Comparability of Pulmonary Nodule Size Measurements among Different Scanners and Protocols: Should Diameter Be Favorized over Volume?
Abstract
1. Introduction
2. Materials and Methods
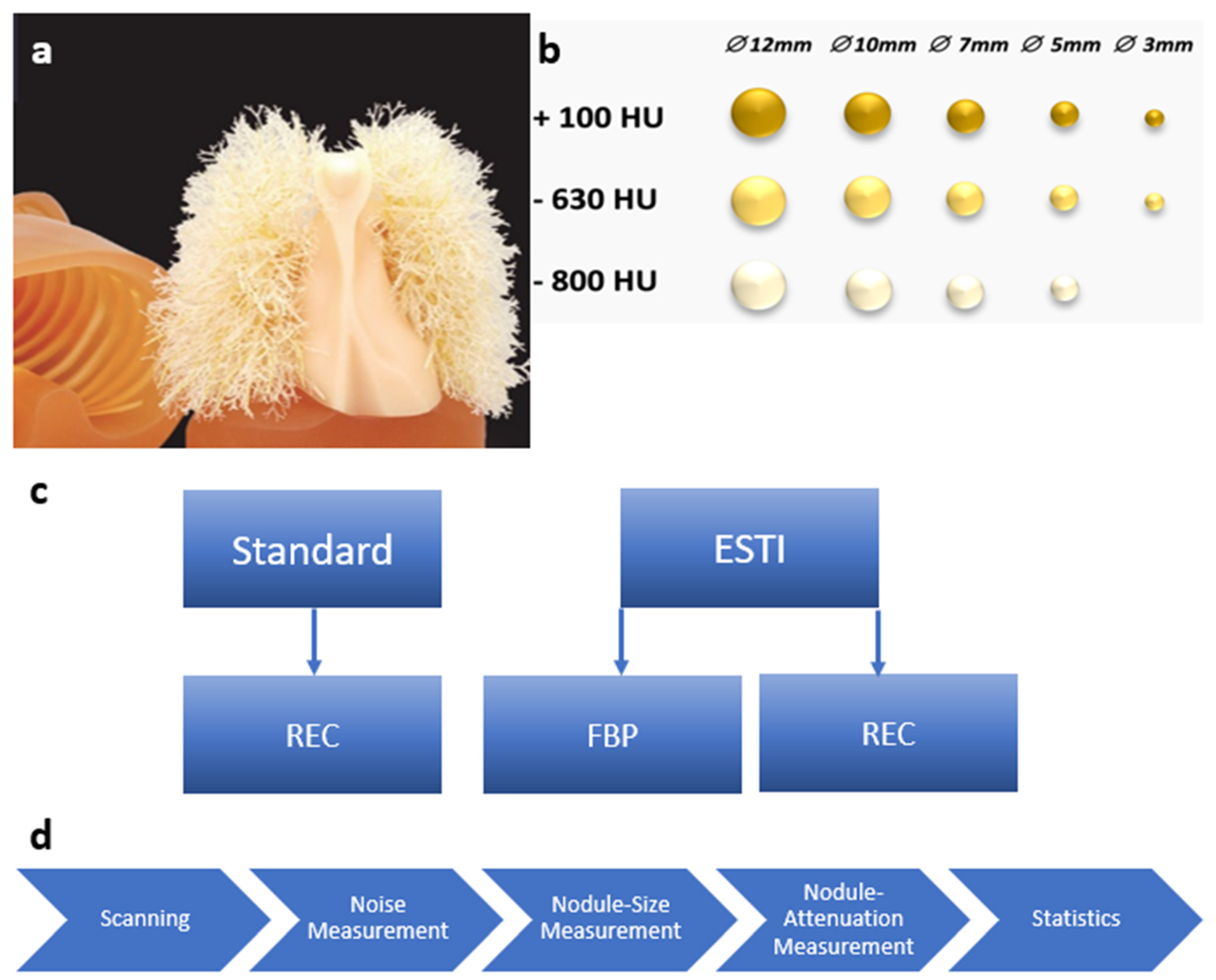
2.1. Chest Phantom
2.2. CT Scanning Protocols
2.3. Image Noise Evaluation
2.4. Pulmonary Nodule Evaluation
2.4.1. Nodule Density
2.4.2. Nodule Size
2.5. Statistical Analysis
3. Results
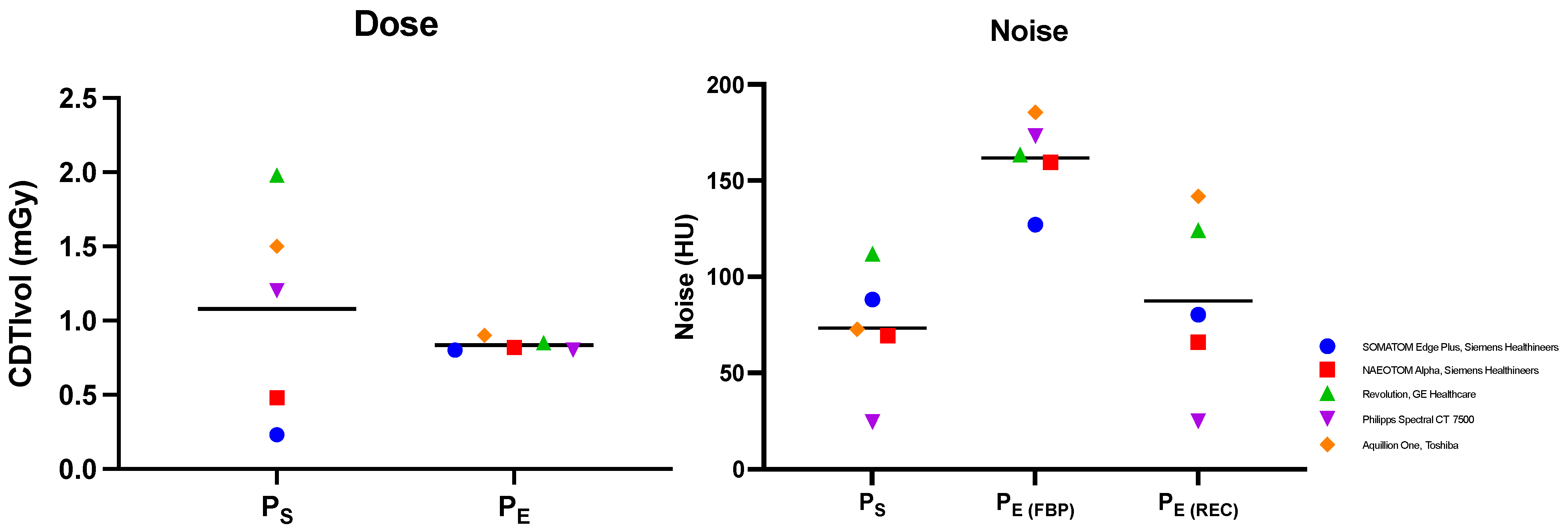
3.1. Dose and Noise Evaluation
3.2. Nodule Size Evaluation
3.2.1. Absolute Percentage Errors of Volume and Diameter Metrics
3.2.2. Differences in Determined Nodule Size between Standard and ESTI Protocols
3.3. CT Attenuation Evaluation
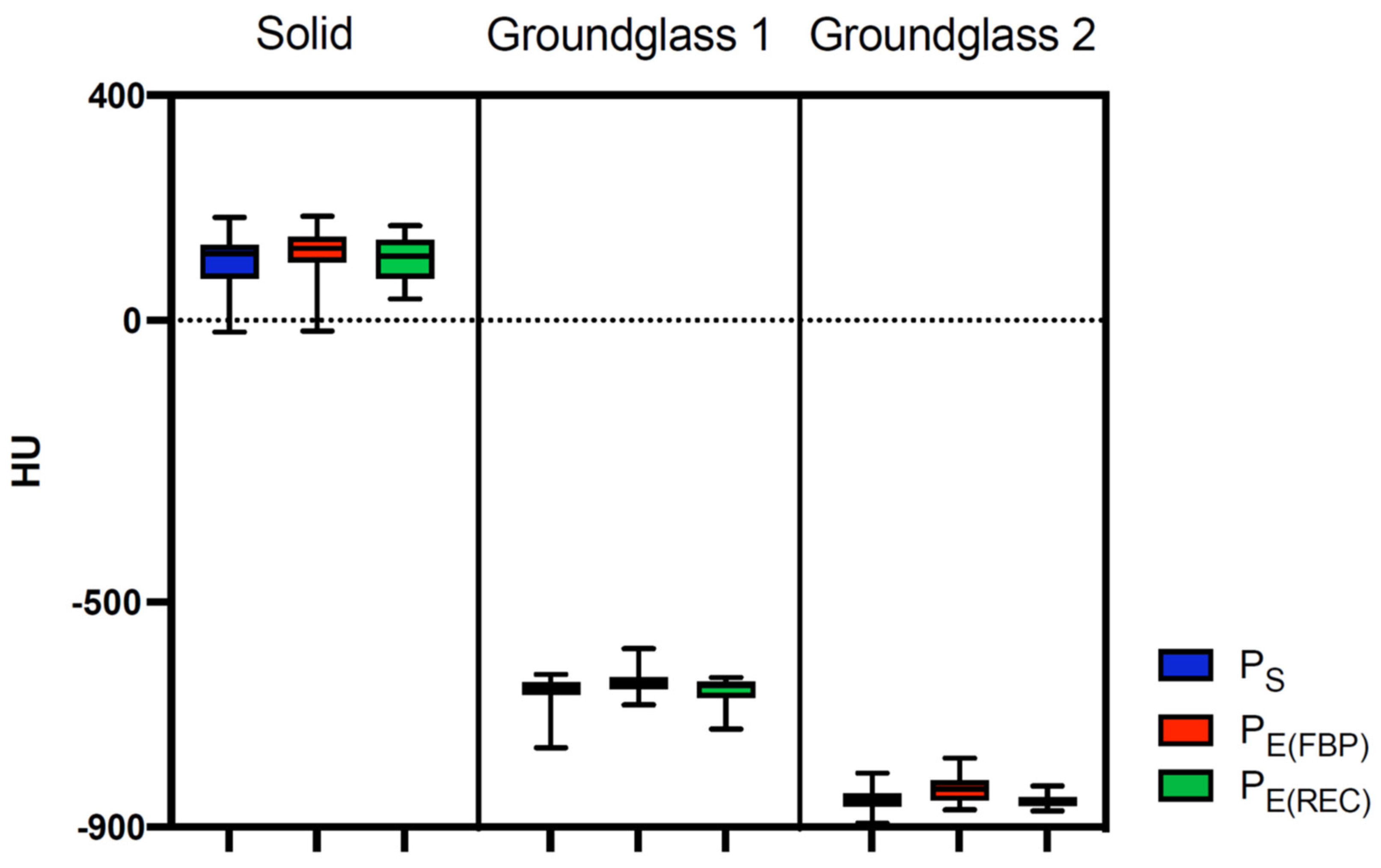
3.3.1. Comparison between Protocol Determined CT Attenuation and Factual Nodule Density
3.3.2. Comparison of CT Attenuation among Different Scan Protocols
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute. SEER*Explorer. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html (accessed on 25 April 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- National Cancer Institute. Financial Burden of Cancer Care. Available online: https://progressreport.cancer.gov/after/economic_burden (accessed on 25 April 2022).
- National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- De Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Martini, K.; Ottilinger, T.; Serrallach, B.; Markart, S.; Glaser-Gallion, N.; Blüthgen, C.; Leschka, S.; Bauer, R.W.; Wildermuth, S.; Messerli, M. Lung cancer screening with submillisievert chest CT: Potential pitfalls of pulmonary findings in different readers with various experience levels. Eur. J. Radiol. 2019, 121, 108720. [Google Scholar] [CrossRef]
- Sui, X.; Meinel, F.G.; Song, W.; Xu, X.; Wang, Z.; Wang, Y.; Jin, Z.; Chen, J.; Vliegenthart, R.; Schoepf, U.J. Detection and size measurements of pulmonary nodules in ultra-low-dose CT with iterative reconstruction compared to low dose CT. Eur. J. Radiol. 2015, 85, 564–570. [Google Scholar] [CrossRef]
- Jungblut, L.; Blüthgen, C.; Polacin, M.; Messerli, M.; Schmidt, B.; Euler, A.; Alkadhi, H.; Frauenfelder, T.; Martini, K. First Performance Evaluation of an Artificial Intelligence-Based Computer-Aided Detection System for Pulmonary Nodule Evaluation in Dual-Source Photon-Counting Detector CT at Different Low-Dose Levels. Investig. Radiol. 2021, 57, 108–114. [Google Scholar] [CrossRef]
- Han, D.; Heuvelmans, M.A.; Oudkerk, M. Volume versus diameter assessment of small pulmonary nodules in CT lung cancer screening. Transl. Lung Cancer Res. 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Eberhard, M.; Stocker, D.; Milanese, G.; Martini, K.; Nguyen-Kim, T.D.L.; Wurnig, M.C.; Frauenfelder, T.; Baumueller, S. Volumetric assessment of solid pulmonary nodules on ultralow-dose CT: A phantom study. J. Thorac. Dis. 2019, 11, 3515–3524. [Google Scholar] [CrossRef]
- Solomon, J.; Ebner, L.; Christe, A.; Peters, A.; Munz, J.; Löbelenz, L.; Klaus, J.; Richards, T.; Samei, E.; Roos, J.E. Minimum perceivable size difference: How well can radiologists visually detect a change in lung nodule size from CT images? Eur. Radiol. 2021, 31, 1947–1955. [Google Scholar]
- Milanese, G.; Eberhard, M.; Martini, K.; De Martini, I.V.; Frauenfelder, T. Vessel suppressed chest Computed Tomography for semi-automated volumetric measurements of solid pulmonary nodules. Eur. J. Radiol. 2018, 101, 97–102. [Google Scholar] [CrossRef]
- De Hoop, B.; Gietema, H.; Van Ginneken, B.; Zanen, P.; Groenewegen, G.; Prokop, M. A comparison of six software packages for evaluation of solid lung nodules using semi-automated volumetry: What is the minimum increase in size to detect growth in repeated CT examinations. Eur. Radiol. 2008, 19, 800–808. [Google Scholar] [CrossRef]
- Schwyzer, M.; Messerli, M.; Eberhard, M.; Skawran, S.; Martini, K.; Frauenfelder, T. Impact of dose reduction and iterative reconstruction algorithm on the detectability of pulmonary nodules by artificial intelligence. Diagn. Interv. Imaging 2022, 103, 273–280. [Google Scholar] [CrossRef]
- Martini, K.; Blüthgen, C.; Eberhard, M.; Schönenberger, A.; De Martini, I.; Huber, F.; Barth, B.; Euler, A.; Frauenfelder, T. Impact of Vessel Suppressed-CT on Diagnostic Accuracy in Detection of Pulmonary Metastasis and Reading Time. Acad. Radiol. 2020, 28, 988–994. [Google Scholar] [CrossRef]
- Mackin, D.; Fave, X.; Zhang, L.; Fried, D.; Yang, J.; Taylor, B.; Rodriguez-Rivera, E.; Dodge, C.; Jones, A.K.; Court, L. Measuring Computed Tomography Scanner Variability of Radiomics Features. Investig. Radiol. 2015, 50, 757–765. [Google Scholar] [CrossRef]
- European Society of Thoracic Imaging. ESTI Lung Cancer Screening Certification Project. Available online: https://www.myesti.org/lungcancerscreeningcertificationproject/ (accessed on 31 October 2022).
- Gordic, S.; Morsbach, F.; Schmidt, B.; Allmendinger, T.; Flohr, T.; Husarik, D. Ultralow-Dose Chest Computed Tomography for Pulmonary Nodule Detection. Investig. Radiol. 2014, 49, 465–473. [Google Scholar] [CrossRef]
- Obuchowski, N.A.; Reeves, A.P.; Huang, E.P.; Wang, X.-F.; Buckler, A.; Kim, H.J.; Barnhart, H.X.; Jackson, E.; Giger, M.; Pennello, G.; et al. Quantitative imaging biomarkers: A review of statistical methods for computer algorithm comparisons. Stat. Methods Med. Res. 2014, 24, 68–106. [Google Scholar] [CrossRef]
- Goldman, L.W. Principles of CT: Radiation Dose and Image Quality. J. Nucl. Med. Technol. 2007, 35, 213–225. [Google Scholar]
- Fareed, A.; Vavere, A.L.; Zimmermann, E.; Tanami, Y.; Steveson, C.; Matheson, M.; Paul, N.; Clouse, M.; Cox, C.; Lima, J.A.C.; et al. Impact of iterative reconstruction vs. filtered back projection on image quality in 320-slice CT coronary angiography: Insights from the CORE320 multicenter study. Medicine 2017, 96, e8452. [Google Scholar]
- Koyama, H.; Ohno, Y.; Nishio, M.; Matsumoto, S.; Sugihara, N.; Yoshikawa, T.; Seki, S.; Sugimura, K. Iterative reconstruction technique vs filter back projection: Utility for quantitative bronchial assessment on low-dose thin-section MDCT in patients with/without chronic obstructive pulmonary disease. Eur. Radiol. 2014, 24, 1860–1867. [Google Scholar] [CrossRef]
- Greffier, J.; Frandon, J.; Larbi, A.; Om, D.; Beregi, J.; Pereira, F. Noise assessment across two generations of iterative reconstruction algorithms of three manufacturers using bone reconstruction kernel. Diagn. Interv. Imaging 2019, 100, 763–770. [Google Scholar] [CrossRef]
- Lederlin, M.; Revel, M.-P.; Khalil, A.; Ferretti, G.; Milleron, B.; Laurent, F. Management strategy of pulmonary nodule in 2013. Diagn. Interv. Imaging 2013, 94, 1081–1094. [Google Scholar] [CrossRef]
- Scholten, E.T.; De Hoop, B.; Jacobs, C.; Vorst, S.V.A.-V.D.; Van Klaveren, R.J.; Oudkerk, M.; Vliegenthart, R.; De Koning, H.J.; Van Der Aalst, C.M.; Mali, W.T.M.; et al. Semi-Automatic Quantification of Subsolid Pulmonary Nodules: Comparison with Manual Measurements. PLoS ONE 2013, 8, e80249. [Google Scholar] [CrossRef]
- Devaraj, A.; Van Ginneken, B.; Nair, A.; Baldwin, D. Use of Volumetry for Lung Nodule Management: Theory and Practice. Radiology 2017, 284, 630–644. [Google Scholar] [CrossRef]
- Gietema, H.A.; Wang, Y.; Xu, D.; van Klaveren, R.J.; de Koning, H.; Scholten, E.; Verschakelen, J.; Kohl, G.; Oudkerk, M.; Prokop, M. Pulmonary Nodules Detected at Lung Cancer Screening: Interobserver Variability of Semiautomated Volume Measurements. Radiology 2006, 241, 251–257. [Google Scholar] [CrossRef]
- Kim, H.; Park, C.M.; Chae, H.-D.; Lee, S.M.; Goo, J.M. Impact of radiation dose and iterative reconstruction on pulmonary nodule measurements at chest CT: A phantom study. Diagn. Interv. Radiol. 2015, 21, 459–465. [Google Scholar] [CrossRef]
- Chu, Z.-G.; Li, W.-J.; Fu, B.-J.; Lv, F.-J. CT Characteristics for Predicting Invasiveness in Pulmonary Pure Ground-Glass Nodules. Am. J. Roentgenol. 2020, 215, 351–358. [Google Scholar] [CrossRef]
- Bak, S.H.; Lee, H.Y.; Kim, J.-H.; Um, S.-W.; Kwon, O.J.; Han, J.; Kim, H.K.; Kim, J.; Lee, K.S. Quantitative CT Scanning Analysis of Pure Ground-Glass Opacity Nodules Predicts Further CT Scanning Change. Chest 2016, 149, 180–191. [Google Scholar] [CrossRef]
- Eguchi, T.; Kondo, R.; Kawakami, S.; Matsushita, M.; Yoshizawa, A.; Hara, D.; Matsuoka, S.; Takeda, T.; Miura, K.; Agatsuma, H.; et al. Computed tomography attenuation predicts the growth of pure ground-glass nodules. Lung Cancer 2014, 84, 242–247. [Google Scholar] [CrossRef]
- Bogot, N.R.; Kazerooni, E.A.; Kelly, A.M.; Quint, L.E.; Desjardins, B.; Nan, B. Interobserver and Intraobserver Variability in the Assessment of Pulmonary Nodule Size on CT Using Film and Computer Display Methods1. Acad. Radiol. 2005, 12, 948–956. [Google Scholar] [CrossRef]
- Held, L.; Rufibach, K.; Seifert, B. Medizinische Statistik: Konzepte, Methoden, Anwendungen; Pearson Deutschland GmbH: Hallbergmoos, Germany, 2013. [Google Scholar]
- American College of Radiology—Lung Cancer Screening. Available online: https://www.acr.org/Clinical-Resources/Lung-Cancer-Screening-Resources/FAQ (accessed on 18 January 2023).
- Wan, Y.-L.; Wu, P.; Huang, P.-C.; Tsay, P.-K.; Pan, K.-T.; Trang, N.; Chuang, W.-Y.; Wu, C.-Y.; Lo, S. The Use of Artificial Intelligence in the Differentiation of Malignant and Benign Lung Nodules on Computed Tomograms Proven by Surgical Pathology. Cancers 2020, 12, 2211. [Google Scholar] [CrossRef]




| CT Scanner | SOMATOM Edge Plus, Siemens Healthineers | NAEOTOM Alpha, Siemens Healthineers | Revolution, GE Healthcare | Aquillion One, Toshiba | Spectral CT 7500, Philips | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scan Protocol | Standard | ESTI | Standard | ESTI | Standard | ESTI | Standard | ESTI | Standard | ESTI | ||||||
| Dose | CTDI (mGy) | 0.23 | 0.8 | 0.48 | 0.82 | 1.98 | 0.85 | 1.5 | 0.9 | 1.2 | 0.8 | |||||
| DLP (mGy*cm) | 8.25 | 29 | 16.3 | 28.2 | 78.24 | 32.61 | 54.8 | 32.3 | 52.1 | 36.7 | ||||||
| Tube voltage (kV) | 100 sn | 100 sn | 120 | 100 sn | 120 | 100 | A 80 | 100 | 120 | 100 | ||||||
| Tube current (mAs) | 100 | 310 | IQ level 5 | IQ level 20 | SmartmA 10–400 | 40 | R 165 | 35 | 15 (10–20) | 17 (11–24) | ||||||
| Pitch | 1.2 | 1.2 | 0.85 | 1 | 0.992 | 0.992 | 0.5 × 80 | 0.5 × 80 | 1.15 | 1.15 | ||||||
| Rotation time (s) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.35 | 0.35 | 0.33 | 0.33 | ||||||
| Slice Thickness (mm) | 1.5 | 1 | 1.5 | 1 | 0.625 | 0.625 | 1 | 1 | 1 | 1 | ||||||
| Increment (mm) | 1 | 0.7 | 1 | 0.7 | 0.625 | 0.7 | 0.8 | 0.7 | 0.5 | 0.7 | ||||||
| Reconstruction algorithm | ADMIRE 3 | ADMIRE 3 | FBP | QIR 3 | QIR 3 | FBP | ASiR-V 50% | ASiR-V 50% | FBP | AIDR 3D standard | AIDR 3D standard | FBP | IMR level 1 | IMR level 1 | FBP | |
| Metric | Protocol | Mean 1/Mean 2 | 95% CI of Difference | p-Value |
|---|---|---|---|---|
| Volume | PS vs. PE(FBP) | 9.098/10.87 | −6.549 to 3.000 | 0.6543 |
| PS vs. PE(REC) | 9.098/7.990 | −3.666 to 5.882 | 0.8472 | |
| PE(FBP) vs. PE(REC) | 10.87/7.990 | −1.892 to 7.657 | 0.3290 | |
| Diameter | PS vs. PE(FBP) | 17.71/21.29 | −7.861 to 0.7025 | 0.1213 |
| PS vs. PE(REC) | 17.71/17.90 | −4.432 to 4.066 | 0.9943 | |
| PE(FBP) vs. PE(REC) | 21.29/17.90 | −0.8522 to 7.645 | 0.1448 |
| Nodule Density | Protocol | Mean [HU] | Mean Difference [HU] | p-Value |
|---|---|---|---|---|
| Solid (100 HU) | Ps | 106.7 ± 49 | −6.68 | 0.8357 |
| PE(FBP) | 120.5 ± 42.1 | −20.51 | 0.0578 | |
| PE(REC) | 110.4 ± 39.3 | −10.4 | 0.4234 | |
| Ground-glass 1 (−630 HU) | Ps | −661.6 ± 30 | 31.6 | <0.0001 |
| PE(FBP) | −642.4 ± 22.3 | 12.36 | 0.0282 | |
| PE(REC) | −655.3 ± 22.1 | 25.32 | <0.0001 | |
| Ground-glass 2 (−800 HU) | Ps | −849.8 ± 20.1 | 49.8 | <0.0001 |
| PE(FBP) | −833.4 ± 22.4 | 33.35 | <0.0001 | |
| PE(REC) | −852.4 ± 11.2 | 52.4 | <0.0001 |
| Nodule Density | Protocol | Mean [HU] | Mean Difference [HU] | p-Value | |
|---|---|---|---|---|---|
| Solid (100 HU) | Ps vs. PE(FBP) | 106.7 | 120.5 | −13.83 | 0.2355 |
| Ps vs. PE(REC) | 106.7 | 110.4 | −3.724 | 0.9738 | |
| PE(FBP) vs. PE(REC) | 120.5 | 110.4 | 10.1 | 0.5162 | |
| Ground-glass 1 (−630 HU) | Ps vs. PE(FBP) | −661.6 | −642.4 | −19.24 | 0.0668 |
| Ps vs. PE(REC) | −661.6 | −655.3 | −6.28 | 0.5699 | |
| PE(FBP) vs. PE(REC) | −642.4 | −655.3 | 12.96 | 0.0194 | |
| Ground-glass 2 (−800 HU) | Ps vs. PE(FBP) | −849.8 | −833.4 | −16.45 | 0.0007 |
| Ps vs. PE(REC) | −849.8 | −852.4 | 2.6 | 0.8715 | |
| PE(FBP) vs. PE(REC) | −833.4 | −852.4 | 19.05 | 0.0003 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gross, C.F.; Jungblut, L.; Schindera, S.; Messerli, M.; Fretz, V.; Frauenfelder, T.; Martini, K. Comparability of Pulmonary Nodule Size Measurements among Different Scanners and Protocols: Should Diameter Be Favorized over Volume? Diagnostics 2023, 13, 631. https://doi.org/10.3390/diagnostics13040631
Gross CF, Jungblut L, Schindera S, Messerli M, Fretz V, Frauenfelder T, Martini K. Comparability of Pulmonary Nodule Size Measurements among Different Scanners and Protocols: Should Diameter Be Favorized over Volume? Diagnostics. 2023; 13(4):631. https://doi.org/10.3390/diagnostics13040631
Chicago/Turabian StyleGross, Colin F., Lisa Jungblut, Sebastian Schindera, Michael Messerli, Valentin Fretz, Thomas Frauenfelder, and Katharina Martini. 2023. "Comparability of Pulmonary Nodule Size Measurements among Different Scanners and Protocols: Should Diameter Be Favorized over Volume?" Diagnostics 13, no. 4: 631. https://doi.org/10.3390/diagnostics13040631
APA StyleGross, C. F., Jungblut, L., Schindera, S., Messerli, M., Fretz, V., Frauenfelder, T., & Martini, K. (2023). Comparability of Pulmonary Nodule Size Measurements among Different Scanners and Protocols: Should Diameter Be Favorized over Volume? Diagnostics, 13(4), 631. https://doi.org/10.3390/diagnostics13040631







