Cardiovascular Magnetic Resonance Imaging Findings in Africans with Idiopathic Dilated Cardiomyopathy
Abstract
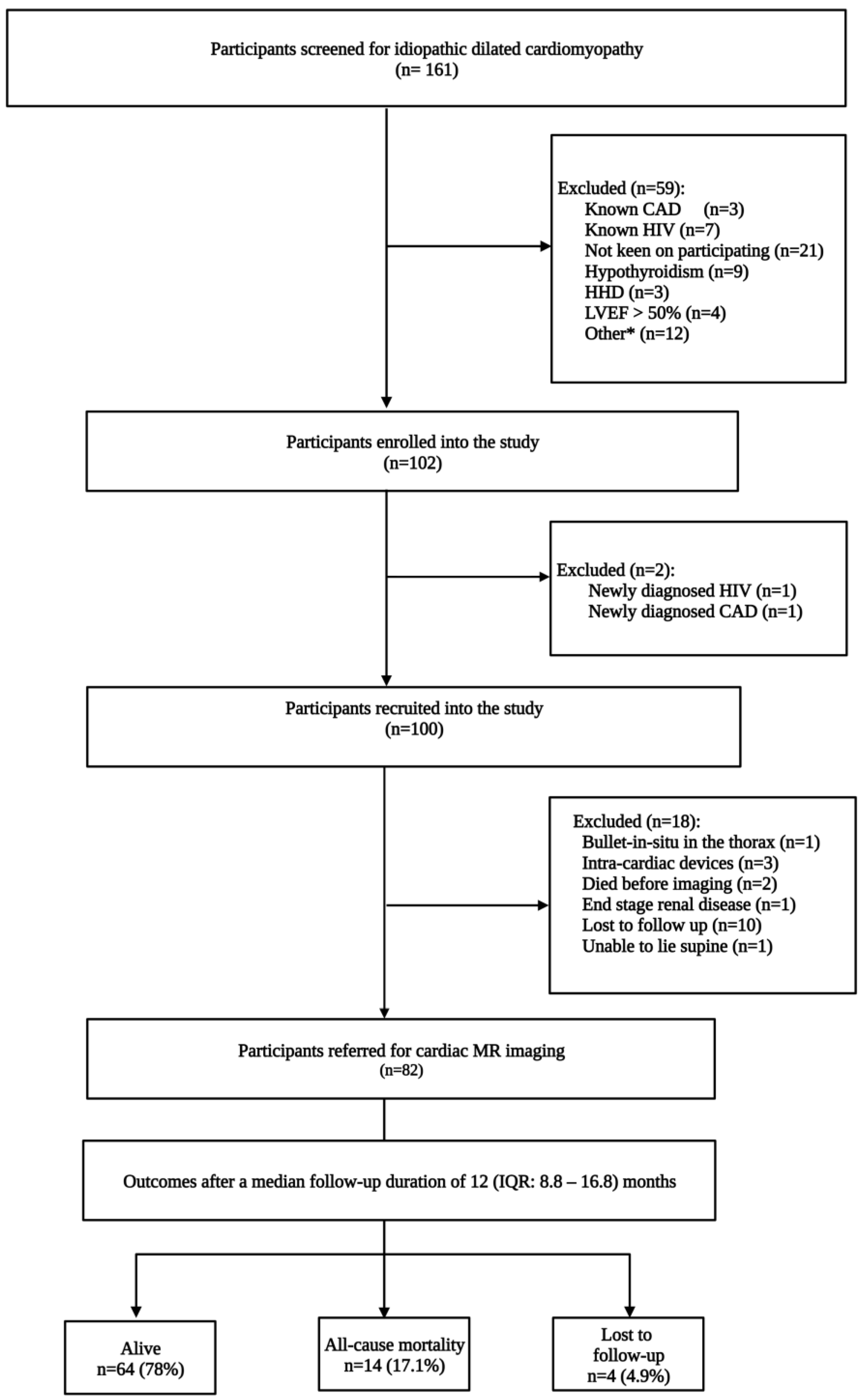
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Cardiac Magnetic Resonance Imaging Protocol and Image Analysis
2.3. Patient Follow-Up and Study Endpoints
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. Cardiovascular Magnetic Resonance Imaging
3.3. Electrocardiogram and Echocardiogram Parameters
3.4. Follow-Up and Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agbor, V.N.; Essouma, M.; Ntusi, N.A.B.; Nyaga, U.F.; Bigna, J.J.; Noubiap, J.J. Heart failure in sub-Saharan Africa: A contemporaneous systematic review and meta-analysis. Int. J. Cardiol. 2018, 257, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Agbor, V.N.; Ntusi, N.A.B.; Noubiap, J.J. An overview of heart failure in low- and middle-income countries. Cardiovasc. Diagn. Ther. 2020, 10, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Hazebroek, M.; Dennert, R.; Heymans, S. Idiopathic dilated cardiomyopathy: Possible triggers and treatment strategies. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2012, 20, 332–335. [Google Scholar] [CrossRef]
- Arnold, J.R.; McCann, G.P. Cardiovascular magnetic resonance: Applications and practical considerations for the general cardiologist. Heart 2020, 106, 174–181. [Google Scholar] [CrossRef]
- Ganesan, A.N.; Gunton, J.; Nucifora, G.; McGavigan, A.D.; Selvanayagam, J.B. Impact of Late Gadolinium Enhancement on mortality, sudden death and major adverse cardiovascular events in ischemic and nonischemic cardiomyopathy: A systematic review and meta-analysis. Int. J Cardiol. 2018, 254, 230–237. [Google Scholar] [CrossRef]
- Kuruvilla, S.; Adenaw, N.; Katwal, A.B.; Lipinski, M.J.; Kramer, C.M.; Salerno, M. Late Gadolinium Enhancement on Cardiac Magnetic Resonance Predicts Adverse Cardiovascular Outcomes in Nonischemic Cardiomyopathy. Circ. Cardiovasc. Imaging 2014, 7, 250–258. [Google Scholar] [CrossRef]
- Tigen, K.; Karaahmet, T.; Kirma, C.; Dundar, C.; Pala, S.; Isiklar, I.; Cevik, C.; Kilicgedik, A.; Basaran, Y. Diffuse late gadolinium enhancement by cardiovascular magnetic resonance predicts significant intraventricular systolic dyssynchrony in patients with non-ischemic dilated cardiomyopathy. J. Am. Soc. Echocardiogr. 2010, 23, 416–422. [Google Scholar] [CrossRef]
- McCrohon, J.A.; Moon, J.C.; Prasad, S.K.; McKenna, W.J.; Lorenz, C.H.; Coats, A.J.; Pennell, D.J. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 2003, 108, 54–59. [Google Scholar] [CrossRef]
- Grigoratos, C.; Pantano, A.; Meschisi, M.; Gaeta, R.; Ait-Ali, L.; Barison, A.; Todiere, G.; Festa, P.; Sinagra, G.; Aquaro, G.D. Clinical importance of late gadolinium enhancement at right ventricular insertion points in otherwise normal hearts. Int. J. Cardiovasc. Imaging 2020, 36, 913–920. [Google Scholar] [CrossRef]
- Yi, J.E.; Park, J.; Lee, H.J.; Shin, D.G.; Kim, Y.; Kim, M.; Kwon, K.; Pyun, W.B.; Kim, Y.J.; Joung, B. Prognostic implications of late gadolinium enhancement at the right ventricular insertion point in patients with non-ischemic dilated cardiomyopathy: A multicenter retrospective cohort study. PLoS ONE 2018, 13, e0208100. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Zhang, Q.; Zeng, Z.; Luo, Y.; Jiang, J.; Chen, Y. Prognostic value of late gadolinium enhancement in dilated cardiomyopathy patients: A meta-analysis. Clin. Radiol. 2015, 70, 999–1008. [Google Scholar] [CrossRef]
- Looi, J.L.; Edwards, C.; Armstrong, G.P.; Scott, A.; Patel, H.; Hart, H.; Christiansen, J.P. Characteristics and prognostic importance of myocardial fibrosis in patients with dilated cardiomyopathy assessed by contrast-enhanced cardiac magnetic resonance imaging. Clin. Med. Insights Cardiol. 2010, 4, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Masci, P.G.; Schuurman, R.; Andrea, B.; Ripoli, A.; Coceani, M.; Chiappino, S.; Todiere, G.; Srebot, V.; Passino, C.; Aquaro, G.D.; et al. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: A contrast-enhanced cardiovascular magnetic study. Circ. Cardiovasc. Imaging 2013, 6, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cho, Y.K.; Jun, D.H.; Nam, C.W.; Han, S.W.; Hur, S.H.; Kim, Y.N.; Kim, K.B. Prognostic implications of the NT-ProBNP level and left atrial size in non-ischemic dilated cardiomyopathy. Circ. J. 2008, 72, 1658–1665. [Google Scholar] [CrossRef]
- Geng, Z.; Huang, L.; Song, M.; Song, Y. N-terminal pro-brain natriuretic peptide and cardiovascular or all-cause mortality in the general population: A meta-analysis. Sci. Rep. 2017, 7, 41504. [Google Scholar] [CrossRef]
- Hombach, V.; Merkle, N.; Torzewski, J.; Kraus, J.M.; Kunze, M.; Zimmermann, O.; Kestler, H.A.; Wohrle, J. Electrocardiographic and cardiac magnetic resonance imaging parameters as predictors of a worse outcome in patients with idiopathic dilated cardiomyopathy. Eur. Heart J. 2009, 30, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Erley, J.; Genovese, D.; Tapaskar, N.; Alvi, N.; Rashedi, N.; Besser, S.A.; Kawaji, K.; Goyal, N.; Kelle, S.; Lang, R.M.; et al. Echocardiography and cardiovascular magnetic resonance based evaluation of myocardial strain and relationship with late gadolinium enhancement. J. Cardiovasc. Magn. Reson. 2019, 21, 46. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.Y.; Gebker, R.; Kelle, S.; Hinojar, R.; Doltra, A.; Varma, N.; Child, N.; et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovasc. Imaging 2016, 9, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Dass, S.; Suttie, J.J.; Piechnik, S.K.; Ferreira, V.M.; Holloway, C.J.; Banerjee, R.; Mahmod, M.; Cochlin, L.; Karamitsos, T.D.; Robson, M.D.; et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, D.; Sirajuddin, A.; He, J.; Xu, J.; Zhuang, B.; Huang, J.; Yin, G.; Fan, X.; Wu, W.; et al. T1 Mapping and Extracellular Volume Fraction in Dilated Cardiomyopathy: A Prognosis Study. JACC Cardiovasc. Imaging 2022, 15, 578–590. [Google Scholar] [CrossRef] [PubMed]


| All Patients | All-Cause Mortality | Alive | ||
|---|---|---|---|---|
| (n = 78) | (n = 14) | (n = 64) | p-Value | |
| Age, years | 47.3 ± 13.3 | 41.6 ± 14.0 | 48.6 ± 13.0 | 0.074 |
| Male | 53 (67.9) | 10 (71.4) | 43 (67.2) | 0.758 |
| Smoking | 15 (19.2) | 6 (42.9) | 9 (14.1) | 0.013 |
| BMI, kg/m2 | 26.1 (23.3–30.5) | 24.1 (21.4–25.3) | 27.7 (23.6–31.3) | 0.045 |
| BSA, m2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.328 |
| Heart rate, bpm | 80 (69–95) | 89 (69–104) | 80 (69–95) | 0.475 |
| Systolic BP, mmHg | 119 (101–129) | 115 (99–122) | 119 (106–133) | 0.263 |
| Diastolic BP, mmHg | 76 (67–88) | 73 (60–91) | 78 (67–88) | 0.442 |
| MAP, mmHg | 89 (78–101) | 81 (73–93) | 92 (80–101) | 0.124 |
| NYHA class | 0.173 | |||
| 1 | 29 (37.2) | 2 (14.3) | 27 (42.2) | |
| 2 | 34 (43.6) | 7 (50.0) | 27 (42.2) | |
| 3 | 12 (15.4) | 4 (28.6) | 8 (12.5) | |
| 4 | 3 (3.8) | 1 (7.1) | 2 (3.1) | |
| Medication | ||||
| Beta-blocker | 73 (93.6) | 13 (92.9) | 60 (93.7) | 0.837 |
| ACE inhibitors | 51 (65.4) | 11 (78.6) | 40 (62.5) | 0.706 |
| ARB | 9 (11.5) | 0 (0) | 9 (14.1) | 0.202 |
| MRA | 55 (70.5) | 10 (71.4) | 45 (70.3) | 0.964 |
| Loop diuretics | 71 (91.0) | 13 (92.9.1) | 58 (90.6) | 0.795 |
| Statin | 22 (28.2) | 3 (21.4) | 19 (29.7) | 0.842 |
| Sodium | 140 (138–143) | 140 (137–141) | 140 (139–143) | 0.234 |
| Potassium | 4.4 ± 0.6 | 4.6 ± 0.8 | 4.4 ± 0.5 | 0.167 |
| eGFR, mL/min | 70.4 (49.0–92.9) | 62.6 (47.0–92.9) | 75.1 (49.0–94.6) | 0.610 |
| Pro BNP | 1842 (526–3860) | 5573 (2471–8188) | 1106 (421–2826) | 0.001 |
| Troponin I | 14 (8–29) | 17 (13–38) | 13 (8–27) | 0.234 |
| CK-MB | 1.9 (1.4–3.0) | 1.6 (1.3–2.6) | 2.1 (1.5–3.2) | 0.181 |
| HbA1C | 6.3 (6.0–6.8) | 6.4 (6.0–6.8) | 6.2 (6.0–6.7) | 0.768 |
| Total cholesterol | 4.1 ± 1.1 | 3.7 ± 0.7 | 4.2 ± 1.2 | 0.137 |
| LDL | 2.6 ± 0.9 | 2.1 ± 0.6 | 2.7 ± 0.9 | 0.040 |
| HDL | 1.0 (0.8–1.4) | 1.0 (0.8–1.3) | 1.0 (0.8–1.4) | 1.000 |
| C-reactive protein | 9 (9–14) | 14.5 (9.5–31) | 9 (9–12) | 0.005 |
| All Patients | All-Cause Mortality | Alive | ||
|---|---|---|---|---|
| (n = 78) | (n = 14) | (n = 64) | p-Value | |
| LVEF (%) | 24 (18–34) | 18 (13–24) | 24 (18–36) | 0.042 |
| LVEDV (mL) | 222 (176–297) | 282 (171–313) | 220 (176–297) | 0.509 |
| LVESV (mL) | 175 (121–243) | 230 (156–305) | 168 (115–238) | 0.091 |
| LVEDVI (mL/min) | 119 (95–153) | 138 (96–181) | 117 (95–147) | 0.482 |
| LVESVI (mL/min) | 93 (63–130) | 123 (89–160) | 88 (63–115) | 0.101 |
| LV stroke volume (mL) | 56.3 ± 17.5 | 50.0 ± 15.3 | 57.8 ± 17.7 | 0.132 |
| LV stroke volume index (mL/m2) | 28.5 (22.8–35.7) | 27.3 (24.1–36.6) | 28.9 (22.9–35.5) | 0.561 |
| LVED wall mass (g) | 131 (106–161) | 156 (134–170) | 123 (96–159) | 0.036 |
| LVED wall mass index (g/m2) | 74.2 (53.6–88.9) | 89.4 (74.5–100.6) | 73.6 (51.9–84.7) | 0.025 |
| LV total mass (g) | 194.3 ± 58.7 | 219.2 ± 70.0 | 188.8 ± 55.3 | 0.080 |
| LV mass index (g/m2) | 99 (85–114) | 111 (90–147) | 98 (82–113) | 0.135 |
| RVEDV (mL) | 143 (103–201) | 232 (161–316) | 132 (96–178) | <0.001 |
| RVESV (mL) | 90 (58–147) | 154 (127–273) | 73 (55–130) | <0.001 |
| RVEF (%) | 34.2 ± 16.3 | 23.1 ± 12.0 | 36.7 ± 16.2 | 0.004 |
| RVEDVI (mL/m2) | 74 (58–109) | 114 (101–147) | 70 (56–94) | <0.001 |
| RVESVI (mL/m2) | 46 (32–82) | 86 (74–105) | 41 (30–71) | <0.001 |
| RV stroke volume (mL) | 48 (29–68) | 50 (27–74) | 48 (30–68) | 0.889 |
| RV ED wall mass (g) | 139 ± 47.8 | 163.6 ± 8.7 | 135.2 ± 50.5 | 0.455 |
| Cardiac output (L/min) | 4.1 (3.2–5.3) | 4.4 (3.2–5.5) | 4.0 (3.1–5.3) | 0.929 |
| Cardiac index (L/min/m2) | 2.5 (2.1–3.8) | 2.5 (2.4–2.7) | 2.4 (2.1–4.0) | 0.883 |
| Cardiac density (g/mL) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.633 |
| n = 43 (%) | |
|---|---|
| Midwall | 28 (65) |
| Right ventricular insertion point (normal variant) and midwall | 4 (9.3) |
| Right ventricular insertion point (normal variant) | 3 (7) |
| Transmural | 3 (7) |
| Focal | 2 (4.6) |
| Subendocardial | 2 (4.6) |
| Midwall and subendocardial | 1 (2.3) |
| Midwall, subendocardial and transmural | 1 (2.3) |
| Midwall and epicardial | 1 (2.3) |
| Subendocardial and transmural | 1 (2.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsabedze, N.; du Plessis, A.; Mpanya, D.; Vorster, A.; Wells, Q.; Scholtz, L.; Manga, P. Cardiovascular Magnetic Resonance Imaging Findings in Africans with Idiopathic Dilated Cardiomyopathy. Diagnostics 2023, 13, 617. https://doi.org/10.3390/diagnostics13040617
Tsabedze N, du Plessis A, Mpanya D, Vorster A, Wells Q, Scholtz L, Manga P. Cardiovascular Magnetic Resonance Imaging Findings in Africans with Idiopathic Dilated Cardiomyopathy. Diagnostics. 2023; 13(4):617. https://doi.org/10.3390/diagnostics13040617
Chicago/Turabian StyleTsabedze, Nqoba, Andre du Plessis, Dineo Mpanya, Anelia Vorster, Quinn Wells, Leonie Scholtz, and Pravin Manga. 2023. "Cardiovascular Magnetic Resonance Imaging Findings in Africans with Idiopathic Dilated Cardiomyopathy" Diagnostics 13, no. 4: 617. https://doi.org/10.3390/diagnostics13040617
APA StyleTsabedze, N., du Plessis, A., Mpanya, D., Vorster, A., Wells, Q., Scholtz, L., & Manga, P. (2023). Cardiovascular Magnetic Resonance Imaging Findings in Africans with Idiopathic Dilated Cardiomyopathy. Diagnostics, 13(4), 617. https://doi.org/10.3390/diagnostics13040617







