The Prospects of Using Structural Phase Analysis of Microcalcifications in Breast Cancer Diagnostics
Abstract
1. Introduction
2. Materials and Methods
2.1. The Ethics Committee
2.2. Retrospective Study
2.3. Samples Collection
2.4. Histopathology Examination
2.5. Histochemistry Examination
2.6. Immunohistochemistry (IHC) of BC Tissue
2.7. Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM-EDS)
2.8. Transmission Electron Microscopy with Electron Diffraction (TEM-ED)
2.9. Statistical Analysis
3. Results
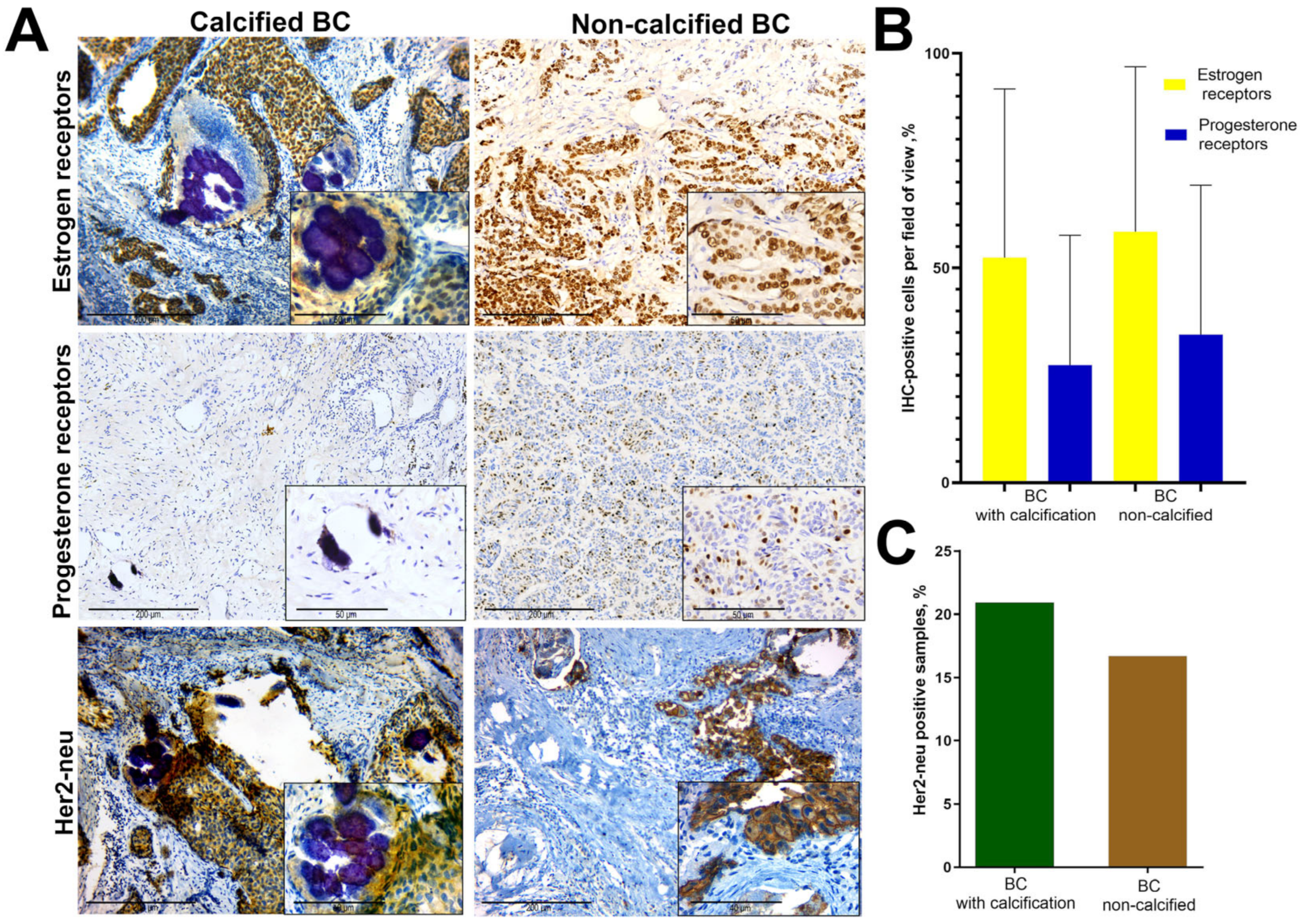
3.1. A Retrospective Study of the ER, PR and Her2-neu Expression in BC Tissue with Calcification
3.2. Macroscopic Study
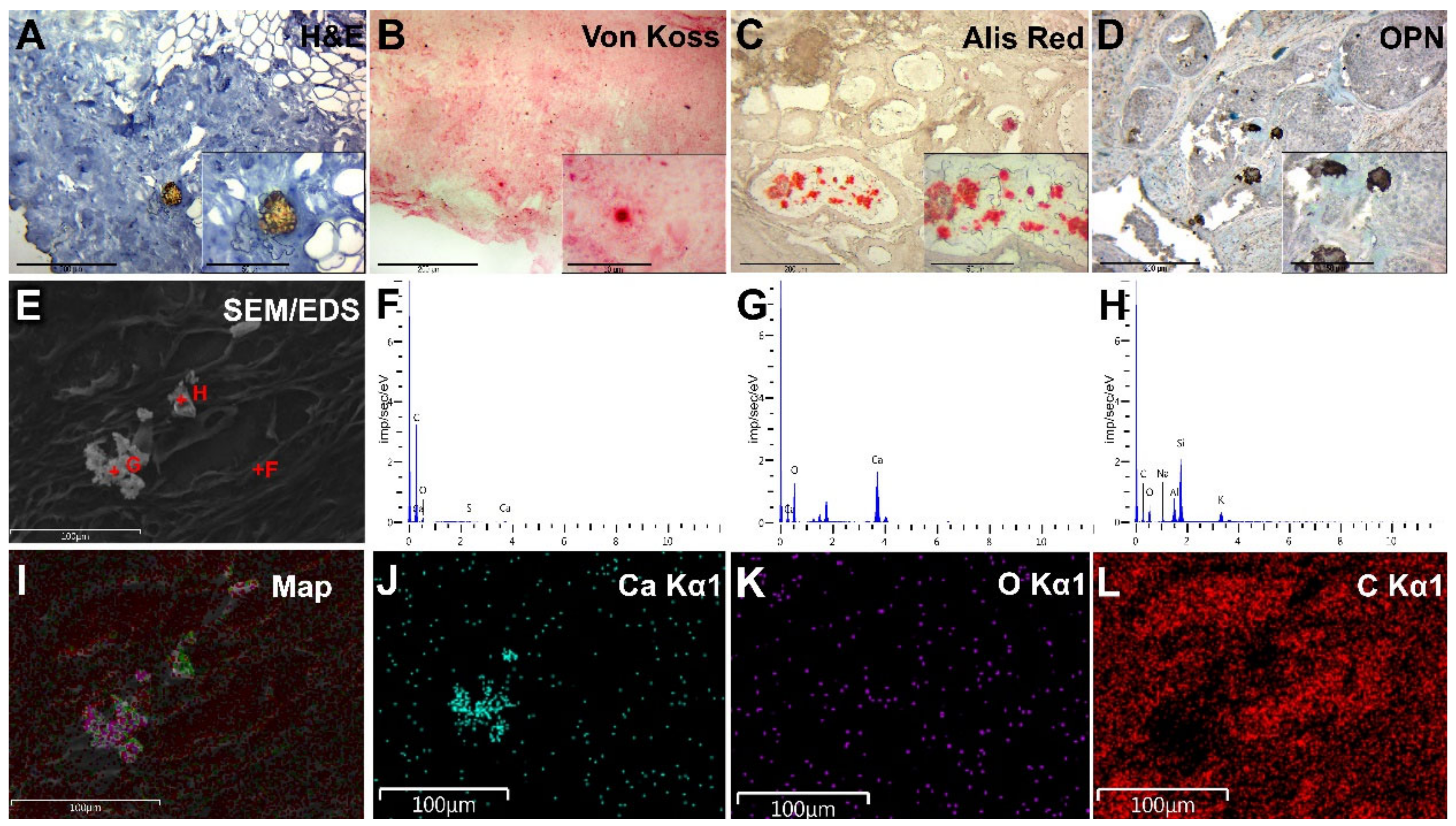
3.3. Histopathology
3.4. Immunohistochemistry
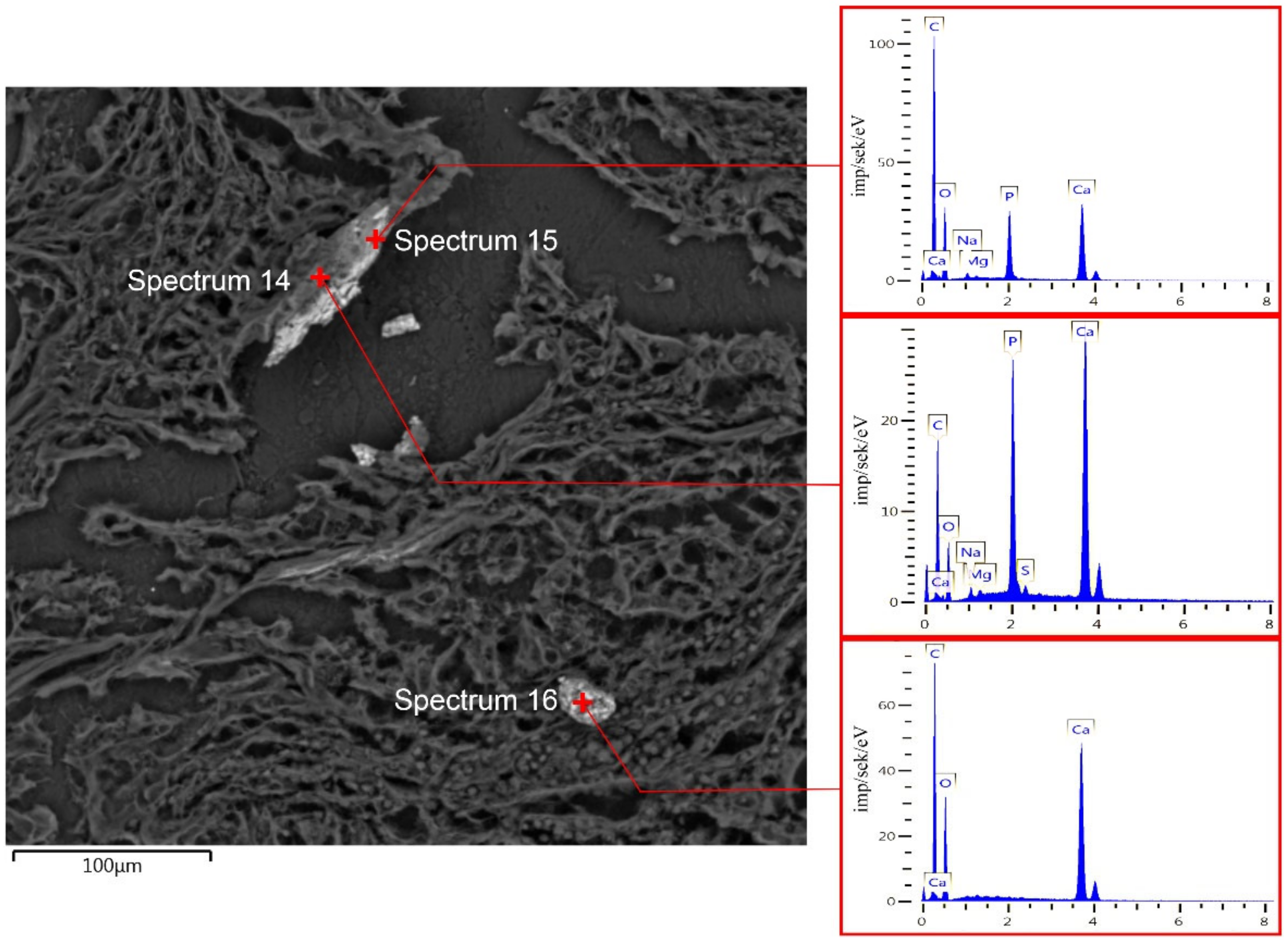
3.5. Scanning Electron Microscopy
3.6. EDX Analysis of BC Microcalcifications
3.7. Transmission Electron Microscopy with Electron Diffraction
3.8. The Problem of Oxalates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A.; Iqbal, J.; Miller, A.B. Why Have Breast Cancer Mortality Rates Declined? J. Cancer Policy 2015, 5, 8–17. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.; Altman, D.G.; Cameron, D.A.; Dewar, J.A.; Thompson, S.G.; Wilcox, M. The Benefits and Harms of Breast Cancer Screening: An Independent Review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- Spak, D.A.; Plaxco, J.S.; Santiago, L.; Dryden, M.J.; Dogan, B.E. BI-RADS® Fifth Edition: A Summary of Changes. Diagn. Interv. Imaging 2017, 98, 179–190. [Google Scholar] [CrossRef]
- Massafra, R.; Comes, M.C.; Bove, S.; Didonna, V.; Gatta, G.; Giotta, F.; Fanizzi, A.; La Forgia, D.; Latorre, A.; Pastena, M.I.; et al. Robustness Evaluation of a Deep Learning Model on Sagittal and Axial Breast DCE-MRIs to Predict Pathological Complete Response to Neoadjuvant Chemotherapy. J. Pers. Med. 2022, 12, 953. [Google Scholar] [CrossRef]
- Romanjuk, A.; Lyndin, M.; Moskalenko, R.; Gortinskaya, O.; Lyndina, Y. The Role of Heavy Metal Salts in Pathological Biomineralization of Breast Cancer Tissue. Adv. Clin. Exp. Med. 2016, 25, 907–910. [Google Scholar] [CrossRef]
- Cox, R.F.; Hernandez-Santana, A.; Ramdass, S.; McMahon, G.; Harmey, J.H.; Morgan, M.P. Microcalcifications in Breast Cancer: Novel Insights into the Molecular Mechanism and Functional Consequence of Mammary Mineralisation. Br. J. Cancer 2012, 106, 525–537. [Google Scholar] [CrossRef]
- Logullo, A.F.; Prigenzi, K.C.K.; Nimir, C.C.B.A.; Franco, A.F.V.; Campos, M.S.D.A. Breast Microcalcifications: Past, Present and Future (Review). Biomed. Rep. 2022, 16, 81. [Google Scholar] [CrossRef]
- Scott, R.; Kendall, C.; Stone, N.; Rogers, K. Elemental vs. Phase Composition of Breast Calcifications. Sci. Rep. 2017, 7, 136. [Google Scholar] [CrossRef]
- Baker, R.; Rogers, K.D.; Shepherd, N.; Stone, N. New Relationships between Breast Microcalcifications and Cancer. Br. J. Cancer 2010, 103, 1034–1039. [Google Scholar] [CrossRef]
- Gosling, S.; Scott, R.; Greenwood, C.; Bouzy, P.; Nallala, J.; Lyburn, I.D.; Stone, N.; Rogers, K. Calcification Microstructure Reflects Breast Tissue Microenvironment. J. Mammary Gland. Biol. Neoplasia 2019, 24, 333–342. [Google Scholar] [CrossRef]
- Vidavsky, N.; Kunitake, J.A.M.R.; Estroff, L.A. Multiple Pathways for Pathological Calcification in the human Body. Adv. Healthc. Mater. 2021, 10, e2001271. [Google Scholar] [CrossRef]
- Chyzhma, R.; Piddubnyi, A.; Danilchenko, S.; Kravtsova, O.; Moskalenko, R. Potential Role of Hydroxyapatite Nanocrystalline for Early Diagnostics of Ovarian Cancer. Diagnostics 2021, 11, 1741. [Google Scholar] [CrossRef]
- Miladinović, M.; Vučković, L.; Klisic, A. Comparison of Dako HercepTest and Ventana PATHWAY Anti-HER2 (4B5) Tests and Their Correlation with Silver in Situ Hybridization in Lung Adenocarcinoma. Open Med. 2021, 16, 1503–1512. [Google Scholar] [CrossRef]
- Radomychelski, I.M.; Piddubnyi, A.; Danilchenko, S.; Maksymova, O.; Moskalenko, Y.; Moskalenko, R. Morphological and Crystal-Chemical Features of Macro- and Microcalcifications of Human Aorta. Microsc. Microanal. 2021, 27, 1539–1546. [Google Scholar] [CrossRef]
- Leborgne, R. Diagnosis of Tumors of the Breast by Simple Roentgenography; Calcifications in Carcinomas. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1951, 65, 1–11. [Google Scholar]
- Venkatesan, A.; Chu, P.; Kerlikowske, K.; Sickles, E.A.; Smith-Bindman, R. Positive Predictive Value of Specific Mammographic Findings According to Reader and Patient Variables. Radiology 2009, 250, 648–657. [Google Scholar] [CrossRef]
- Hofvind, S.; Iversen, B.F.; Eriksen, L.; Styr, B.M.; Kjellevold, K.; Kurz, K.D. Mammographic Morphology and Distribution of Calcifications in Ductal Carcinoma in Situ Diagnosed in Organized Screening. Acta Radiol. 2011, 52, 481–487. [Google Scholar] [CrossRef]
- Frappart, L.; Boudeulle, M.; Boumendil, J.; Lin, H.C.; Martinon, I.; Palayer, C.; Mallet-Guy, Y.; Raudrant, D.; Bremond, A.; Rochet, Y.; et al. Structure and Composition of Microcalcifications in Benign and Malignant Lesions of the Breast: Study by Light Microscopy, Transmission and Scanning Electron Microscopy, Microprobe Analysis, and X-Ray Diffraction. Hum. Pathol. 1984, 15, 880–889. [Google Scholar] [CrossRef]
- Haka, A.S.; Shafer-Peltier, K.E.; Fitzmaurice, M.; Crowe, J.; Dasari, R.R.; Feld, M.S. Identifying Microcalcifications in Benign and Malignant Breast Lesions by Probing Differences in Their Chemical Composition Using Raman Spectroscopy. Cancer Res. 2002, 62, 5375–5380. [Google Scholar] [PubMed]
- Zhang, Y.; Wang, C.; Li, Y.; Lu, A.; Meng, F.; Ding, H.; Mei, F.; Liu, J.; Li, K.; Yang, C.; et al. Carbonate and Cation Substitutions in Hydroxylapatite in Breast Cancer Micro-Calcifications. Mineral. Mag. 2021, 85, 321–331. [Google Scholar] [CrossRef]
- Wilkinson, L.; Thomas, V.; Sharma, N. Microcalcification on Mammography: Approaches to Interpretation and Biopsy. Br. J. Radiol. 2017, 90, 20160594. [Google Scholar] [CrossRef] [PubMed]
- Castellaro, A.M.; Tonda, A.; Cejas, H.H.; Ferreyra, H.; Caputto, B.L.; Pucci, O.A.; Gil, G.A. Oxalate Induces Breast Cancer. BMC Cancer 2015, 15, 1. [Google Scholar] [CrossRef]
- Holme, T.C.; Reis, M.M.; Thompson, A.; Robertson, A.; Parham, D.; Hickman, P.; Preece, P.E. Is Mammographic Microcalcification of Biological Significance? Eur. J. Surg. Oncol. 1993, 19, 250–253. [Google Scholar]
- Chae, E.Y.; Moon, W.K.; Kim, H.H.; Kim, W.H.; Cha, J.H.; Shin, H.J.; Choi, W.J.; Han, W.; Noh, D.Y.; Lee, S.B.; et al. Association between Ultrasound Features and the 21-Gene Recurrence Score Assays in Patients with Oestrogen Receptor-Positive, HER2-Negative, Invasive Breast Cancer. PLoS ONE 2016, 11, e0158461. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J.; Zhou, Y.; Mao, F.; Shen, S.; Sun, Q. Mammographic casting-type calcification is an independent prognostic factor in invasive breast cancer. Sci. Rep. 2019, 9, 10544. [Google Scholar] [CrossRef]
- Clemenceau, A.; Michou, L.; Diorio, C.; Durocher, F. Breast Cancer and Microcalcifications: An Osteoimmunological Disorder? Int. J. Mol. Sci. 2020, 21, 8613. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, H.; Zheng, C.; Gu, M.; Zheng, X. Secretion of BMP-2 by tumor-associated macrophages (TAM) promotes microcalcifications in breast cancer. BMC Cancer 2022, 22, 34. [Google Scholar] [CrossRef]
- Steitz, S.A.; Speer, M.Y.; McKee, M.D.; Liaw, L.; Almeida, M.; Yang, H.; Giachelli, C.M. Osteopontin Inhibits Mineral Deposition and Promotes Regression of Ectopic Calcification. Am. J. Pathol. 2002, 161, 2035–2046. [Google Scholar] [CrossRef]
- Feng, M.; Grice, D.M.; Faddy, H.M.; Nguyen, N.; Leitch, S.; Wang, Y.; Muend, S.; Kenny, P.A.; Sukumar, S.; Roberts-Thomson, S.J.; et al. Store-Independent Activation of Orai1 by SPCA2 in Mammary Tumors. Cell 2010, 143, 84–98. [Google Scholar] [CrossRef]
- Allan, A.L.; George, R.; Vantyghem, S.A.; Lee, M.W.; Hodgson, N.C.; Engel, C.J.; Holliday, R.L.; Girvan, D.P.; Scott, L.A.; Postenka, C.O.; et al. Role of the Integrin-Binding Protein Osteopontin in Lymphatic Metastasis of Breast Cancer. Am. J. Pathol. 2006, 169, 233–246. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and Growth of Extracellular-Vesicle-Derived Microcalcification in Atherosclerotic Plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef]
- Scimeca, M.; Giannini, E.; Antonacci, C.; Pistolese, C.A.; Spagnoli, L.G.; Bonanno, E. Microcalcifications in Breast Cancer: An Active Phenomenon Mediated by Epithelial Cells with Mesenchymal Characteristics. BMC Cancer 2014, 14, 286. [Google Scholar] [CrossRef]




| Feature | Hydroxyapatite | Calcium Oxalate |
|---|---|---|
| Localization | Tumor masses, vessels, necrotic masses | The lumen of glands, detritus in the ducts |
| Hematoxylin-eosin staining | Dark blue | Amber |
| Polarization | − | + |
| Von Koss staining | + | Doubtful |
| Alizarin red staining | + | + |
| IHC OPN expression | + | Doubtful |
| Crystalline form | Hexagonal | Rectangular crystals, parallelepiped |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piddubnyi, A.; Kolomiiets, O.; Danilchenko, S.; Stepanenko, A.; Moskalenko, Y.; Moskalenko, R. The Prospects of Using Structural Phase Analysis of Microcalcifications in Breast Cancer Diagnostics. Diagnostics 2023, 13, 737. https://doi.org/10.3390/diagnostics13040737
Piddubnyi A, Kolomiiets O, Danilchenko S, Stepanenko A, Moskalenko Y, Moskalenko R. The Prospects of Using Structural Phase Analysis of Microcalcifications in Breast Cancer Diagnostics. Diagnostics. 2023; 13(4):737. https://doi.org/10.3390/diagnostics13040737
Chicago/Turabian StylePiddubnyi, Artem, Olena Kolomiiets, Sergey Danilchenko, Andriy Stepanenko, Yuliia Moskalenko, and Roman Moskalenko. 2023. "The Prospects of Using Structural Phase Analysis of Microcalcifications in Breast Cancer Diagnostics" Diagnostics 13, no. 4: 737. https://doi.org/10.3390/diagnostics13040737
APA StylePiddubnyi, A., Kolomiiets, O., Danilchenko, S., Stepanenko, A., Moskalenko, Y., & Moskalenko, R. (2023). The Prospects of Using Structural Phase Analysis of Microcalcifications in Breast Cancer Diagnostics. Diagnostics, 13(4), 737. https://doi.org/10.3390/diagnostics13040737