The Role of PET/CT in Breast Cancer
Abstract
1. Introduction
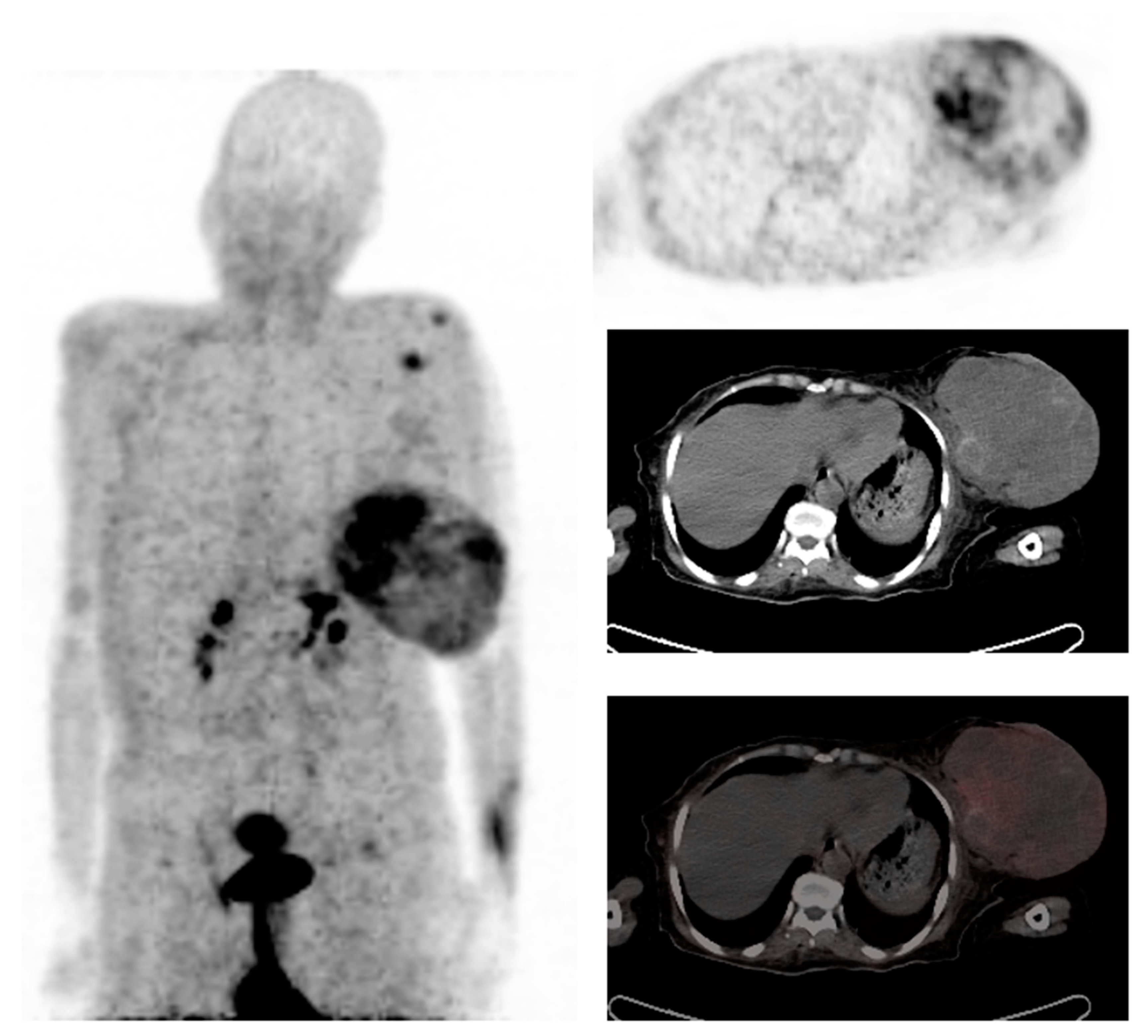
2. The Role of 18F-FDG PET/CT in Breast Cancer
2.1. Diagnosis
2.2. Staging
2.2.1. Axillary Lymph Node Staging
2.2.2. Distant Metastasis Staging
2.3. Prognosis
2.4. Treatment Response Evaluation
2.5. Recurrence
2.6. Radiotherapy Planning
2.7. Positron Emission Mammography
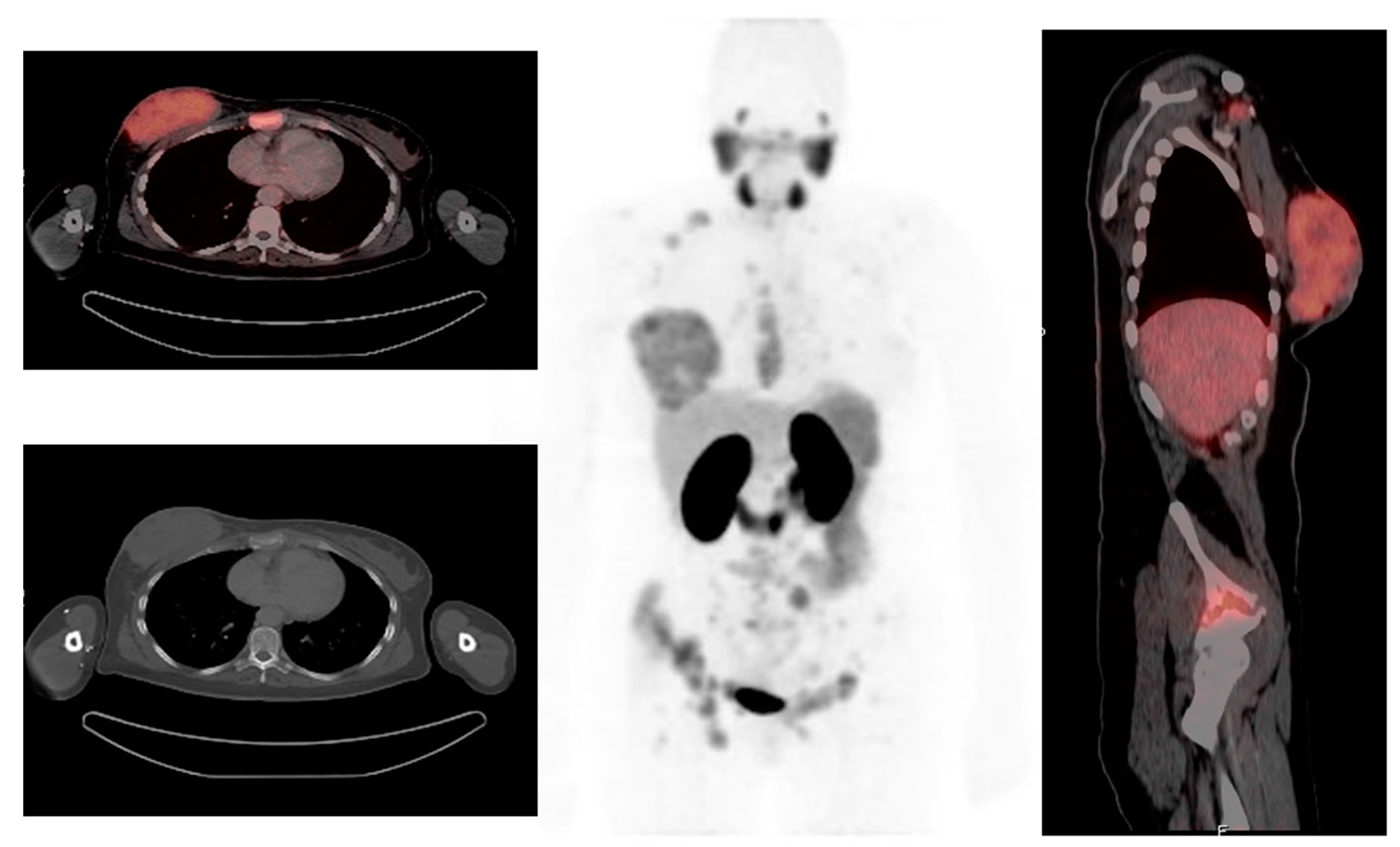
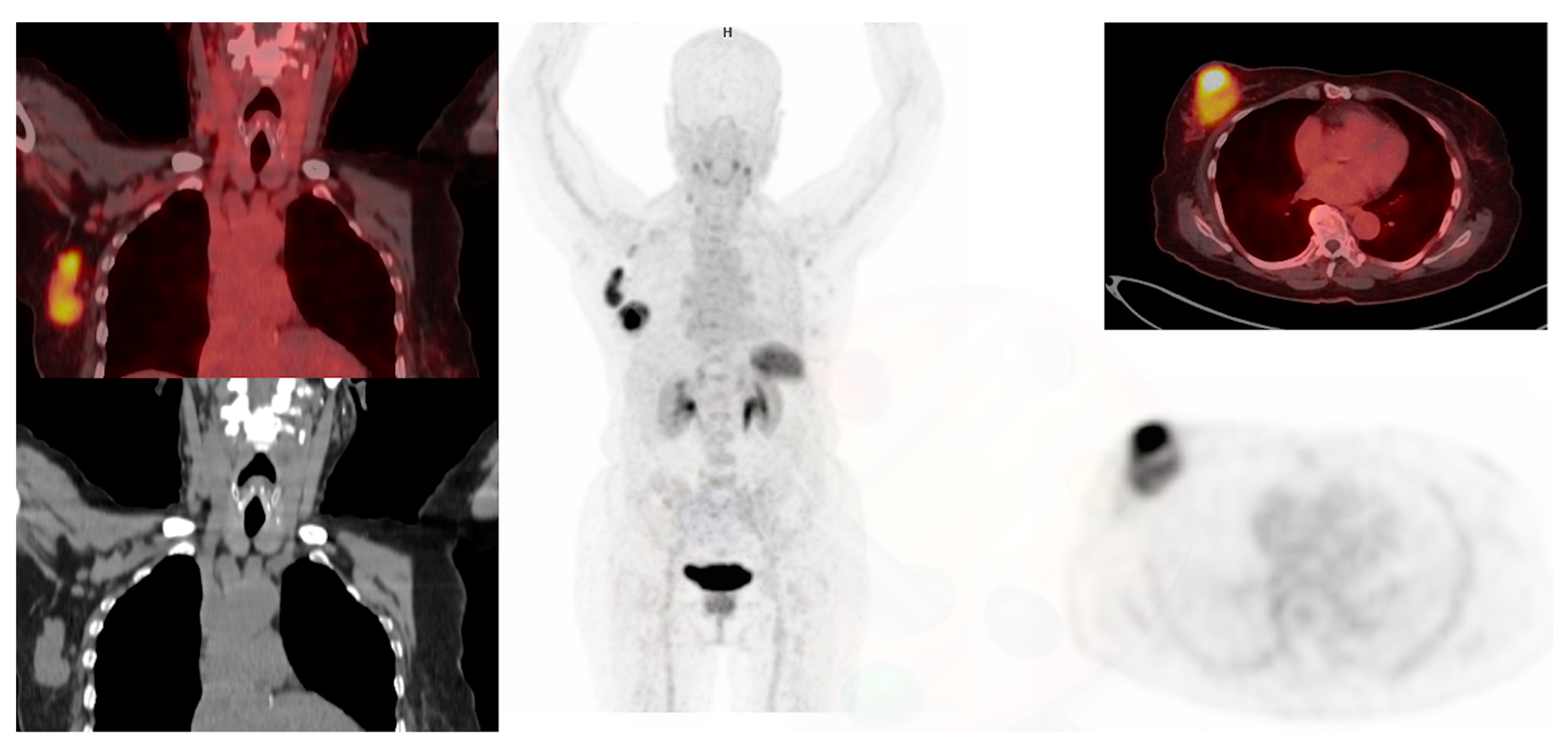
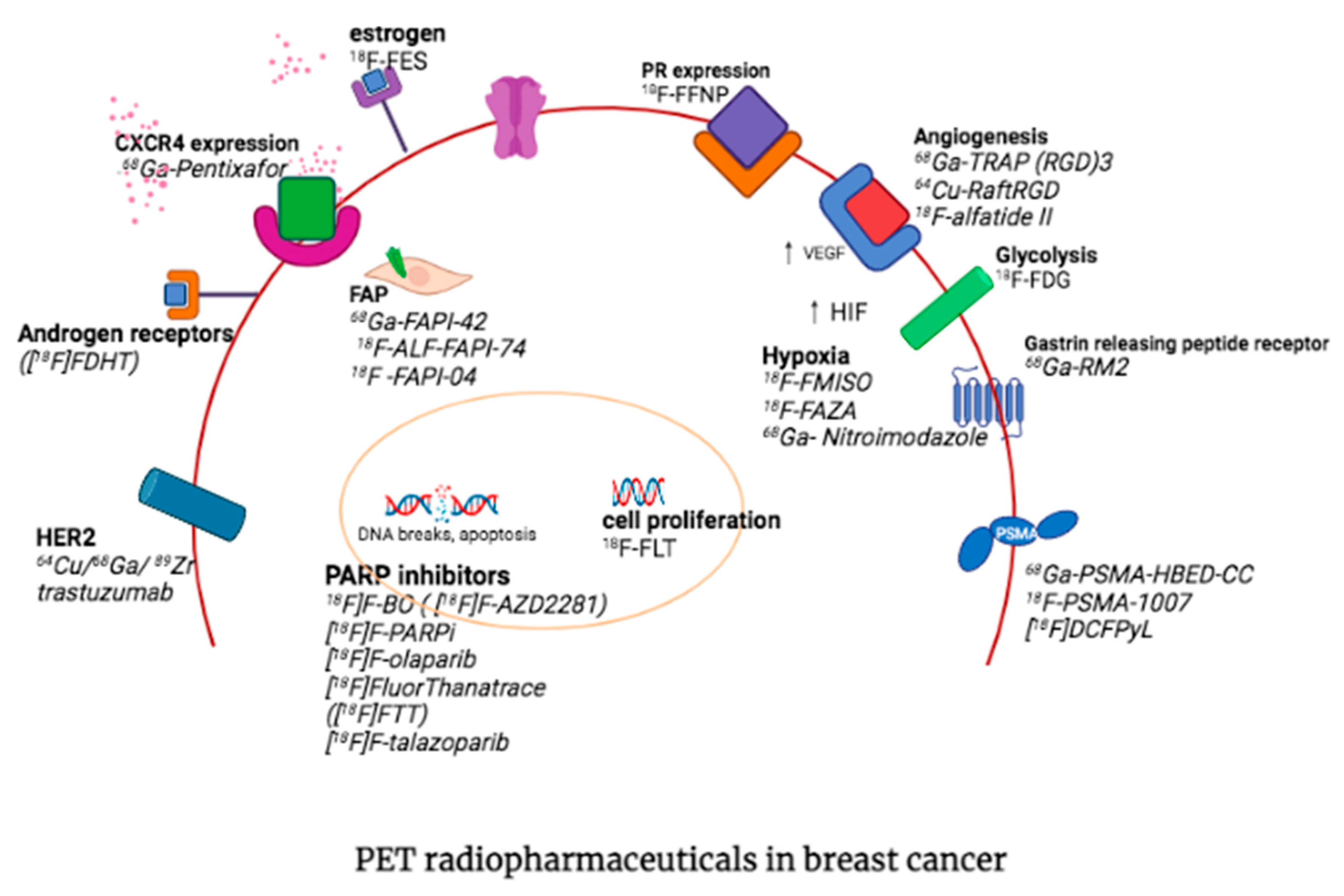
3. Other PET Radiopharmaceuticals in Molecular Imaging of Breast Cancer
3.1. Fibroblast Activation Protein
3.2. Prostate-Specific Membrane Antigen
3.3. Chemokine Receptor 4
3.4. Estrogen Receptor Imaging
3.5. Progesterone Receptor Imaging
3.6. Human Epidermal Growth Factor Receptor 2 (HER2)
3.7. Androgen Receptor
3.8. Somatostatin Receptor Expression
3.9. Integrins
3.10. Gastrin Releasing Peptide Receptor
3.11. PARP Inhibitors
3.12. Hypoxia Imaging
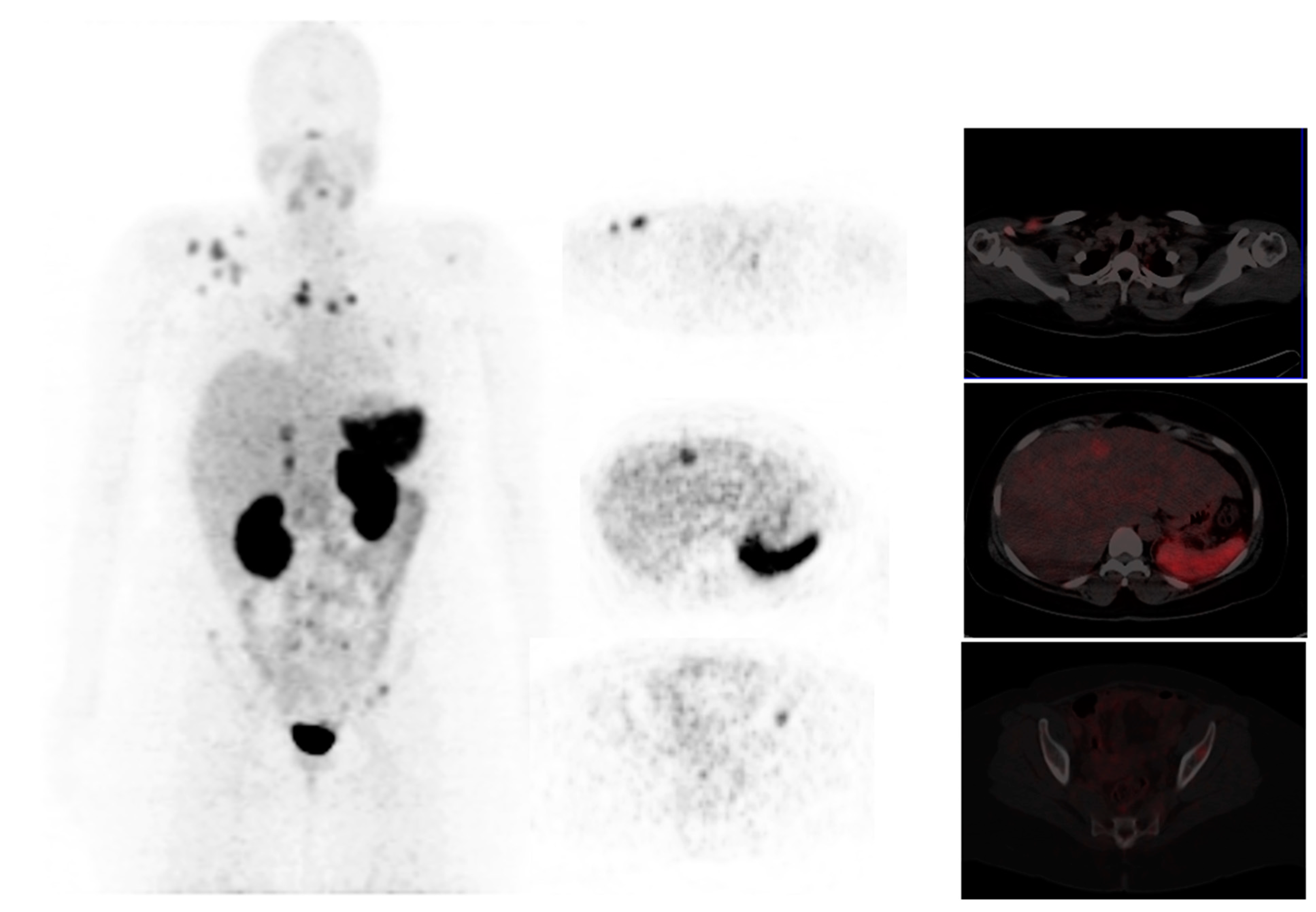
3.13. PET/CT Imaging of Bone Metastasis
3.13.1. 18F-NaF Bone Imaging
3.13.2. 68Ga- Zoledronate
4. Targeted Therapies in Breast Cancer
4.1. FAPI
4.2. CXCR4 Antagonists
4.3. 177Lu-Trastuzumab
5. Concluding Remarks and Future Perspectives
| PET Tracers | Class | Biochemical Mechanism | Clinical Application | Level of Evidence |
|---|---|---|---|---|
| 68Ga-PSMA-HBED-CC 18F-PSMA-1007 [18F]DCFPyL | Prostate-specific membrane antigen | PSMA inhibitors angiogenesis | Staging Potential for treatment response monitoring [56,57,100] | Systematic review |
| 18F-FLT (flurothymidine) | Cell proliferation | Substrates for cytosolic thymidinekinase-1 (TK1), which catalyzes the initial metabolic step of thymidine triphosphate synthesis. | Staging, monitoring, and prediction of response to treatment Uptake correlates with proliferation index ki-67 [101,102] | Systematic review and meta-analysis |
| −11C-choline or 18F-choline | Membrane Lipid Synthesis | Intracellular phosphorylation by choline kinase to phosphorylcholine. Associated with phospholipids of the cell membrane and tumor growth. | Assessment of tumor progression and Monitoring response to therapy [103,104] | Peer review |
| 11C-methionine 18F-Fluciclovine | Amino Acid Transport | Uptake related to amino acid transport in tumor cells | Assessment of disease, response to therapy and distinguishing responders from non-responders [104] | Peer review |
| 68Ga FAPI-42 18F-ALF-FAPI-74 18F-FAPI-04 | Fibroblast activation protein | Overexpressing fibroblast activation protein by cancer-associated fibroblasts (CAFs) | Staging Monitoring response to therapy Potential for selection of treatment response [88,89,90] | Peer review |
| F16a-[18F]fluoro-17b-estradiol (18F-FES) | Estrogen receptor imaging | Establish the ER status | Non-invasive detection of ER status in primary and metastasis Select candidates for anti-estrogen therapy [61,62,63] | Peer review |
| [18F]-fluorofuranyl norprogesterone ([18F]FFNP) | Progesterone receptor imaging | Progesterone analogue | Non-invasive detection of PR status predict response to endocrine therapy [64,65] | Peer review |
| 16β-[18F]fluoro-5α-dihydrotestosterone ([18F]FDHT) | Androgen receptor | Testosterone analog | Non-invasive alternative to biopsy imaging biomarker for evaluating response to SARM therapy [69,70,71] | Peer review |
| 64Cu trastuzumab 68Ga trastuzumab 89Zr trastuzumab | HER-2 receptor | Humanized monoclonal antibody | Prognostic information assessing the expression of HER2 in tumors prediction of response to HER2 targeted therapy [67,68,99] | Peer review |
| 68Ga-DOTATATE | Somatostatin receptor expression | Somatostatin receptor analog | May have a role in ER+ and PR+ breast cancer with low FDG uptake Lower detection of visceral lesions than FDG [72,73] | Peer review |
| 68Ga-TRAP (RGD)3 64Cu-RaftRGD 18F-alfatide II | Integrin alpha v beta (RDG) | Angiogenesis | Complementary to FDG PET Select patients who may benefit from therapies targeting angiogenesis Monitor treatment response Prognosis [74,75,76] | Peer review |
| 68Ga-RM2 | Gastrin-releasing peptide receptor | Overexpression of the physiologic ligand gastrin-releasing peptide in breast cancer | Assess disease extentPotential for selecting candidates for GRPR antagonists Superior to FDG (less uptake in inflammation, infection, and background) [77,78] | Peer review |
| [18F]F-BO (also known as [18F]F-AZD2281 [18F]F-PARPi [18F]F-olaparib [18F]FluorThanatrace ([18F]FTT) [18F]F-talazoparib | PARP inhibitors | Blocking the repair pathway of DNA double-strand breaks and promoting cell apoptosis | Patient selection for treatment with PARPi treatment monitoring [79,80] | Peer review |
| 18F-FMISO 18F-FAZA 18F-FETNIM 18F-HX4 60/64Ga-ATSM 68Ga- Nitroimidazole | Hypoxia | Selective accumulation in viable hypoxic cells | Non-invasively provides hypoxic information [81] Helps identify patients with a risk of early recurrence Identify patients eligible for antiangiogenic therapy [87,88] | Peer review |
| 8F-sodium fluoride (18F-NaF) | Fluoride | ion exchange with hydroxyl ions on the outside of the hydroxyapatite that converts hydroxyapatite to fluorapatite | Detection of bone metastasis [79,82] | Peer review |
| 68Ga- Zoledronate | Bisphosphonate | Accumulates in areas of high bone turnover | Detection of bone metastasis Selection for [177Lu]Lu-DOTAZOL and [225Ac]Ac-DOTAZOL [86,87] | Peer review |
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BC | breast cancer |
| CEA | carcinoembryonic antigen |
| DCIS | ductal carcinoma in situ |
| HER 2+ | Human Epidermal growth factor Receptor 2 oncogene |
| IBC | Inflammatory breast cancer |
| SARM | nonsteroidal elective AR modulation |
| LS | lymphoscintigraphy |
| MDP | methylene diphosphonate |
| NCCN | national embryonic cancer network |
| SLN | sentinel lymph node |
| SLNB | sentinel lymph node biopsy |
| TNBC | triple-negative breast cancer |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17 (Suppl. 3), 43–46. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef] [PubMed]
- Reis-Filho, J.S.; Pusztai, L. Gene expression profiling in breast cancer: Classification, prognostication, and prediction. Lancet 2011, 378, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Vercher-Conejero, J.L.; Pelegrí-Martinez, L.; Lopez-Aznar, D.; Cózar-Santiago, M.D.P. Positron Emission Tomography in Breast Cancer. Diagnostics 2015, 5, 61–83. [Google Scholar] [CrossRef]
- Paydary, K.; Seraj, S.M.; Zadeh, M.Z.; Emamzadehfard, S.; Shamchi, S.P.; Gholami, S.; Werner, T.J.; Alavi, A. The Evolving Role of FDG-PET/CT in the Diagnosis, Staging, and Treatment of Breast Cancer. Mol. Imaging Biol. 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Taori, K.; Dhakate, S.; Rathod, J.; Hatgaonkar, A.; Disawal, A.; Wavare, P.; Bakare, V.; Puri, R.P. Evaluation of breast masses using mammography and sonography as first line investigations. Open J. Med. Imaging 2013, 3, 40. [Google Scholar] [CrossRef]
- Rong, J.; Wang, S.; Ding, Q.; Yun, M.; Zheng, Z.; Ye, S. Comparison of 18FDG PET-CT and bone scintigraphy for detection of bone metastases in breast cancer patients. A meta-analysis. Surg. Oncol. 2013, 22, 86–91. [Google Scholar] [CrossRef]
- Kitajima, K.; Miyoshi, Y. Present and future role of FDG-PET/CT imaging in the management of breast cancer. Jpn. J. Radiol. 2016, 34, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Bertagna, F.; Treglia, G.; Orlando, E.; Dognini, L.; Giovanella, L.; Sadeghi, R.; Giubbini, R. Prevalence and clinical significance of incidental F18-FDG breast uptake: A systematic review and meta-analysis. Jpn. J. Radiol. 2014, 32, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A. PET/CT for patients with breast cancer: Where is the clinical impact? Am. J. Roentgenol. 2019, 213, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Choi, J.Y. Impact of 18F-FDG PET, PET/CT, and PET/MRI on staging and management as an initial staging modality in breast cancer: A systematic review and meta-analysis. Clin. Nucl. Med. 2021, 46, 271. [Google Scholar] [CrossRef] [PubMed]
- Kasem, J.; Wazir, U.; Mokbel, K. Sensitivity, Specificity and the Diagnostic Accuracy of PET/CT for Axillary Staging in Patients with Stage I–III Cancer: A Systematic Review of the Literature. Vivo J. 2021, 35, 23–30. [Google Scholar] [CrossRef]
- Groheux, D.; Hindié, E.; Giacchetti, S. Early assessment with 18F-fluorodeoxyglucose positron emission to-mography/computed tomography can help predict the outcome of neoadjuvant chemotherapy in triple negative breast cancer. Eur. J. Cancer 2014, 50, 1864–1871. [Google Scholar] [CrossRef]
- Peare, R.; Staff, R.T.; Heys, S.D. The use of FDG-PET in assessing axillary lymph node status in breast cancer: A systematic review and meta-analysis of the literature. Breast Cancer Res. Treat. 2010, 123, 281–290. [Google Scholar] [CrossRef]
- Ko, H.; Baghdadi, Y.; Love, C.; Sparano, J.A. Clinical Utility of 18F-FDG PET/CT in Staging Localized Breast Cancer Before Initiating Preoperative Systemic Therapy. J. Natl. Compr. Cancer Netw. 2020, 18, 1240–1246. [Google Scholar] [CrossRef]
- Cachin, F.; Prince, H.M.; Hogg, A. Powerful prognostic stratification by [18F]fluorodeoxyglucose positron emission tomography in patients with metastatic breast cancer treated with high-dose chem-otherapy. J. Clin. Oncol. 2006, 24, 3026–3031. [Google Scholar] [CrossRef]
- Hong, S.; Li, J.; Wang, S. 18FDG PET-CT for diagnosis of distant metastases in breast cancer patients. A meta-analysis. Surg. Oncol. 2013, 22, 139–143. [Google Scholar] [CrossRef]
- Blake, G.M.; Park-Holohan, S.J.; Cook, G.J.; Fogelman, I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin. Nucl. Med. 2001, 31, 28–49. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Y.L.; Liu, Y.; Xiong, J.P.; He, C.Z. Comparison of whole-body PET/PET-CT and conventional imaging procedures for distant metastasis staging in patients with breast cancer: A meta-analysis. Eur. J. Gynaecol. Oncol. 2015, 36, 672–676. [Google Scholar]
- Hildebrandt, M.G.; Naghavi-Behzad, M.; Vogsen, M. A role of FDG-PET/CT for response evaluation in metastatic breast cancer? Semin. Nuclear Med. 2022, 52, 520–530. [Google Scholar] [CrossRef]
- Plunkett, T.; Smith, P.; Rubens, R. Risk of complications from bone metastases in breast cancer: Implications for management. Eur. J. Cancer 2000, 36, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Mammatas, L.H.; Aras, T.; Vogel, W.V.; van de Brug, T.; Oprea-Lager, D.E.; Verheul, H.M.; Hoekstra, O.S.; Boellaard, R.; Menke-van der Houven van Oordt, C.W. Diagnostic Performance of [18F] FDG PET in Staging Grade 1–2, Estrogen Receptor Positive Breast Cancer. Diagnostics 2021, 11, 1954. [Google Scholar] [CrossRef] [PubMed]
- Tural, D.; Kivrak Salim, D.; Mutlu, H. Is there any relation between PET-CT SUVmax value and prognostic factors in locally advanced breast cancer? J. Buon 2015, 20, 1282–1286. [Google Scholar]
- Lei, L.; Wang, X.; Chen, Z. PET/CT Imaging for Monitoring Recurrence and Evaluating Response to Treatment in Breast Cancer. Adv. Clin. Exp. Med. 2016, 25, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Zucchini, G.; Quercia, S.; Zamagni, C.; Santini, D.; Taffurelli, M.; Fanti, S.; Martoni, A. Potential utility of early metabolic response by 18F-2-fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography in a selected group of breast cancer patients receiving preoperative chemotherapy. Eur. J. Cancer 2013, 49, 1539–1545. [Google Scholar] [CrossRef]
- Asaoka, M.; Gandhi, S.; Ishikawa, T.; Takabe, K. Neoadjuvant Chemotherapy for Breast Cancer: Past, Present, and Future. Breast Cancer Basic Clin. Res. 2020, 14, 1178223420980377. [Google Scholar] [CrossRef]
- Gennari, A.; Donati, S.; Salvadori, B. Role of 2-[18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) in the early assessment of response to chemotherapy in metastatic breast cancer patients. Clin. Breast Cancer 2000, 1, 156–161; discussion 162–163. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Robertson, C.; Maisonneuve, P.; et al. A Randomized Comparison of Sentinel-Node Biopsy with Routine Axillary Dissection in Breast Cancer. N. Engl. J. Med. 2003, 349, 546–553. [Google Scholar] [CrossRef]
- Cook, G.J.; Azad, G.K.; Goh, V. Imaging bone metastases in breast cancer: Staging and response assessment. J. Nucl. Med. 2016, 57 (Suppl. 1), 27S–33S. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Jiang, X. Diagnostic efficacy of 18F-FDG-PET or PET/CT in breast cancer with suspected recurrence: A systematic review and meta-analysis. Nucl. Med. Commun. 2016, 37, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hou, H.; Wang, C.; Li, J.; Yao, Q.; Amer, S.; Tian, M. The Diagnostic Value of18F-FDG PET/CT in Association with Serum Tumor Marker Assays in Breast Cancer Recurrence and Metastasis. BioMed Res. Int. 2015, 2015, 489021. [Google Scholar] [CrossRef]
- Jacene, H.A.; DiPiro, P.J.; Bellon, J.; Hu, J.; Cheng, S.-C.; Warren, L.; Schlosnagle, E.; Nakhlis, F.; Rosenbluth, J.M.; Yeh, E.; et al. Discrepancy between FDG-PET/CT and contrast-enhanced CT in the staging of patients with inflammatory breast cancer: Implications for treatment planning. Breast Cancer Res. Treat. 2020, 181, 383–390. [Google Scholar] [CrossRef]
- Ford, E.C.; Herman, J.; Yorke, E.; Wahl, R.L. 18F-FDG PET/CT for Image-Guided and Intensity-Modulated Radiotherapy. J. Nucl. Med. 2009, 50, 1655–1665. [Google Scholar] [CrossRef]
- Glass, S.B.; Shah, Z.A. Clinical utility of positron emission mammography. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon, UK, 2013; Volume 26, pp. 314–319. [Google Scholar]
- Weinberg, I.N.; Beylin, D.; Zavarzin, V. Positron emission mammography: High-resolution bio-chemical breast imaging. Technol. Cancer Res. Treat. 2005, 4, 55–60. [Google Scholar] [CrossRef]
- Yano, F.; Itoh, M.; Hirakawa, H.; Yamamoto, S.; Yoshikawa, A.; Hatazawa, J. Diagnostic Accuracy of Positron Emission Mammography with 18F-fluorodeoxyglucose in Breast Cancer Tumor of Less than 20 mm in Size. Asia Ocean J. Nucl. Med. Biol. 2018, 7, 13–21. [Google Scholar] [CrossRef]
- Schilling, K.; Narayanan, D.; Kalinyak, J.E.; The, J.; Velasquez, M.V.; Kahn, S.; Saady, M.; Mahal, R.; Chrystal, L. Positron emission mammography in breast cancer presurgical planning: Comparisons with magnetic resonance imaging. Eur. J. Nucl. Med. 2011, 38, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Yanai, A.; Itoh, M.; Hirakawa, H.; Yanai, K.; Tashiro, M.; Harada, R.; Yoshikawa, A.; Yamamoto, S.; Ohuchi, N.; Ishida, T. Newly-Developed Positron Emission Mammography (PEM) Device for the Detection of Small Breast Cancer. Tohoku J. Exp. Med. 2018, 245, 13–19. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom.-Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Heirbaut, L.; Cheng, J.D.; Joossens, J.; Ryabtsova, O.; Cos, P.; Maes, L.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med. Chem. Lett. 2013, 4, 491–496. [Google Scholar] [CrossRef]
- Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. USA 1990, 87, 7235–7239. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Heesch, A.; Maurer, J.; Stickeler, E.; Beheshti, M.; Mottaghy, F.M.; Morgenroth, A. Development of Radiotracers for Breast Cancer—The Tumor Microenvironment as an Emerging Target. Cells 2020, 9, 2334. [Google Scholar] [CrossRef]
- Komek, H.; Can, C.; Guzel, Y.; Oruc, Z.; Gundogan, C.; Yildirim, O.A.; Kaplan, I.; Erdur, E.; Yildirim, M.S.; Cakabay, B. (68)Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: A comparative pilot study with the (18)F-FDG PET/CT. Ann. Nucl. Med. 2021, 35, 744–752. [Google Scholar] [CrossRef]
- Elboga, U.; Sahin, E.; Kus, T.; Cayirli, Y.; Aktas, G.; Uzun, E.; Cinkir, H.; Teker, F.; Sever, O.; Alper, A.; et al. Superiority of 68Ga-FAPI PET/CT scan in detecting additional lesions compared to 18FDG PET/CT scan in breast cancer. Ann. Nucl. Med. 2021, 35, 1321–1331. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Hu, K.; Wang, L.; Wu, H.; Huang, S.; Tian, Y.; Wang, Q.; Xiao, C.; Han, Y.; Tang, G. [18F] FAPI-42 PET imaging in cancer patients: Optimal acquisition time, biodistribution, and comparison with [68Ga] Ga-FAPI-04. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2833–2843. [Google Scholar] [CrossRef]
- Li, Z.; Aboian, M.S.; Zhu, X.; Marquez-Nostra, B. Clinical Evaluation of Nuclear Imaging Agents in Breast Cancer. Cancers 2022, 14, 2103. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Lengana, T.; Modiselle, M.; Vorster, M.; Zeevaart, J.; Maes, A.; Ebenhan, T.; Van deWiele, C. 68Ga-PSMA-HBED-CC PET imaging in breast carcinoma patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Modiselle, M.; Vorster, M.; Mokgoro, N.; Nyakale, N.; Mokaleng, B.; Ebenhan, T. 68Ga-PSMA imaging of metastatic breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1482–1483. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.D.; Jacobson, O. Molecular Imaging of Chemokine Receptor CXCR4. Theranostics 2013, 3, 76–84. [Google Scholar] [CrossRef]
- Lapidot, T.; Dar, A.; Kollet, O. How do stem cells find their way home? Blood 2005, 106, 1901–1910. [Google Scholar] [CrossRef]
- Vag, T.; Steiger, K.; Rossmann, A.; Keller, U.; Noske, A.; Herhaus, P.; Ettl, J.; Niemeyer, M.; Wester, H.-J.; Schwaiger, M. PET imaging of chemokine receptor CXCR4 in patients with primary and recurrent breast carcinoma. EJNMMI Res. 2018, 8, 90. [Google Scholar] [CrossRef]
- Kurland, B.F.; Peterson, L.M.; Lee, J.H.; Schubert, E.K.; Currin, E.R.; Link, J.M.; Krohn, K.A.; Mankoff, D.A.; Linden, H.M. Estrogen Receptor Binding (18F-FES PET) and Glycolytic Activity (18F-FDG PET) Predict Progression-Free Survival on Endocrine Therapy in Patients with ER+ Breast CancerFDG PET and FES PET Predict PFS on Endocrine Therapy. Clin. Cancer Res. 2017, 23, 407–415. [Google Scholar] [CrossRef]
- Piccardo, A.; Fiz, F.; Treglia, G.; Bottoni, G.; Trimboli, P. Head-to-Head Comparison between 18F-FES PET/CT and 18F-FDG PET/CT in Oestrogen Receptor-Positive Breast Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1919. [Google Scholar] [CrossRef] [PubMed]
- van Kruchten, M.; Glaudemans, A.W.; de Vries, E.F.; Beets-Tan, R.G.; Schröder, C.P.; Dierckx, R.A.; de Vries, E.G.; Hospers, G.A. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J. Nucl. Med. 2012, 53, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Lebron, L.; Greenspan, D.; Pandit-Taskar, N. PET Imaging of Breast Cancer. Role Patient Manag. PET Clin. 2015, 10, 159–195. [Google Scholar] [CrossRef]
- Dehdashti, F.; Wu, N.; Ma, C.X.; Naughton, M.J.; Katzenellenbogen, J.A.; Siegel, B.A. Association of PET-based estradiol-challenge test for breast cancer progesterone receptors with response to endocrine therapy. Nat. Commun. 2021, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Mammatas, L.H.; Venema, C.M.; Schröder, C.P.; de Vet, H.C.W.; van Kruchten, M.; Glaudemans, A.W.J.M.; Yaqub, M.M.; Verheul, H.M.W.; Boven, E.; van der Vegt, B.; et al. Visual and quantitative evaluation of [18F]FES and [18F]FDHT PET in patients with metastatic breast cancer: An interobserver variability study. EJNMMI Res. 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Laforest, R.; Lapi, S.E.; Oyama, R.; Bose, R.; Tabchy, A.; Marquez-Nostra, B.V.; Burkemper, J.; Wright, B.D.; Frye, J.; Frye, S.; et al. [89Zr] trastuzumab: Evaluation of radiation dosimetry, safety, and optimal im-aging parameters in women with HER2-positive breast cancer. Mol. Imaging Biol. 2016, 18, 952–959. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Bading, J.R.; Park, J.M.; Frankel, P.H.; Carroll, M.I.; Tran, T.T.; Poku, E.K.; Rockne, R.C.; Raubitschek, A.A.; Shively, J.E.; et al. Tumor uptake of 64Cu-DOTA-trastuzumab in patients with metastatic breast cancer. J. Nucl. Med. 2018, 59, 38–43. [Google Scholar] [CrossRef]
- Venema, C.M.; Mammatas, L.H.; Schröder, C.P.; Van Kruchten, M.; Apollonio, G.; Glaudemans, A.W.J.M.; Bongaerts, A.H.; Hoekstra, O.S.; Verheul, H.M.; Boven, E.; et al. Androgen and Estrogen Receptor Imaging in Metastatic Breast Cancer Patients as a Surrogate for Tissue Biopsies. J. Nucl. Med. 2017, 58, 1906–1912. [Google Scholar] [CrossRef]
- Overmoyer, B.; Rugo, H.S.; Johnston, S.; O’Shaughnessy, J.A.; Palmieri, C.; Schwartzberg, L.S.; Taylor, R.P.; Young, D.C.; Johnston, M.A. First stage of an on-going phase 2, open label, international, randomized, parallel design study investigating efficacy+ safety of GTx-024 for advanced ER+/AR+ breast cancer (BC). Ann. Oncol. 2017, 28, i7–i8. [Google Scholar] [CrossRef]
- Jacene, H.A.; Liu, M.; Cheng, S.-C.; Abbott, A.; Dubey, S.; McCall, K.; Young, D.; Johnston, M.; Abbeele, A.D.V.D.; Overmoyer, B. Imaging Androgen Receptors in Breast Cancer with 18F-Fluoro-5α-Dihydrotestosterone PET: A Pilot Study. J. Nucl. Med. 2022, 63, 22–28. [Google Scholar] [CrossRef]
- Lee, H.; Suh, M.; Choi, H.; Ha, S.; Paeng, J.C.; Cheon, G.J.; Kang, K.W.; Lee, D.S. A pan-cancer analysis of the clinical and genetic portraits of somatostatin receptor expressing tumor as a potential target of peptide receptor imaging and therapy. EJNMMI Res. 2020, 10, 42. [Google Scholar] [CrossRef]
- Nguyen, A.; Fullard, K.; Sheehan-Dare, G.; Tang, R.; Chan, L.; Ho, B.; Dear, R.; Keane, J.; Hickey, A.; Nandurkar, R.; et al. Diagnostic value of 68Ga-DOTATATE PET-CT imaging for staging of ER+/PR+ HER2-breast cancer patients with metastatic disease: Comparison with conventional imaging with bone scan, diagnostic CT and 18F-FDG PET-CT in a prospective pilot trial. J. Med. Imaging Radiat. Oncol. 2022, 66, 731–737. [Google Scholar] [CrossRef]
- Miladinova, D. Molecular imaging in breast cancer. Nucl. Med. Mol. Imaging 2019, 53, 313–319. [Google Scholar] [CrossRef]
- Wu, J.; Wang, S.; Zhang, X.; Teng, Z.; Wang, J.; Yung, B.C.; Niu, G.; Zhu, H.; Lu, G.; Chen, X. 18F-Alfatide II PET/CT for Identification of Breast Cancer: A Preliminary Clinical Study. J. Nucl. Med. 2018, 59, 1809–1816. [Google Scholar] [CrossRef]
- Kazmierczak, P.M. ⁶⁸Ga-TRAP-(RGD)3 Hybrid Imaging for the In Vivo Monitoring of α (v) β₃-Integrin Expression as Biomarker of Anti-Angiogenic Therapy Effects in Experimental Breast Cancer. PLoS ONE 2016, 11, e0168248. [Google Scholar] [CrossRef]
- Chao, C.; Ives, K.; Hellmich, H.L.; Townsend, C.M., Jr.; Hellmich, M.R. Gastrin-releasing peptide receptor in breast cancer mediates cellular migration and interleukin-8 expression. J. Surg. Res. 2009, 156, 26–31. [Google Scholar] [CrossRef]
- Stoykow, C.; Erbes, T.; Maecke, H.R.; Bulla, S.; Bartholomä, M.; Mayer, S.; Drendel, V.; Bronsert, P.; Werner, M.; Gitsch, G.; et al. Gastrin-releasing Peptide Receptor Imaging in Breast Cancer Using the Receptor Antagonist 68Ga-RM2 And PET. Theranostics 2016, 6, 1641–1650. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J. Current status and progress in using radiolabelled PARP-1 inhibitors for imaging PARP-1 expression in tumours. Eur. J. Med. Chem. 2022, 242, 114690. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.S.; Gillard, M.; Lipkowitz, S.; Lee, J.-M. Update on PARP Inhibitors in Breast Cancer. Curr. Treat. Options Oncol. 2018, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Asano, A.; Ueda, S.; Kuji, I.; Yamane, T.; Takeuchi, H.; Hirokawa, E.; Sugitani, I.; Shimada, H.; Hasebe, T.; Osaki, A.; et al. Intracellular hypoxia measured by 18F-fluoromisonidazole positron emission tomography has prognostic impact in patients with estrogen receptor-positive breast cancer. Breast Cancer Res. 2018, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Dadgar, H.; Norouzbeigi, N.; Jokar, N.; Zareizadeh, J.; Gholamrezanezhad, A.; Ahmadzadehfar, H.; Abbaszadeh, M.; Assadi, M. Comparison of 18F-NaF Imaging, 99mTc-MDP Scintigraphy, and 18F-FDG for Detecting Bone Metastases. World J. Nucl. Med. 2022, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Puntoni, M.; Morbelli, S.; Massollo, M.; Bongioanni, F.; Paparo, F.; Altrinetti, V.; Gonella, R.; Gennari, A.; Iacozzi, M.; et al. 18F-FDG PET/CT is a prognostic biomarker in patients affected by bone metastases from breast cancer in comparison with 18F-NaF PET/CT. Nukl.-Nucl. 2015, 54, 163–172. [Google Scholar] [CrossRef]
- Roop, M.J.; Singh, B.; Singh, H.; Watts, A.; Kohli, P.S.; Mittal, B.R.; Singh, G. Incremental value of cocktail 18F-FDG and 18F-NaF PET/CT over 18F-FDG PET/CT alone for characterization of skeletal metastases in breast cancer. Clin. Nucl. Med. 2017, 42, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.I.; Rao, J.E.; Mittra, E.S.; Nallapareddy, K.; Chengapa, A.; Dick, D.W.; Gambhir, S.S.; Iagaru, A. Prospective comparison of combined 18F-FDG and 18F-NaF PET/CT vs. 18F-FDG PET/CT imaging for detection of malignancy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Khawar, A.; Eppard, E.; Roesch, F.; Ahmadzadehfar, H.; Kürpig, S.; Meisenheimer, M.; Gaertner, F.C.; Essler, M.; Bundschuh, R.A. Preliminary results of biodistribution and dosimetric analysis of [68Ga]Ga-DOTAZOL: A new zoledronate-based bisphosphonate for PET/CT diagnosis of bone diseases. Ann. Nucl. Med. 2019, 33, 404–413. [Google Scholar] [CrossRef]
- Lawal, I.O.; Mokoala, K.M.; Mahapane, J.; Kleyhans, J.; Meckel, M.; Vorster, M.; Ebenhan, T.; Rösch, F.; Sathekge, M.M. A prospective intra-individual comparison of [68Ga] Ga-PSMA-11 PET/CT, [68Ga] Ga-NODAGAZOL PET/CT, and [99mTc] Tc-MDP bone scintigraphy for radionuclide imaging of prostate cancer skeletal metastases. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. 2021, 48, 942–944. [Google Scholar] [CrossRef]
- Lindner, T.; Altmann, A.; Giesel, F.; Kratochwil, C.; Kleist, C.; Krämer, S.; Mier, W.; Cardinale, J.; Kauczor, H.-U.; Jäger, D.; et al. Additional file 1 of 18F-labeled tracers targeting fibroblast activation protein. EJNMMI Radiopharm. Chem. 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Baum, R.P.; Schuchardt, C.; Singh, A.; Chantadisai, M.; Robiller, F.C.; Zhang, J.; Mueller, D.; Eismant, A.; Al-maguel, F.; Zboralski, D.; et al. Feasibility, biodistribution, and preliminary dosimetry in pep-tide-targeted radionuclide therapy of diverse adenocarcinomas using 177Lu-FAP-2286: First-in-humans results. J. Nucl. Med. 2022, 63, 415–423. [Google Scholar] [CrossRef]
- Pernas, S.; Martin, M.; Kaufman, P.A.; Gil-Martin, M.; Pardo, P.G.; Lopez-Tarruella, S.; Manso, L.; Ciruelos, E.; Perez-Fidalgo, J.A.; Hernando, C.; et al. Balixafortide plus eribulin in HER2-negative meta-static breast cancer: A phase 1, single-arm, dose-escalation trial. Lancet Oncol. 2018, 19, 812–824. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, C.; Zhao, H.; Chen, H. CXCR4 in breast cancer: Oncogenic role and therapeutic targeting. Drug Des. Dev. Ther. 2015, 9, 4953–4964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gong, S.; Wu, J.; Li, H.; Oupicky, D.; Sun, M. CXCR4-Targeted and Redox Responsive Dextrin Nanogel for Metastatic Breast Cancer Therapy. Biomacromolecules 2017, 18, 1793–1802. [Google Scholar] [CrossRef]
- Kotb, R.M.; Ibrahim, S.S.; Mostafa, O.M.; Shahin, N.N. Potential role of CXCR4 in trastuzumab resistance in breast cancer patients. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166520. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, L.; Peng, X.; Guo, Q.; Wu, Q.; Wang, W.; Zhang, G.; Wu, J.; Du, C. CXCR4 antagonist AMD3100 enhances the response of MDA-MB-231 triple-negative breast cancer cells to ionizing radiation. Cancer Lett. 2018, 418, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lefort, S.; Thuleau, A.; Kieffer, Y.; Sirven, P.; Bieche, I.; Marangoni, E.; Vincent-Salomon, A.; Mechta-Grigoriou, F. CXCR4 inhibitors could benefit to HER2 but not to triple-negative breast cancer patients. Oncogene 2017, 36, 1211–1222. [Google Scholar] [CrossRef]
- Zhou, M.; Luo, C.; Zhou, Z.; Li, L.; Huang, Y. Improving anti-PD-L1 therapy in triple negative breast cancer by polymer-enhanced immunogenic cell death and CXCR4 blockade. J. Control. Release 2021, 334, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Bhusari, P.; Vatsa, R.; Singh, G.; Parmar, M.; Bal, A.; Dhawan, D.K.; Mittal, B.R.; Shukla, J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int. J. Cancer 2017, 140, 938–947. [Google Scholar] [CrossRef]
- Sorace, A.G.; Quarles, C.C.; Whisenant, J.G.; Hanker, A.B.; McIntyre, J.O.; Sanchez, V.M.; Yankeelov, T.E. Trastuzumab improves tumor perfusion and vascular delivery of cytotoxic therapy in a murine model of HER2+ breast cancer: Preliminary results. Breast Cancer Res. Treat. 2016, 155, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Bertagna, F.; Albano, D.; Cerudelli, E.; Gazzilli, M.; Tomasini, D.; Bonù, M.; Giubbini, R.; Treglia, G. Radiolabelled PSMA PET/CT in breast cancer. A systematic review. Nucl. Med. Rev. 2020, 23, 32–35. [Google Scholar]
- Woolf, D.K.; Beresford, M.; Li, S.P.; Dowsett, M.; Sanghera, B.; Wong, W.L.; Sonoda, L.; Detre, S.; Amin, V.; Ah-See, M.-L.; et al. Evaluation of FLT-PET-CT as an imaging biomarker of proliferation in primary breast cancer. Br. J. Cancer 2014, 110, 2847–2854. [Google Scholar] [CrossRef]
- Chalkidou, A.; Landau, D.; Odell, E.; Cornelius, V.; O’Doherty, M.; Marsden, P. Correlation between Ki-67 immunohistochemistry and 18F-Fluorothymidine uptake in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2012, 48, 3499–3513. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Saad, F.F.; Zakaria, M.H.; Appanna, B. PET/CT analysis of 21 patients with breast cancer: Physiological distribution of 18F-choline and diagnostic pitfalls. J. Int. Med. Res. 2018, 46, 3138–3148. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Schuster, D.M. Amino Acid Metabolism as a Target for Breast Cancer Imaging. PET Clin. 2018, 13, 437–444. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadebe, B.; Harry, L.; Ebrahim, T.; Pillay, V.; Vorster, M. The Role of PET/CT in Breast Cancer. Diagnostics 2023, 13, 597. https://doi.org/10.3390/diagnostics13040597
Hadebe B, Harry L, Ebrahim T, Pillay V, Vorster M. The Role of PET/CT in Breast Cancer. Diagnostics. 2023; 13(4):597. https://doi.org/10.3390/diagnostics13040597
Chicago/Turabian StyleHadebe, Bawinile, Lerwine Harry, Tasmeera Ebrahim, Venesen Pillay, and Mariza Vorster. 2023. "The Role of PET/CT in Breast Cancer" Diagnostics 13, no. 4: 597. https://doi.org/10.3390/diagnostics13040597
APA StyleHadebe, B., Harry, L., Ebrahim, T., Pillay, V., & Vorster, M. (2023). The Role of PET/CT in Breast Cancer. Diagnostics, 13(4), 597. https://doi.org/10.3390/diagnostics13040597






