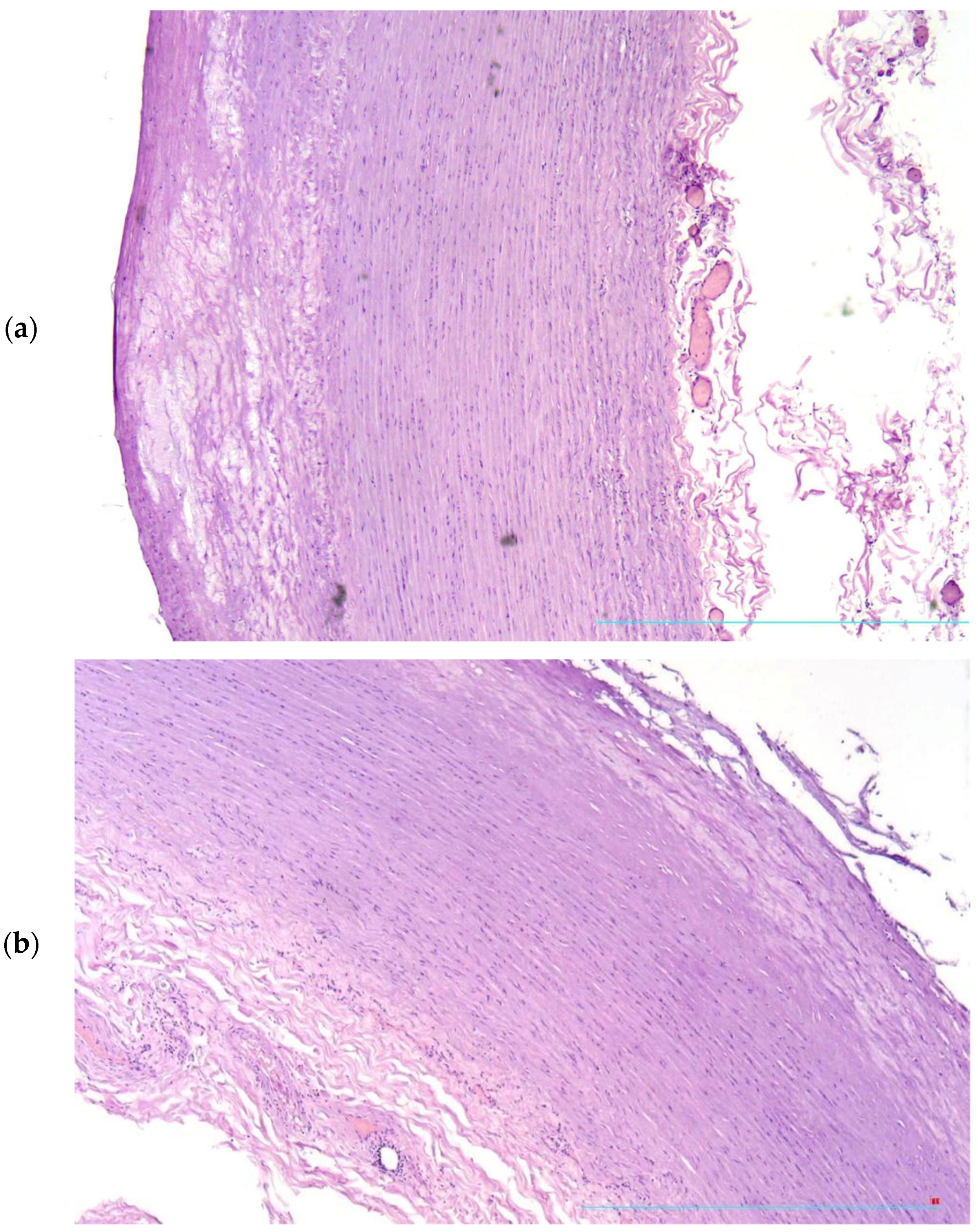
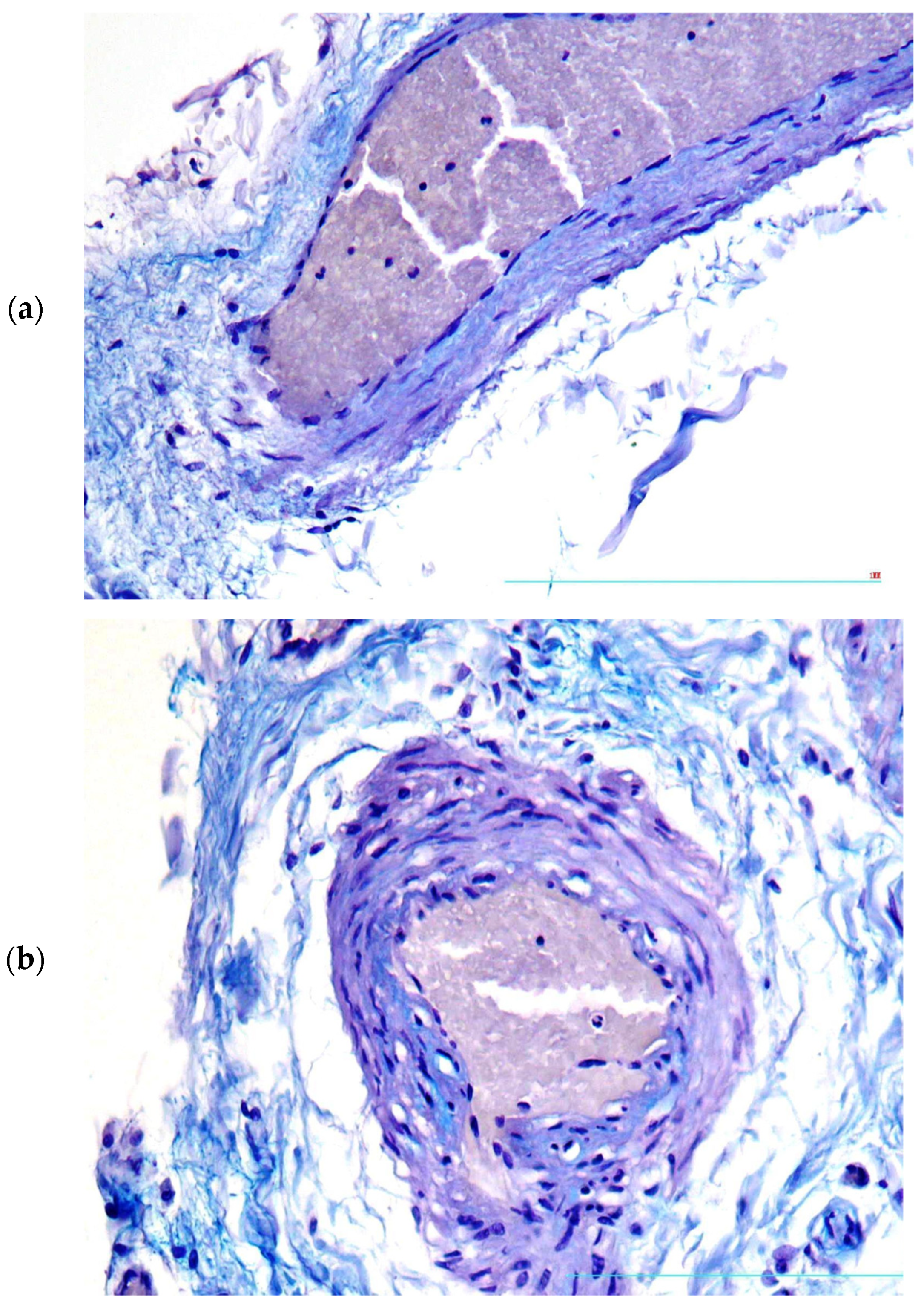
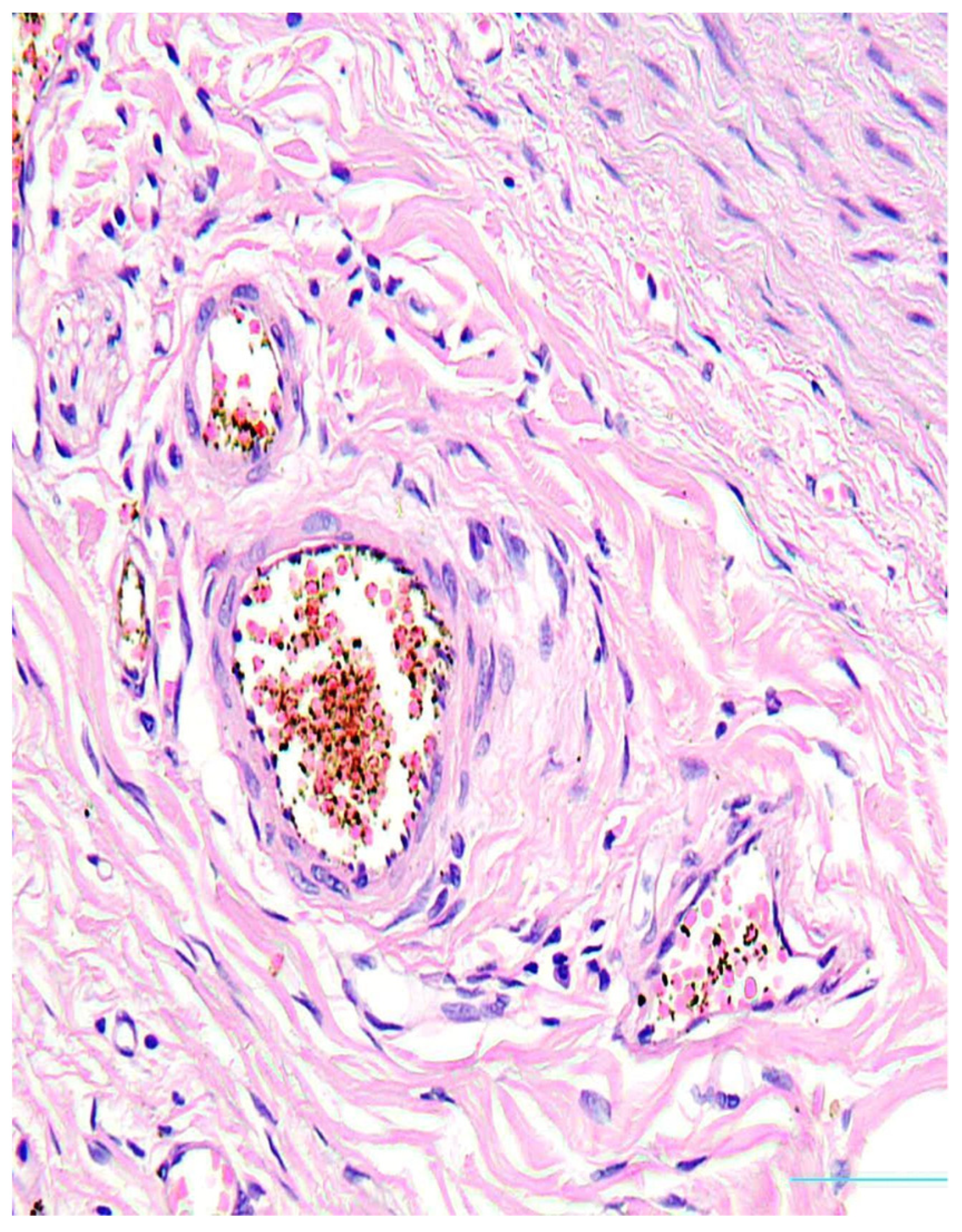
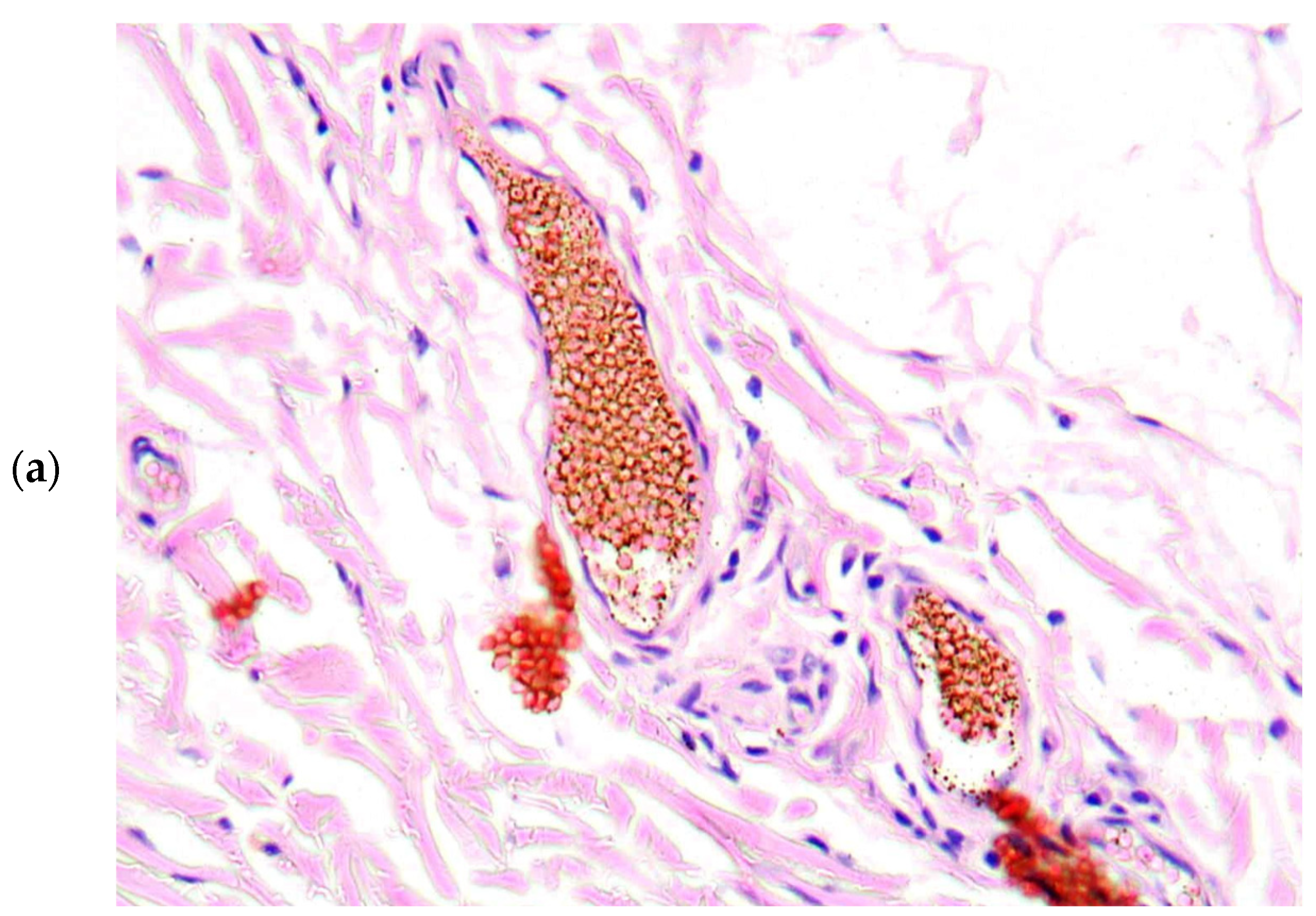
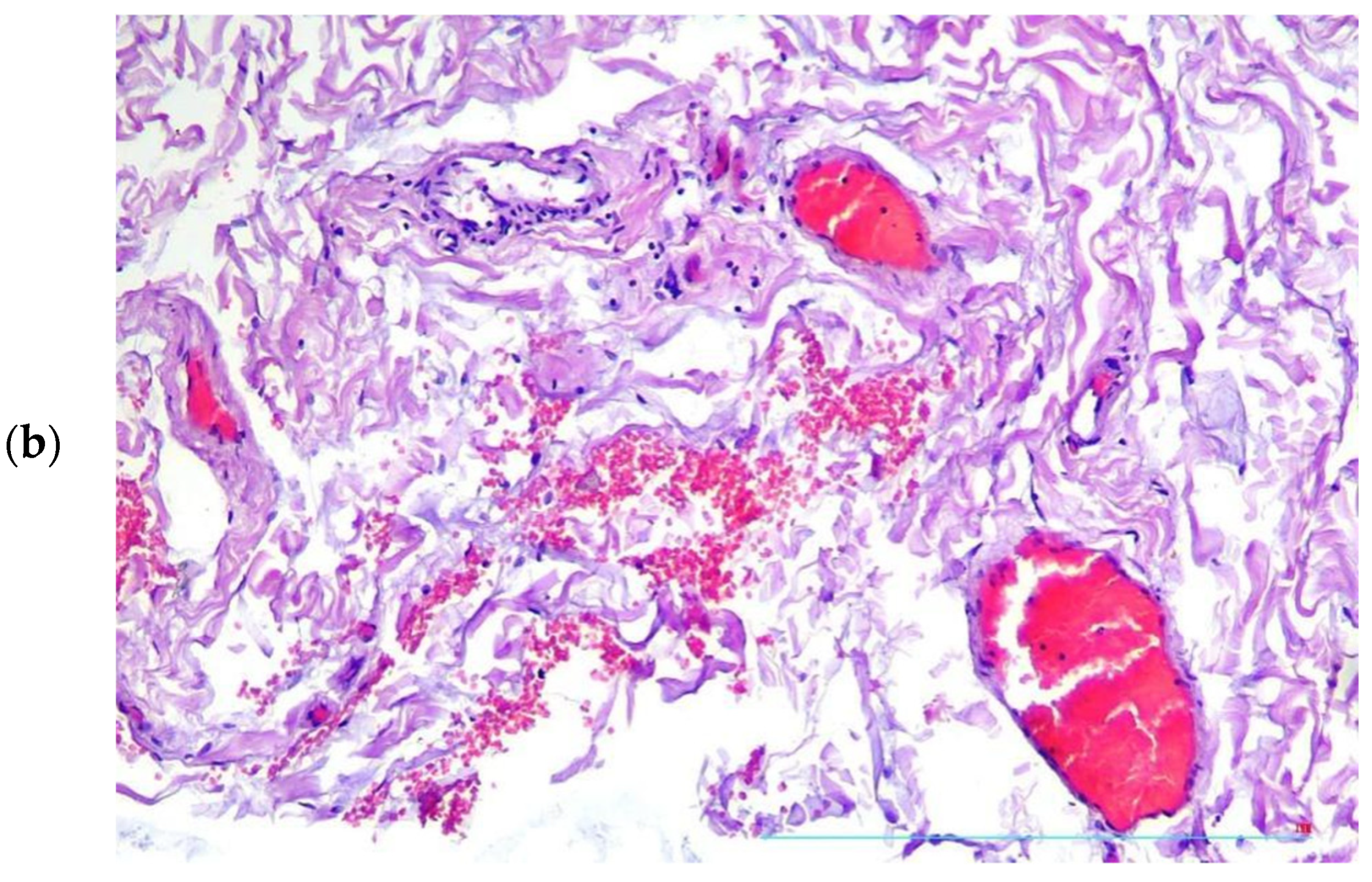
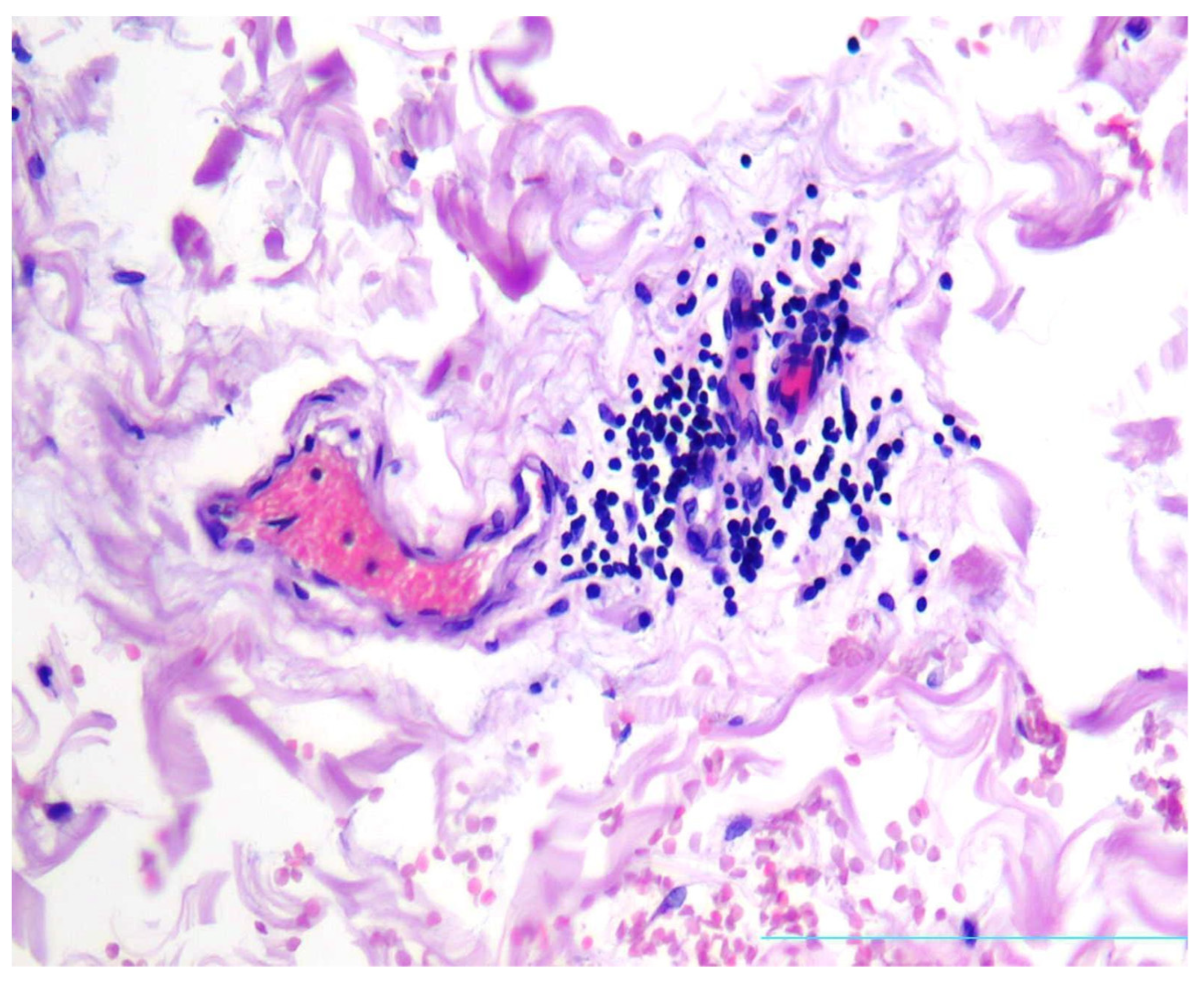
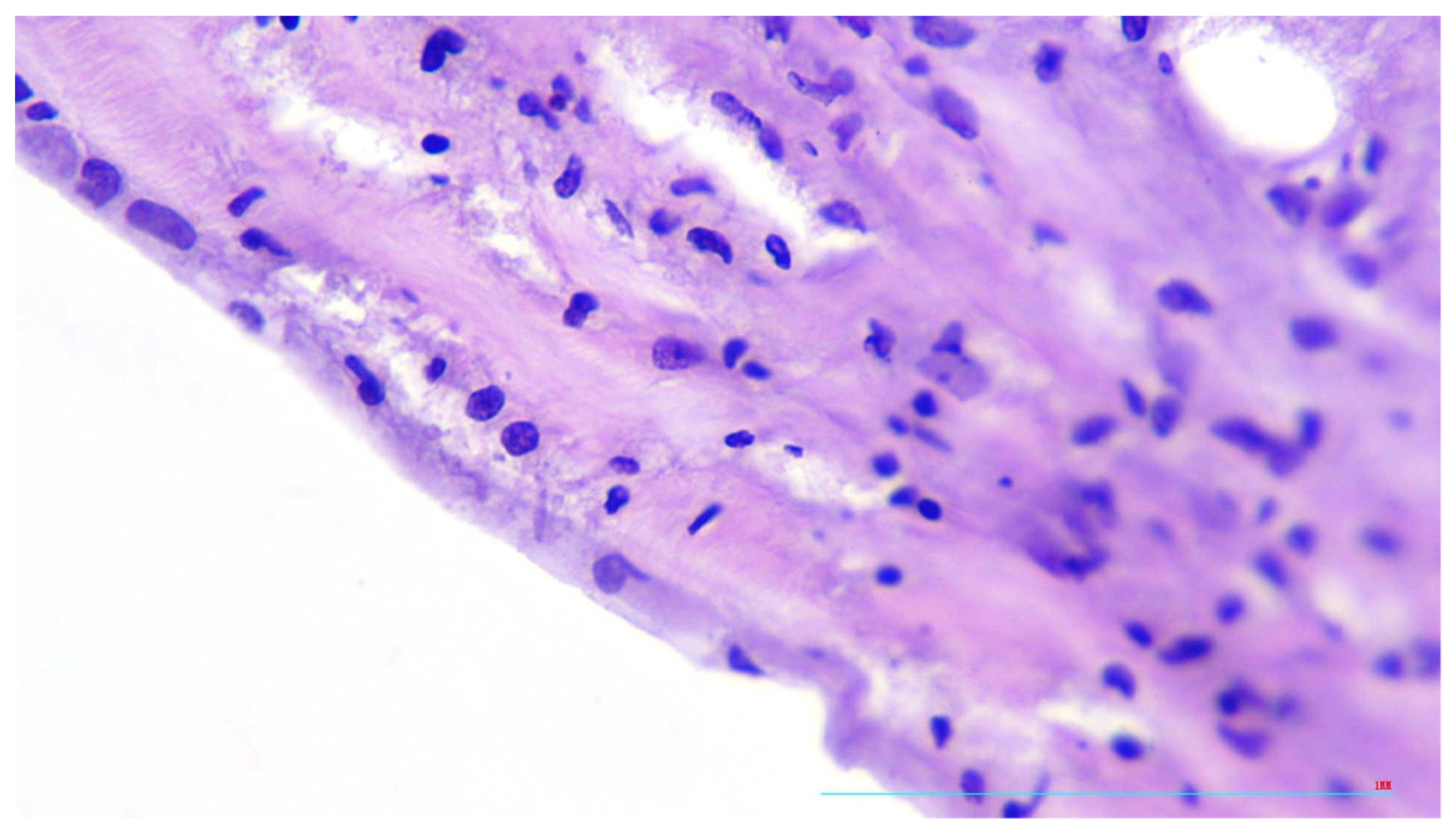
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19) Outbreak. Available online: https://www.who.int (accessed on 1 March 2020).
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Tsioufis, C.; Oikonomou, E.; Antoniades, C.; Crea, F.; Kaski, J.C.; Tousoulis, D. Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives. Int. J. Mol. Sci. 2021, 22, 6607. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long Covid 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights from TUN-EndCOV Study. Front. Cardiovasc. Med. 2021, 8, 745–758. [Google Scholar]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long Covid/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Tanaka, H.; Zaima, N.; Sasaki, T.; Sano, M.; Yamamoto, N.; Saito, T.; Inuzuka, K.; Hayasaka, T.; Goto-Inoue, N.; Sugiura, Y.; et al. Hypoperfusion of the adventitial vasa vasorum develops an abdominal aortic aneurysm. PLoS ONE 2015, 10, e0134386. [Google Scholar] [CrossRef]
- Faa, G.; Gerosa, C.; Fanni, D.; Barcellona, D.; Cerrone, G.; Orru, G.; Scano, A.; Marongiu, F.; Suri, G.S.; Demontis, R.; et al. Aortic vulnerability to COVID-19: Is the microvasculature of vasa vasorum a key factor? A case report and a review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6439–6442. [Google Scholar]
- Boyle, E.C.; Haverich, A. Microvasculature dysfunction as the common thread between atherosclerosis, Kawasaki disease, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) associated multi-system inflammatory syndrome in children. Eur. J. Cardiothorac. Surg. 2020, 58, 1109–1110. [Google Scholar] [CrossRef]
- Haverich, A. A surgeon’s view on the pathogenesis of atherosclerosis. Circulation 2017, 135, 205–207. [Google Scholar] [CrossRef]
- Saba, L.; Gerosa, C.; Fanni, D.; Marongiu, F.; La Nasa, G.; Caocci, G.; Barcellona, D.; Balestrieri, A.; Coghe, F.; Orru, G.; et al. Molecular pathways triggered by COVID-19 in different organs: ACE2 receptor-expressing cells under attack? A review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12609–12622. [Google Scholar] [PubMed]
- Wirth, K.J.; Löhn, M. Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect? Medicina 2022, 58, 1807. [Google Scholar] [CrossRef] [PubMed]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.F.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef] [Green Version]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Der Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Otifi, H.M.; Adiga, B.K. Endothelial Dysfunction in Covid-19 Infection. Am. J. Med. Sci. 2022, 363, 281–287. [Google Scholar] [CrossRef]
- Hogea, T.; Suciu, B.A.; Ivănescu, A.D.; Caras, C.; Chinezu, L.; Arbănas, E.M.; Russu, E.; Kaller, R.; Arbănas, E.M.; Mures, A.V.; et al. Increased Epicardial Adipose Tissue (EAT), Left Coronary Artery Plaque Morphology, and Valvular Atherosclerosis as Risks Factors for Sudden Cardiac Death from a Forensic Perspective. Diagnostics 2023, 13, 142. [Google Scholar] [CrossRef]
- Daisley, H., Jr.; Rampersad, A.; Daisley, M.; Ramdin, A.; Acco, O.; Narinesingh, F.; Humphrey, O.; James, E. The vasa vasorum of the large pulmonary vessels are involved in COVID-19. Autops. Case Rep. 2021, 11, e2021304. [Google Scholar] [CrossRef]
- Haverich, A.; Boyle, E.C. Aortic dissection is a disease of the vasa vasorum. JTCVS Open 2021, 5, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Gerosa, C.; Wintermark, M.; Hedin, U.; Fanni, D.; Suri, J.S.; Balestrieri, A.; Faa, G. Can COVID19 trigger the plaque vulnerability-a Kounis syndrome warning for ‘asymptomatic subjects’. Cardiovasc. Diagn. Ther. 2020, 10, 1352–1355. [Google Scholar] [CrossRef]
- Giryes, S.; McGonagle, D. Immune and non-immune mechanisms that determine vasculitis and coronary artery aneurysm topography in Kawasaki disease and MIS-C. Autoimmun. Rev. 2023, 22, 103240. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.; Dotan, A.; Fritzler, M.J.; Bogdanos, D.P.; Meroni, P.L.; Roggenbuck, D.; Goldman, M.; Landegren, N.; Bastard, P.; Shoenfeld, Y.; et al. Autoantibodies and SARS-CoV2 infection: The spectrum from association to clinical implication: Report of the 15th Dresden Symposium on Autoantibodies. Autoimmun. Rev. 2022, 21, 103012. [Google Scholar] [CrossRef] [PubMed]
- Grzegorowska, O.; Lorkowski, J. Possible correlations between atherosclerosis, acute coronary syndromes and COVID-19. J. Clin. Med. 2020, 9, 3746. [Google Scholar] [CrossRef] [PubMed]
- Leali, M.; Rossi, A.; Gaskill, M.; Sengupta, S.; Zhang, B.; Carriero, A.; Bachir, S.; Crivelli, P.; Paschè, A.; Premi, E.; et al. Imaging of Neurologic Disease in Hospitalized Patients with COVID-19: An Italian Multicenter Retrospective Observational Study. Radiology 2020, 297, E270–E273. [Google Scholar]
- Savastano, M.C.; Santoro, L.; Crincoli, E.; Fossataro, C.; Gambini, G.; Savastano, A.; De Vico, U.; Santoliquido, A.; Nesci, A.; Landi, F.; et al. Radial Peripapillary Capillary Plexus Perfusion and Endothelial Dysfunction in Early Post-SARS-CoV-2 Infection. Vision 2022, 6, 26. [Google Scholar] [CrossRef]
- Indes, J.E.; Koleilat, I.; Hatch, A.N.; Choinski, K.; Jones, D.B.; Aldailami, H.; Billett, H.; Denesopolis, J.M.; Lipsitz, E. Early experience with arterial thromboembolic complications in patients with COVID-19. J. Vasc. Surg. 2021, 73, 381–389. [Google Scholar] [CrossRef]
- Ryabkova, V.A.; Churilov, L.P.; Shoenfeld, Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm—The common denominator and the lessons to be learned. Clin. Immunol. 2021, 223, 108652. [Google Scholar] [CrossRef]
- Ritman, E.L.; Lerman, A. The dynamic vasa vasorum. Cardiovasc. Res. 2007, 75, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Krieg, C.; Létourneau, S.; Pantaleo, G.; Boyman, O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11906–11911, Erratum in Proc. Natl. Acad. Sci. USA 2012, 109, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarova, Y.A.; Ryabkova, V.A.; Salukhov, V.V.; Sagun, B.V.; Korovin, A.E.; Churilov, L.P. Atherosclerosis, Cardiovascular Disorders and COVID-19: Comorbid Pathogenesis. Diagnostics 2023, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Vasuri, F.; Ciavarella, C.; Collura, S.; Mascoli, C.; Valente, S.; Degiovanni, A.; Gargiulo, M.; Capri, M.; Pasquinelli, G. Adventitial Microcirculation Is a Major Target of SARS-CoV-2-Mediated Vascular Inflammation. Biomolecules 2021, 11, 1063. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mures, A.V.; Hălmaciu, I.; Arbănas, E.V.; Kaller, R.; Arbănas, E.M.; Budis, O.A.; Melinte, R.M.; Vunvulea, V.; Rares, C.F.; Mărginean, L.; et al. Prognostic Nutritional Index, Controlling Nutritional Status (CONUT) Score, and Inflammatory Biomarkers as Predictors of Deep Vein Thrombosis, Acute Pulmonary Embolism, and Mortality in COVID-19 Patients. Diagnostics 2022, 12, 2757. [Google Scholar] [CrossRef]
- Brott, T.G.; Hobson, R.W.; Howard, G.; Roubin, G.S.; Clark, W.M.; Brooks, W.; Mackey, A.; Hill, M.D.; Leimgruber, P.P.; Sheffet, A.J.; et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. 2010, 363, 11–23. [Google Scholar] [CrossRef]
- Fuster, V.; Badimon, L.; Badimon, J.J.; Chesebro, J.H. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N. Engl. J. Med. 1992, 326, 242–250. [Google Scholar] [CrossRef]
- Fuster, V.; Badimon, L.; Badimon, J.J.; Chesebro, J.H. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N. Engl. J. Med. 1992, 326, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; Cheng, Z.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Maiellaro, K.; Taylor, W.R. The role of the adventitia in vascular inflammation. Cardiovasc. Res. 2007, 75, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Sangawa, A.; Sasaki, Y.; Yamashita, M.; Tanaka-Shintani, M.; Shintaku, M.; Ishikawa, Y. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J. Atheroscler. Thromb. 2007, 14, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarzaeim, M.; Rezaei, N. Kawasaki Disease and Multisystem Inflammatory Syndrome in Children with COVID-19. SN Compr. Clin. Med. 2020, 2, 2096–2101. [Google Scholar] [CrossRef]
- Hervier, B.; Masseau, A.; Bossard, C.; Agard, C.; Hamidou, M. Vasa-vasoritis of the aorta and fatal myocarditis in fulminant Churg-Strauss syndrome. Rheumatology 2008, 47, 1728–1729. [Google Scholar] [CrossRef] [Green Version]
- Numano, F. Vasa vasoritis, vasculitis and atherosclerosis. Int. J. Cardiol. 2000, 75 (Suppl. S1), S1–S8; Discussion S17–S19. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macarova, J.A.; Malakhova, S.A.; Novitskaya, T.A.; Shapkina, V.A.; Churilov, L.P. COVID-19 and Vasa vasorum: New Atherogenic Factor? A Case Report and Autopsy Findings. Diagnostics 2023, 13, 1097. https://doi.org/10.3390/diagnostics13061097
Macarova JA, Malakhova SA, Novitskaya TA, Shapkina VA, Churilov LP. COVID-19 and Vasa vasorum: New Atherogenic Factor? A Case Report and Autopsy Findings. Diagnostics. 2023; 13(6):1097. https://doi.org/10.3390/diagnostics13061097
Chicago/Turabian StyleMacarova, Julia A., Sofia A. Malakhova, Tatiana A. Novitskaya, Valeria A. Shapkina, and Leonid P. Churilov. 2023. "COVID-19 and Vasa vasorum: New Atherogenic Factor? A Case Report and Autopsy Findings" Diagnostics 13, no. 6: 1097. https://doi.org/10.3390/diagnostics13061097




