Digital Tomosynthesis as a Problem-Solving Technique to Confirm or Exclude Pulmonary Lesions in Hidden Areas of the Chest
Abstract
1. Introduction
2. Materials and Methods
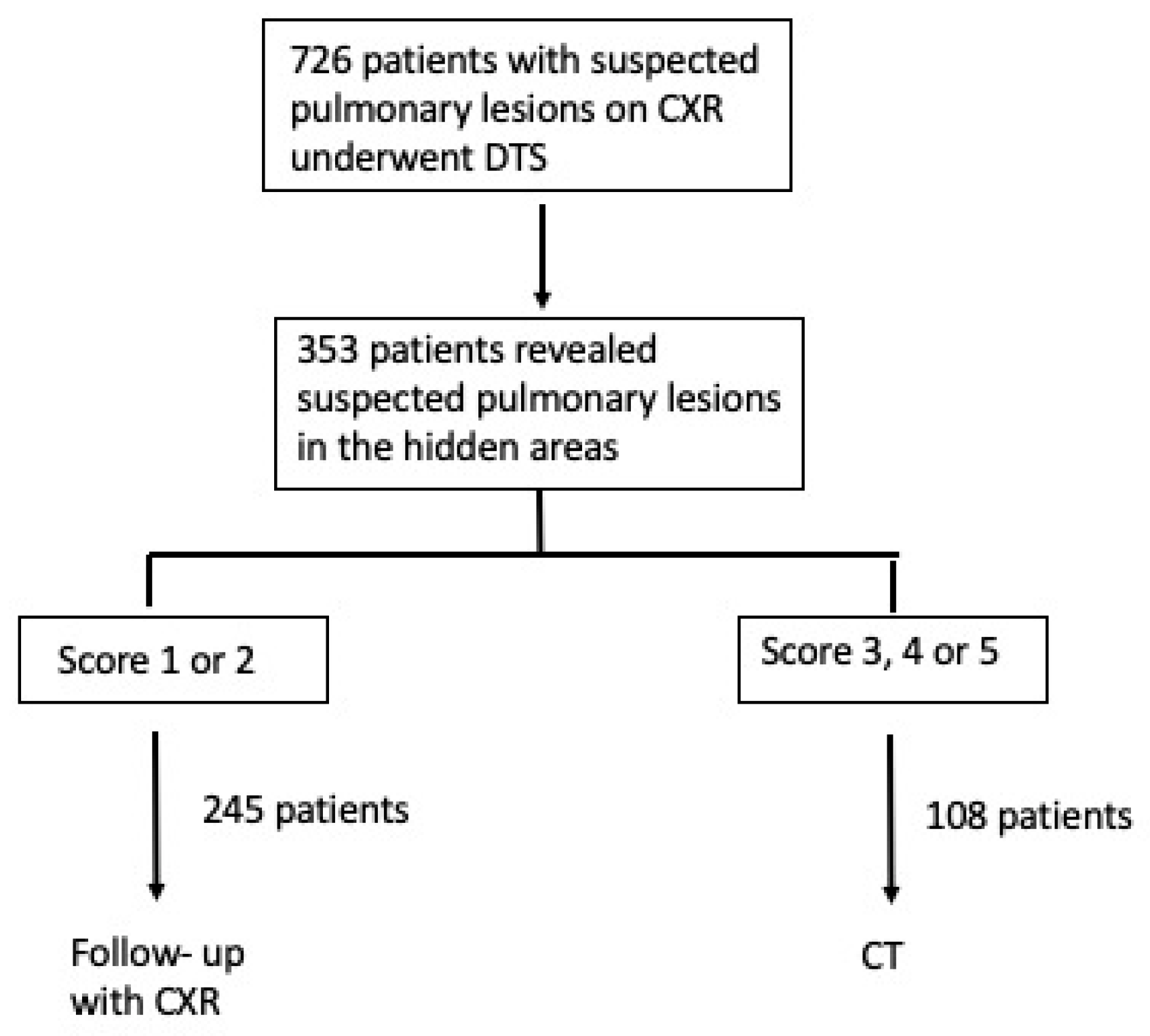
2.1. Patients Population
2.2. Acquisition Protocols
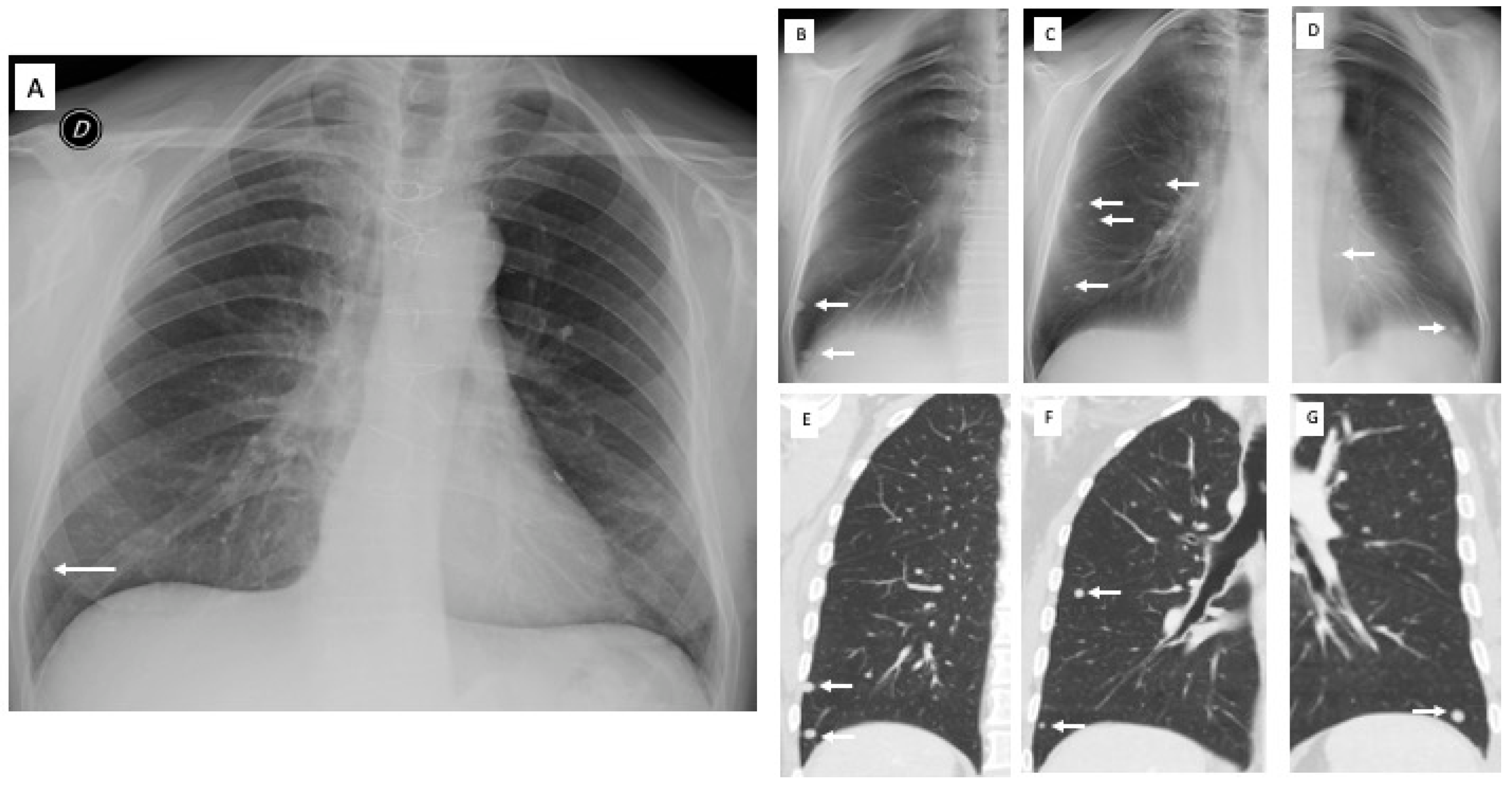
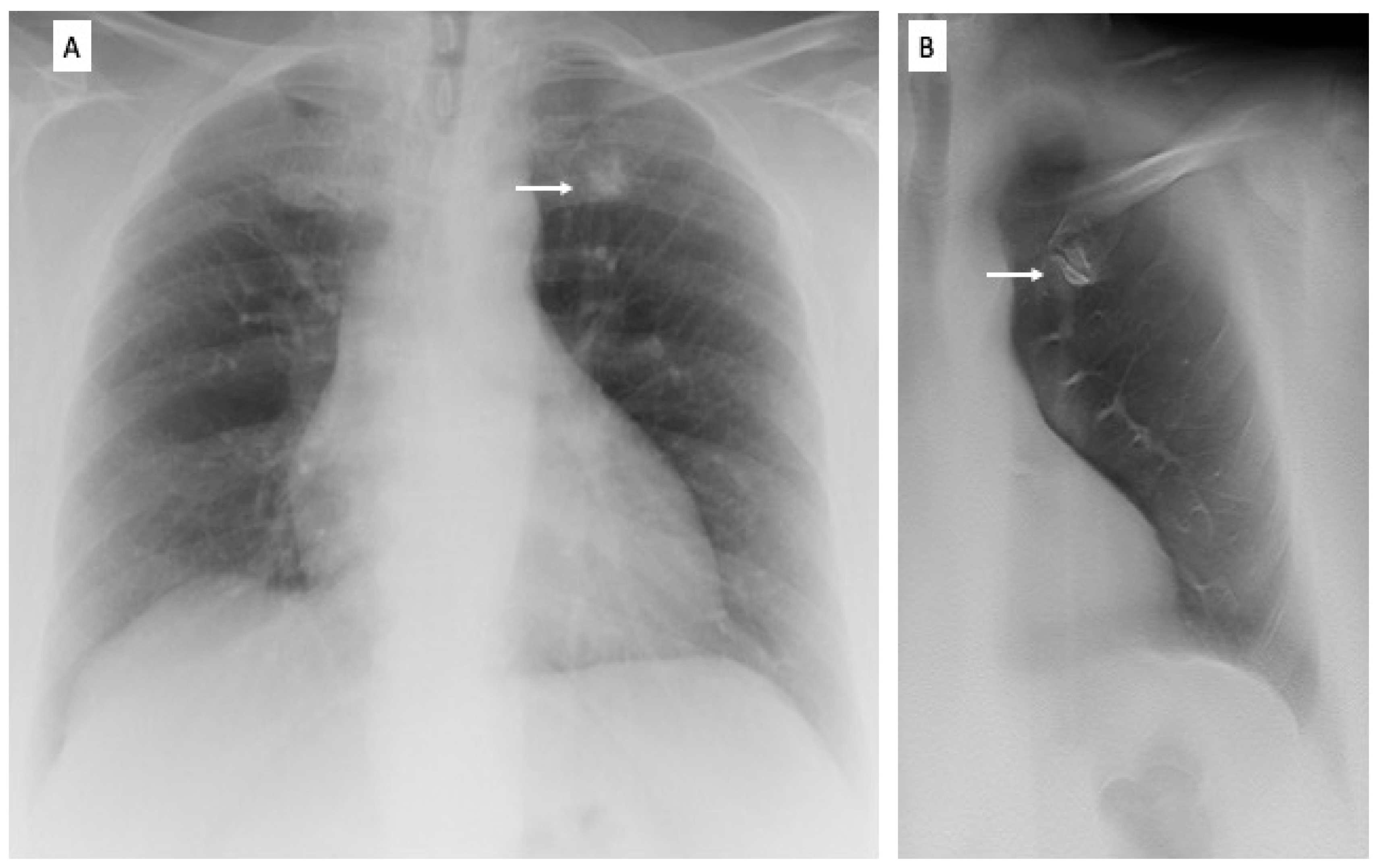
2.3. Image Analysis
2.4. Diagnostic Workup
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erasmus, J.J.; Connolly, J.E.; McAdams, H.P.; Roggli, V.L. Solitary Pulmonary Nodules: Part I. Morphologic Evaluation for Differentiation of Benign and Malignant Lesions. Radiographics 2000, 20, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Remy-Jardin, M.; Remy, J.; Giraud, F.; Marquette, C.H. Pulmonary nodules: Detection with thick-section spiral CT versus conventional CT. Radiology 1993, 187, 513–520. [Google Scholar] [CrossRef]
- Samei, E.; Flynn, M.J.; Eyler, W.R. Detection of Subtle Lung Nodules: Relative Influence of Quantum and Anatomic Noise on Chest Radiographs. Radiology 1999, 213, 727–734. [Google Scholar] [CrossRef]
- Samei, E.; Flynn, M.J.; Peterson, E.; Eyler, W.R. Subtle Lung Nodules: Influence of Local Anatomic Variations on Detection. Radiology 2003, 228, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Bley, T.A.; Baumann, T.; Saueressig, U.; Pache, G.; Treier, M.; Schaefer, O.; Neitzel, U.; Langer, M.; Kotter, E. Comparison of Radiologist and CAD Performance in the Detection of CT-confirmed Subtle Pulmonary Nodules on Digital Chest Radiographs. Investig. Radiol. 2008, 43, 343–348. [Google Scholar] [CrossRef] [PubMed]
- McAdams, H.P.; Samei, E.; Dobbins, J.; Tourassi, G.; Ravin, C.E. Recent Advances in Chest Radiography. Radiology 2006, 241, 663–683. [Google Scholar] [CrossRef]
- Dobbins, J.T.; Godfrey, D.J. Digital x-ray tomosynthesis: Current state of the art and clinical potential. Phys. Med. Biol. 2003, 48, R65–R106. [Google Scholar] [CrossRef]
- Dobbins, J.T.; McAdams, H.P.; Godfrey, D.J.; Li, C.M. Digital Tomosynthesis of the Chest. J. Thorac. Imaging 2008, 23, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Vikgren, J.; Zachrisson, S.; Svalkvist, A.; Johnsson, Å.A.; Boijsen, M.; Flinck, A.; Kheddache, S.; Båth, M. Comparison of Chest Tomosynthesis and Chest Radiography for Detection of Pulmonary Nodules: Human Observer Study of Clinical Cases. Radiology 2008, 249, 1034–1041. [Google Scholar] [CrossRef]
- Quaia, E.; Baratella, E.; Cioffi, V.; Bregant, P.; Cernic, S.; Cuttin, R.; Cova, M.A. The Value of Digital Tomosynthesis in the Diagnosis of Suspected Pulmonary Lesions on Chest Radiography: Analysis of Diagnostic Accuracy and Confidence. Acad. Radiol. 2010, 17, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Baratella, E.; Cernic, S.; Lorusso, A.; Casagrande, F.; Cioffi, V.; Cova, M.A. Analysis of the impact of digital tomosynthesis on the radiological investigation of patients with suspected pulmonary lesions on chest radiography. Eur. Radiol. 2012, 22, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Baratella, E.; Poillucci, G.; Kus, S.; Cioffi, V.; Cova, M.A. Digital Tomosynthesis as a Problem-Solving Imaging Technique to Confirm or Exclude Potential Thoracic Lesions Based on Chest X-Ray Radiography. Acad. Radiol. 2013, 20, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Grisi, G.; Baratella, E.; Cuttin, R.; Poillucci, G.; Kus, S.; Cova, M.A. Diagnostic imaging costs before and after digital tomosynthesis implementation in patient management after detection of suspected thoracic lesions on chest radiography. Insights Imaging 2014, 5, 147–155. [Google Scholar] [CrossRef]
- Machida, H.; Yuhara, T.; Mori, T.; Ueno, E.; Moribe, Y.; Sabol, J.M. Optimizing Parameters for Flat-Panel Detector Digital Tomosynthesis. Radiographics 2010, 30, 549–562. [Google Scholar] [CrossRef]
- Dobbins, J.T.; McAdams, H.P.; Song, J.-W.; Li, C.M.; Godfrey, D.J.; DeLong, D.M.; Paik, S.-H.; Martinez-Jimenez, S. Digital tomosynthesis of the chest for lung nodule detection: Interim sensitivity results from an ongoing NIH-sponsored trial. Med. Phys. 2008, 35, 2554–2557. [Google Scholar] [CrossRef]
- Gomi, T.; Nakajima, M.; Fujiwara, H.; Umeda, T. Comparison of Chest Dual-energy Subtraction Digital Tomosynthesis Imaging and Dual-energy Subtraction Radiography to Detect Simulated Pulmonary Nodules with and without Calcifications: A Phantom Study. Acad. Radiol. 2011, 18, 191–196. [Google Scholar] [CrossRef]
- Yamada, Y.; Jinzaki, M.; Hasegawa, I.; Shiomi, E.; Sugiura, H.; Abe, T.; Sato, Y.; Kuribayashi, S.; Ogawa, K. Fast Scanning Tomosynthesis for the Detection of Pulmonary Nodules: Diagnostic performance compared with chest radiography, using multidetector-row computed tomography as the reference. Investig. Radiol. 2011, 46, 471–477. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sone, S.; Takashima, S.; Li, F.; Yang, Z.G.; Maruyama, Y.; Watanabe, T. Growth rate of small lung cancers detected on mass CT screening. Br. J. Radiol. 2000, 73, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Machin, D. Medical Statistics: A Commonsense Approach. Biometrics 1993, 49, 1286. [Google Scholar] [CrossRef]
- Beck, J.R.; Shultz, E.K. The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch. Pathol. Lab. Med. 1986, 110, 13–20. [Google Scholar] [PubMed]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Servomaa, A.; Tapiovaara, M. Organ Dose Calculation in Medical X Ray Examinations by the Program PCXMC. Radiat. Prot. Dosim. 1998, 80, 213–219. [Google Scholar] [CrossRef]
- Cristy, M.; Eckerman, K.F. Specific Adsorbed Fractions of Energy at Various Ages from Internal Photon Sources: 1, Methods. No. ORNL/TM-8381/V1; Oak Ridge National Lab.: Oak Ridge, TN, USA, 1987. [Google Scholar]
- Sabol, J.M. A Monte Carlo estimation of effective dose in chest tomosynthesis. Med. Phys. 2009, 36, 5480–5487. [Google Scholar] [CrossRef]
- European Commission. European Guidelines on Quality Criteria for Computed Tomography European Guidelines on Quality Criteria; European Commission: Brussels, Belgium, 1999. [Google Scholar]
- Kim, E.; Bista, A.; Kim, T.; Park, S.; Park, K.; Kang, D.; Sun, J. The advantage of digital tomosynthesis for pulmonary nodule detection concerning influence of nodule location and size: A phantom study. Clin. Radiol. 2017, 72, 796.e1–796.e8. [Google Scholar] [CrossRef]
- Lee, K.H.; Goo, J.M.; Lee, S.M.; Park, C.M.; Bahn, Y.E.; Kim, H.; Song, Y.S.; Hwang, E.J. Digital Tomosynthesis for Evaluating Metastatic Lung Nodules: Nodule Visibility, Learning Curves, and Reading Times. Korean J. Radiol. 2015, 16, 430–439. [Google Scholar] [CrossRef]
- Langer, S.G.; Graner, B.D.; Schueler, B.A.; Fetterly, K.A.; Kofler, J.M.; Mandrekar, J.N.; Bartholmai, B.J. Sensitivity of Thoracic Digital Tomosynthesis (DTS) for the Identification of Lung Nodules. J. Digit. Imaging 2016, 29, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Bertolaccini, L.; Solli, P.; Di Salvia, P.O.; Scaradozzi, D. Digital chest tomosynthesis: The 2017 updated review of an emerging application. Ann. Transl. Med. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Galea, A.; Dubbins, P.; Riordan, R.; Adlan, T.; Roobottom, C.; Gay, D. The value of digital tomosynthesis of the chest as a problem-solving tool for suspected pulmonary nodules and hilar lesions detected on chest radiography. Eur. J. Radiol. 2015, 84, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yu, J.; Huang, Z. Low-Dose Chest CT: Optimizing Radiation Protection for Patients. Am. J. Roentgenol. 2004, 183, 809–816. [Google Scholar] [CrossRef]
- Johnsson, Å.A.; Vikgren, J.; Båth, M. A Retrospective Study of Chest Tomosynthesis as a Tool for Optimizing the use of Computed Tomography Resources and Reducing Patient Radiation Exposure. Acad. Radiol. 2014, 21, 1427–1433. [Google Scholar] [CrossRef]
- Siegelman, J.W.; Supanich, M.P.; Gavrielides, M.A. Pulmonary Nodules with Ground-Glass Opacity Can Be Reliably Measured with Low-Dose Techniques Regardless of Iterative Reconstruction: Results of a Phantom Study. Am. J. Roentgenol. 2015, 204, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
| Diagnoses | n | Mean Size (cm) ± SD | Size Range (cm) |
|---|---|---|---|
| Pulmonary opacities | |||
| Non-tumoral lung opacities | 32 | 2.3 ± 0.3 | 0.5–3 |
| Squamous cell carcinomas | 5 | 2.5 ± 0.5 | 2–3 |
| Benign lung nodules | 28 | 1.1 ± 0.3 | 0.5–1.5 |
| Peripheral adenocarcinomas (nodules) | 5 | 2 ± 0.7 | 0.5–1.5 |
| Lung metastases | 7 | 1.6 ± 0.7 | 1–1.8 |
| Pulmonary scars (#) | 26 | 1.1 ± 0.3 | 0.5–1.5 |
| Pleural plaques | 12 | 2.4 ± 0.6 | 1–3 |
| Pulmonary pseudolesions | |||
| Areas of increased opacity | 92 | - | - |
| Vascular kinking or ectasia | 15 | - | - |
| Auricula or mediastinal profiles | 10 | - | - |
| Lung variants | 5 | - | - |
| Total | 237 | 2.3 ± 1.1 | 0.5–3 |
| CXR | 95% CI | DTS | 95% CI | p | |
|---|---|---|---|---|---|
| Sensitivity (%) | 15 (16/103) | 9.15–24 | 92 (95/103) | 85.27–96.59 | 0.0001 |
| Specificity (%) | 9 (13/134) | 5.27–16.02 | 91 (122/134) | 84.88–95.29 | 0.0001 |
| Positive likelihood ratio | (*) | (*) | 10.3 (95/91) | 5.99 to 17.72 | |
| Negative likelihood ratio | (*) | (*) | 0.09 (8/91) | 0.04 to 0.17 | |
| PPV (%) | 12 (16/137) | 6.82–18.27 | 88 (95/107) | 81.23–94.07 | 0.0001 |
| NPV (%) | 13 (13/100) | 7.11–21.2 | 93 (122/130) | 88.23–97.31 | 0.0001 |
| Accuracy (%) | 12 (29/237) | 8.35–17.09 | 91 (217/237) | 87.26–94.76 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratella, E.; Quaia, E.; Crimì, F.; Minelli, P.; Cioffi, V.; Ruaro, B.; Cova, M.A. Digital Tomosynthesis as a Problem-Solving Technique to Confirm or Exclude Pulmonary Lesions in Hidden Areas of the Chest. Diagnostics 2023, 13, 1010. https://doi.org/10.3390/diagnostics13061010
Baratella E, Quaia E, Crimì F, Minelli P, Cioffi V, Ruaro B, Cova MA. Digital Tomosynthesis as a Problem-Solving Technique to Confirm or Exclude Pulmonary Lesions in Hidden Areas of the Chest. Diagnostics. 2023; 13(6):1010. https://doi.org/10.3390/diagnostics13061010
Chicago/Turabian StyleBaratella, Elisa, Emilio Quaia, Filippo Crimì, Pierluca Minelli, Vincenzo Cioffi, Barbara Ruaro, and Maria Assunta Cova. 2023. "Digital Tomosynthesis as a Problem-Solving Technique to Confirm or Exclude Pulmonary Lesions in Hidden Areas of the Chest" Diagnostics 13, no. 6: 1010. https://doi.org/10.3390/diagnostics13061010
APA StyleBaratella, E., Quaia, E., Crimì, F., Minelli, P., Cioffi, V., Ruaro, B., & Cova, M. A. (2023). Digital Tomosynthesis as a Problem-Solving Technique to Confirm or Exclude Pulmonary Lesions in Hidden Areas of the Chest. Diagnostics, 13(6), 1010. https://doi.org/10.3390/diagnostics13061010









