Preanalytical Errors in a Hematology Laboratory: An Experience from a Tertiary Care Center
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chawla, R.; Goswami, B.; Tayal, D.; Mallika, V. Identification of the Types of Preanalytical Errors in the Clinical Chemistry Laboratory: 1-Year Study at G.B. Pant Hospital. Lab. Med. 2010, 41, 89–92. [Google Scholar] [CrossRef]
- Noordin, S.; Isa, S. Evaluation of Blood Sample Rejection in a Clinical Laboratory of an Oncology Institute. Malays. J. Med. Health Sci. 2021, 17, 49–54. [Google Scholar]
- Shirlyn, B. McKenzie, Introduction. In Clinical Laboratory Hematology, 2nd ed.; Zeibig, E., Ed.; Pearson: London, UK, 2010; pp. 29–36. ISBN 978-1-2920-2794-4. [Google Scholar]
- HarsimranKaur, V.N.; Selhi, P.K.; Sood, N.; Singh, A. Preanalytical Errors in Hematology Laboratory—An Avoidable Incompetence. Iran. J. Pathol. 2016, 11, 151–154. [Google Scholar] [PubMed]
- B.Shukla, D.K.; Kanetkar, S.R.; Ingale, S.H. Study of Preanalytical errors in hematology laboratory of a tertiary care hospital. Asian Pac. J. Health Sci. 2017, 4, 4–6. [Google Scholar] [CrossRef]
- Alavi, N.; Khan, S.H.; Saadia, A.; Naeem, T. Challenges in Preanalytical Phase of Laboratory Medicine: Rate of Blood Sample Nonconformity in a Tertiary Care Hospital. EJIFCC 2020, 31, 21–27. [Google Scholar]
- Cornes, M.P.; Atherton, J.; Pourmahram, G.; Borthwick, H.; Kyle, B.; West, J.; Costelloe, S.J. Monitoring and reporting of preanalytical errors in laboratory medicine: The UK situation. Ann. Clin. Biochem. 2016, 53, 279–284. [Google Scholar] [CrossRef]
- Aadil, S. Study of the errors in hematology laboratory in a tertiary care hospital. Eur. J. Mol. Clin. Med. 2020, 7, 1366–1368. [Google Scholar]
- Arul, P.; Pushparaj, M.; Pandian, K.; Chennimalai, L.; Rajendran, K.; Selvaraj, E.; Masilamani, S. Prevalence and types of preanalytical error in hematology laboratory of a tertiary care hospital in South India. J. Lab. Physicians 2018, 10, 237–240. [Google Scholar] [CrossRef]
- Venkat Raghavan, A.T.M.; Sweta, K.; Shanmugasamy, K.; Sowmya, S. Risk assessment of pre-analytical errors and their impact on patient safety in a tertiary care centre in South India. IP J. Diagn. Pathol. Oncol. 2020, 5, 415–418. [Google Scholar] [CrossRef]
- Parco, S.; Visconti, P.; Vascotto, F. Hematology point of care testing and laboratory errors: An example of multidisciplinary management at a children’s hospital in northeast Italy. J. Multidiscip. Healthc. 2014, 7, 45–50. [Google Scholar] [CrossRef]
- De la Salle, B. Pre- and postanalytical errors in haematology. Int. J. Lab. Hematol. 2019, 41 (Suppl. 1), 170–176. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, C.; Yıldız, U.; Akkaya, N.; Taneli, F. Preanalytical Phase Errors: Experience of a Central Laboratory. Cureus 2020, 12, e7335. [Google Scholar] [CrossRef] [PubMed]
- Mehndiratta, M.; Pasha, E.H.; Chandra, N.; Almeida, E.A. Quality Indicators for Evaluating Errors in the Preanalytical Phase. J. Lab. Physicians 2021, 13, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Upreti, S.; Upreti, S.; Bansal, R.; Jeelani, N.; Bharat, V. Types and Frequency of Preanalytical Errors in Haematology Lab. J. Clin. Diagn. Res. 2013, 7, 2491–2493. [Google Scholar] [CrossRef]
- Shukla, D.; Kanetkar, S.; Ingale, S. Study of Pre-Analytical and Post-Analytical Errors in Hematology Laboratory in A Tertiary Care Hospital. J. Med. Sci. Clin. Res. 2016, 4, 14964–14967. [Google Scholar] [CrossRef]
- Chandra, H.; Gupta, A.K.; Singh, N. Study of pre-analytical errors in haematology laboratory: A single-centre experience. J. Med. Evid. 2020, 1, 92–95. [Google Scholar] [CrossRef]
- West, J.; Atherton, J.; Costelloe, S.J.; Pourmahram, G.; Stretton, A.; Cornes, M. Preanalytical errors in medical laboratories: A review of the available methodologies of data collection and analysis. Ann. Clin. Biochem. 2016, 54, 14–19. [Google Scholar] [CrossRef]
- Green, S.F. The cost of poor blood specimen quality and errors in preanalytical processes. Clin. Biochem. 2013, 46, 1175–1179. [Google Scholar] [CrossRef]
- Puri, V.; Gaur, K.; Shukla, S.; Sharma, S.; Suman, S.; Singh, R. Finish before the start: Analyzing preanalytical sample errors in a tertiary care hematology laboratory. Indian J. Pathol. Microbiol. 2020, 63, 435–440. [Google Scholar] [CrossRef]
- Aggarwal, K.; Jhajharia, S.; Pradhan, T.; Acharya, V.; Patra, S.; Mahapatra, S.K. Analysis of Errors in a Clinical Laboratory of a Tertiary Care Hospital, Odisha, India. J. Clin. Diagn. Res. 2021, 15, 27–30. [Google Scholar] [CrossRef]
- Goswami, B.; Tayal, D.; Chawla, R.; Mallika, V. Pre-analytical factors that influence the interpretation of prothrombin time in the clinical laboratory: One year experience in a super speciality hospital in India. Clin. Chim. Acta 2009, 410, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Rachana, G.; Manjunatha, R. A study on identifying the pre-analytical phase errors leading to biochemistry and hematology sample rejection by using fmea & paretos principle. Int. J. Adv. Res. 2019, 7, 811–820. [Google Scholar] [CrossRef]
- Chen, B.H.; Fong, J.F.; Chiang, C.H. Effect of different anticoagulant, underfilling of blood sample and storage stability on selected hemogram. Kaohsiung J. Med. Sci. 1999, 15, 87–93. [Google Scholar] [PubMed]
- Ye, Y.; Wang, W.; Zhao, H.; He, F.; Zhong, K.; Yuan, S.; Du, Y.; Chen, B.; Wang, Z. Haematology specimen acceptability: A national survey in Chinese laboratories. Biochem. Med. 2018, 28, 030704. [Google Scholar] [CrossRef]
- Lippi, G.; Cervellin, G.; Mattiuzzi, C. Critical review and meta-analysis of spurious hemolysis in blood samples collected from intravenous catheters. Biochem. Med. 2013, 23, 193–200. [Google Scholar] [CrossRef]
- Ercan, M.; Akbulut, E.D.; Bayraktar, N.; Ercan, Ş. Effects of specimen haemolysis on complete blood count results by Abbott Alinity hq System. Biochem. Med. 2021, 31, 030706. [Google Scholar] [CrossRef]
- Vives-Corrons, J.-L.; Briggs, C.; Simon-Lopez, R.; Albarede, S.; De La Salle, B.; Flegar-Meatrii, Z.; Nazor, A.; Guyard, A.; Lipsic, T.; Nagai, Y.; et al. Effect of EDTA-anticoagulated whole blood storage on cell morphology examination. A need for standardization. Int. J. Lab. Hematol. 2014, 36, 222–226. [Google Scholar] [CrossRef]
- Pavani, B.; Sri Manvitha, V. Evaluation of errors in clinical hematology practice. MedPulse Int. J. Pathol. 2017, 4, 7–12. [Google Scholar] [CrossRef]
- Arslan, F.D.; Karakoyun, I.; Basok, B.I.; Aksit, M.Z.; Celik, E.; Dogan, K.; Duman, C. The Effects of Education and Training Given to Phlebotomists for Reducing Preanalytical Errors. J. Med. Biochem. 2018, 37, 172–180. [Google Scholar] [CrossRef]
- Hjelmgren, H.; Nilsson, A.; Myrberg, I.H.; Andersson, N.; Ygge, B.; Nordlund, B. Capillary blood sampling increases the risk of preanalytical errors in pediatric hospital care: Observational clinical study. J. Spéc. Pediatr. Nurs. 2021, 26, e12337. [Google Scholar] [CrossRef]
- Ashavaid, T.F.; Dandekar, S.P.; Khodaiji, S.; Ansari, M.H.; Singh, A.P. Influence of method of specimen collection on various preanalytical sample quality indicators in EDTA blood collected for cell counting. Indian J. Clin. Biochem. 2009, 24, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Le, R.D.; Melanson, S.E.F.; Petrides, A.K.; Goonan, E.M.; Bixho, I.; Landman, A.B.; Brogan, A.M.; Bates, D.W.; Tanasijevic, M.J. Significant Reduction in Preanalytical Errors for Nonphlebotomy Blood Draws After Implementation of a Novel Integrated Specimen Collection Module. Am. J. Clin. Pathol. 2016, 146, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yang, Y.C.; Huang, W.F.; Li, Y.F.; Song, P.; Chen, L.; Lan, Y. Reduction of preanalytical errors in laboratory by establishment and application of training system. J. Evid.-Based Med. 2014, 7, 258–262. [Google Scholar] [CrossRef]
| Preanalytical Variables | Number of Samples N (%) | |
|---|---|---|
| 1 | Inappropriate tube | 108 (12.19 %) |
| 2 | Clotted sample | 178 (20.09%) |
| 3 | Insufficient sample | 480 (54.18%) |
| 4 | Hemolyzed sample | 41 (4.63%) |
| 5 | Diluted sample | 16 (1.80%) |
| 6 | Excessive sample | 10 (1.13%) |
| 7 | Labeling error | 29 (3.27%) |
| 8 | Delay in sample transfer to lab | 20 (2.26%) |
| 9 | Empty tube/damaged tube | 4 (0.45%) |
| Total | 886 (100%) |
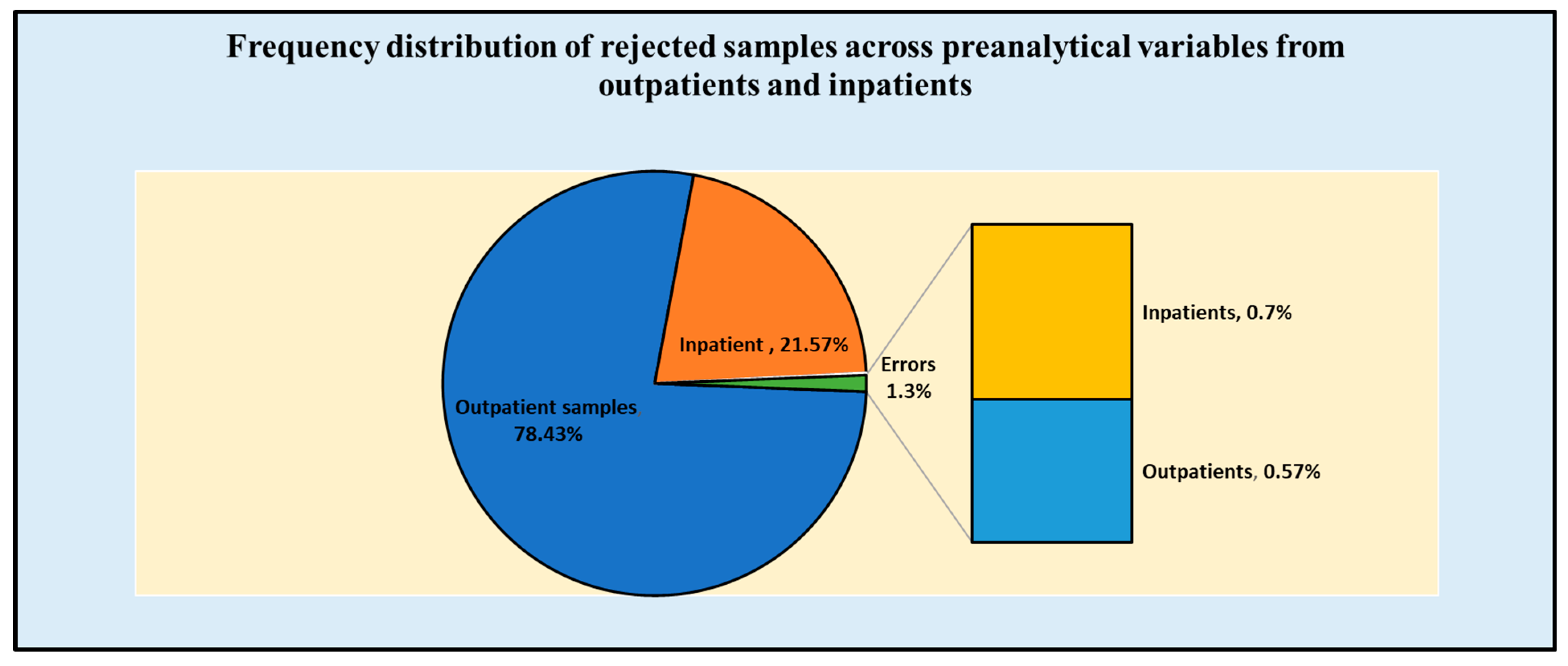
| Preanalytical Variables | Samples from Outpatients 53,249 (78.43%) | Samples from Inpatients 14,643 (21.57%) | Total Number of Hematology Samples 67,892 (100%) | |
|---|---|---|---|---|
| 1 | Inappropriate tube | 47 (12.08%) | 61 (12.27%) | 108 (12.18%) |
| 2 | Clotted sample | 81 (20.83%) | 97 (19.51%) | 178 (20.09%) |
| 3 | Insufficient sample | 213 (54.75%) | 267 (53.72%) | 480 (54.17%) |
| 4 | Hemolyzed sample | 24 (6.17%) | 17 (3.43%) | 41 (4.60%) |
| 5 | Diluted sample | 3 (0.77%) | 13 (2.62%) | 16 (1.80%) |
| 6 | Excessive sample | 7 (1.8%) | 3 (0.60%) | 10 (1.10%) |
| 7 | Labeling error | 11 (2.83%) | 18 (3.63%) | 29 (3.20%) |
| 8 | Delay in sample transfer to lab | 3 (0.77%) | 17 (3.42%) | 20 (2.20%) |
| 9 | Empty tube/damaged tube | 0 (0.00%) | 4 (0.80%) | 4 (0.40%) |
| Total | 389 (0.57%) | 497 (0.73%) | 886 (1.30%) |
| Inpatient Departments | Total | |||||
|---|---|---|---|---|---|---|
| Preanalytical Variables | Medicine | Surgery | Pediatrics | Emergency | ||
| 1 | Inappropriate tube | 15 | 15 | 2 | 29 | 61 |
| 2 | Clotted sample | 25 | 11 | 21 | 40 | 97 |
| 3 | Insufficient sample | 38 | 45 | 136 | 48 | 267 |
| 4 | Hemolyzed sample | 4 | 3 | 1 | 9 | 17 |
| 5 | Diluted sample | 1 | 2 | 10 | 0 | 13 |
| 6 | Excessive sample | 2 | 1 | 0 | 0 | 3 |
| 7 | Labeling error | 4 | 4 | 3 | 7 | 18 |
| 8 | Delay in sample transfer to lab | 5 | 6 | 6 | 0 | 17 |
| 9 | Empty tube/damaged tube | 1 | 2 | 1 | 0 | 4 |
| 95 (19.11%) | 89 (17.90%) | 180 (36.22%) | 133 (26.77%) | 497 (100%) | ||
| Study (Year) | Total Number of Samples | Total Errors/Error Frequency (%) | Most Common Error |
|---|---|---|---|
| Upreti et al. (2013) [15] | 135,808 | 1339 (1%) | Wrong label, insufficient sample |
| Narang et al. (2014) [4] | 471,006 | 1802 (0.38%) | Clotted, insufficient sample |
| Arul et al. (2018) [9] | 118,732 | 513 (0.43%) | Insufficient sample, clotted sample |
| Gaur et al. (2020) [20] | 189,104 | 4052 (2.14%) | Insufficient sample, clotted sample, |
| Present study | 67,892 | 886 (1.3%) | Insufficient sample, clotted sample |
| Arul et al. [9] | Gaur et al. [20] | Present Study | ||||
|---|---|---|---|---|---|---|
| Number of Samples (%) | Number of Samples (%) | Number of Samples (%) | ||||
| Outpatients | Inpatients | Outpatients | Inpatients | Outpatients | Inpatients | |
| Unlabeled/Misidentification | 9 (0.01) | 14 (0.02) | 27 (0.05) | 110 (0.08) | 11 (0.01) | 18 (0.02) |
| Incorrect vials | 28 (0.04) | 32 (0.06) | 5 (0.01) | 0 (0.01) | 47 (0.06) | 61 (0.08) |
| Inadequate samples | 104 (0.17) | 131 (0.23) | 422 (0.75) | 1695 (1.27) | 213 (0.30) | 267 (0.39) |
| Clotted sample | 67 (0.11) | 72 (0.13) | 815 (1.46) | 857 (0.64) | 79 (0.10) | 97 (0.14) |
| Diluted sample | 0 | 23 (0.04) | 4 (0.01) | 9 (0.01) | 3 (0.0) | 13 (0.01) |
| Hemolyzed sample | 15 (0.02) | 18 (0.03) | 20 (0.04) | 54 (0.04) | 24 (0.03) | 17 (0.02) |
| Excessive sample | - | - | 2 (0.00) | 2 (0) | 7 (0.0) | 3 (0.0) |
| Total error (%) | 0.43% | 2.33% | 1.30% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.S.; Tabassum, A.; Arbaeen, A.F.; Qasem, A.H.; Elshemi, A.G.; Almasmoum, H. Preanalytical Errors in a Hematology Laboratory: An Experience from a Tertiary Care Center. Diagnostics 2023, 13, 591. https://doi.org/10.3390/diagnostics13040591
Iqbal MS, Tabassum A, Arbaeen AF, Qasem AH, Elshemi AG, Almasmoum H. Preanalytical Errors in a Hematology Laboratory: An Experience from a Tertiary Care Center. Diagnostics. 2023; 13(4):591. https://doi.org/10.3390/diagnostics13040591
Chicago/Turabian StyleIqbal, Mohammad Shahid, Aisha Tabassum, Ahmad Fawzi Arbaeen, Ahmed H. Qasem, Adel G. Elshemi, and Hibah Almasmoum. 2023. "Preanalytical Errors in a Hematology Laboratory: An Experience from a Tertiary Care Center" Diagnostics 13, no. 4: 591. https://doi.org/10.3390/diagnostics13040591
APA StyleIqbal, M. S., Tabassum, A., Arbaeen, A. F., Qasem, A. H., Elshemi, A. G., & Almasmoum, H. (2023). Preanalytical Errors in a Hematology Laboratory: An Experience from a Tertiary Care Center. Diagnostics, 13(4), 591. https://doi.org/10.3390/diagnostics13040591






