Evaluation of the Efficacy of BBIBP-CorV Inactivated Vaccine Combined with BNT62b2 mRNA Booster Vaccine
Abstract
:1. Introduction
2. Subjects, Methods, and Study Design
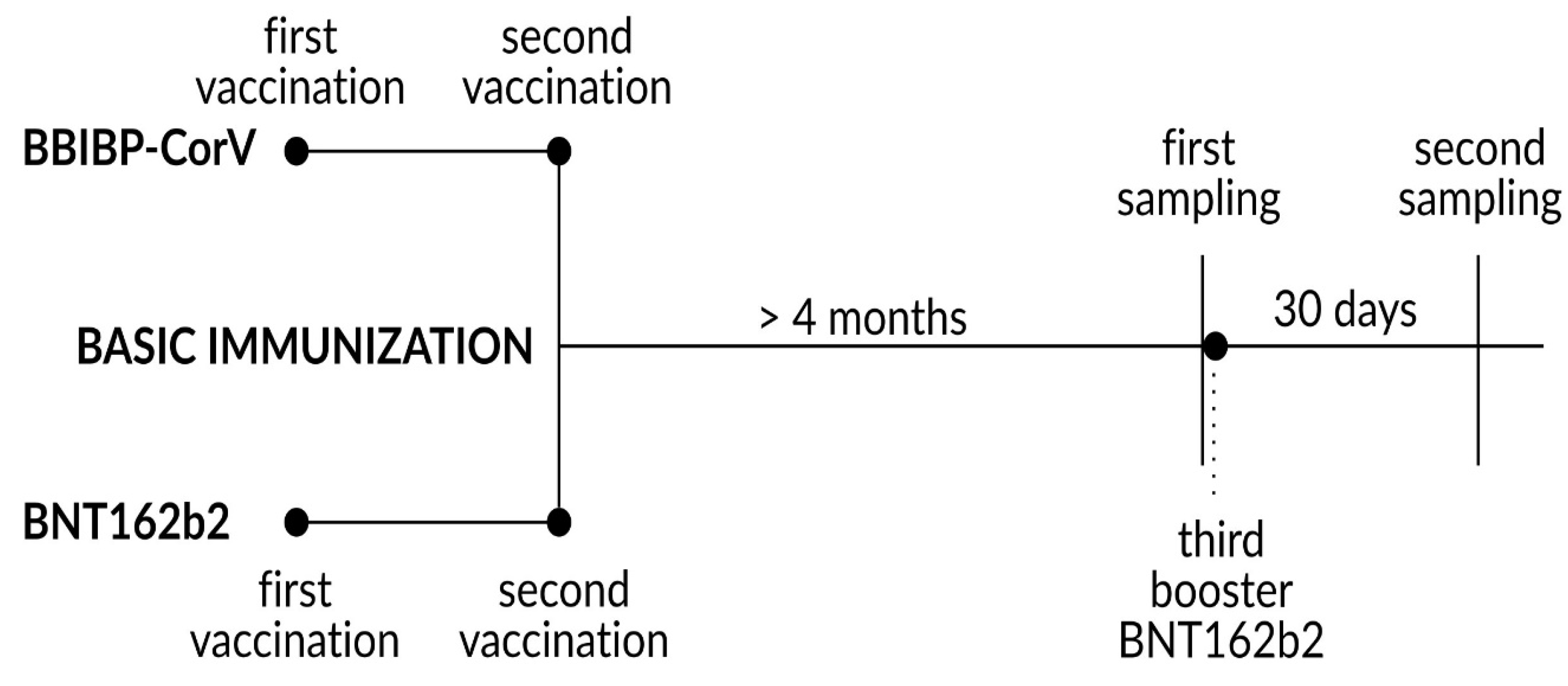
2.1. Study Design
2.2. Laboratory Methods
2.3. Statistical Analysis
3. Results
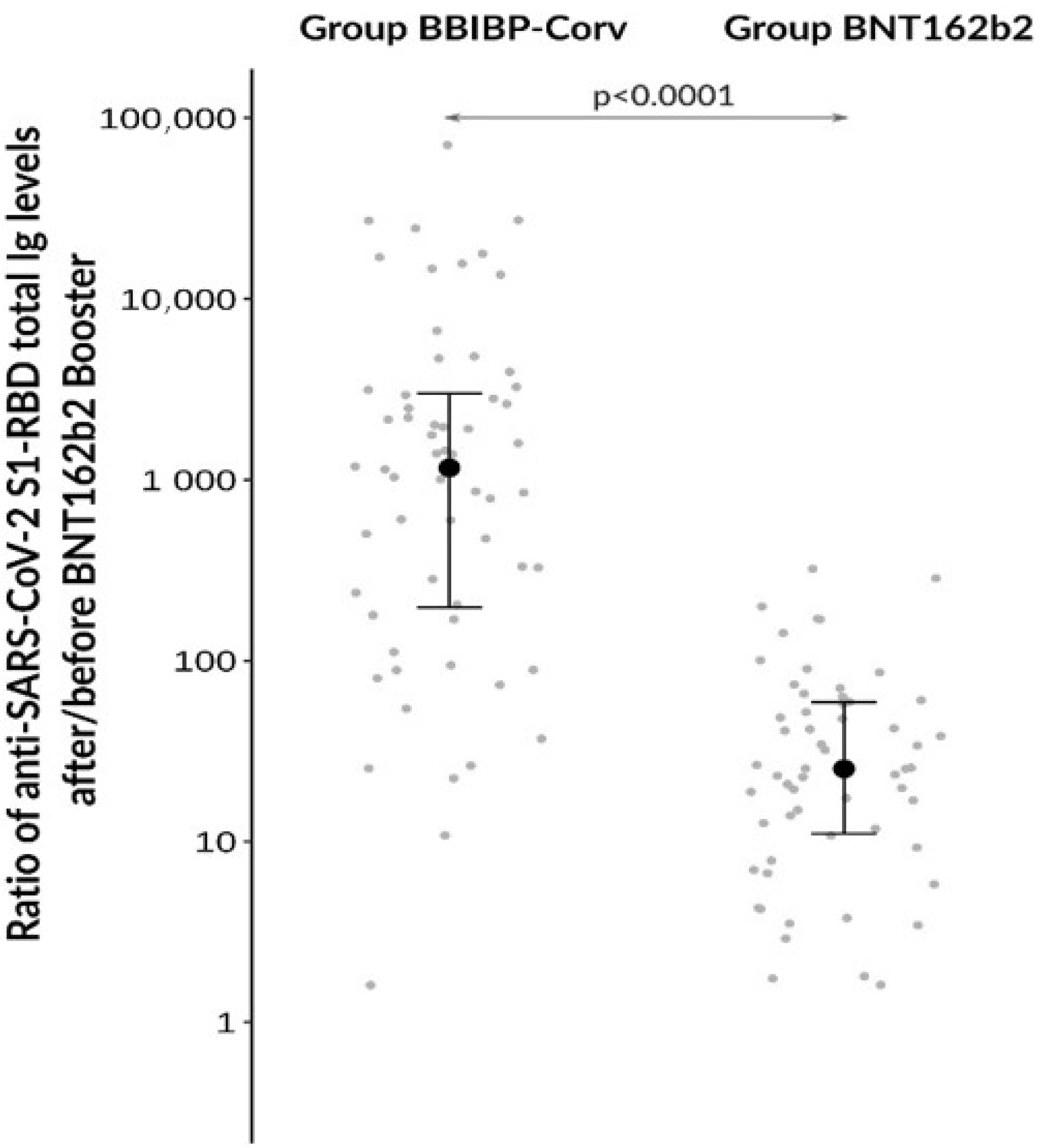
3.1. Antibody Responses after Different Booster Vaccination Regimens
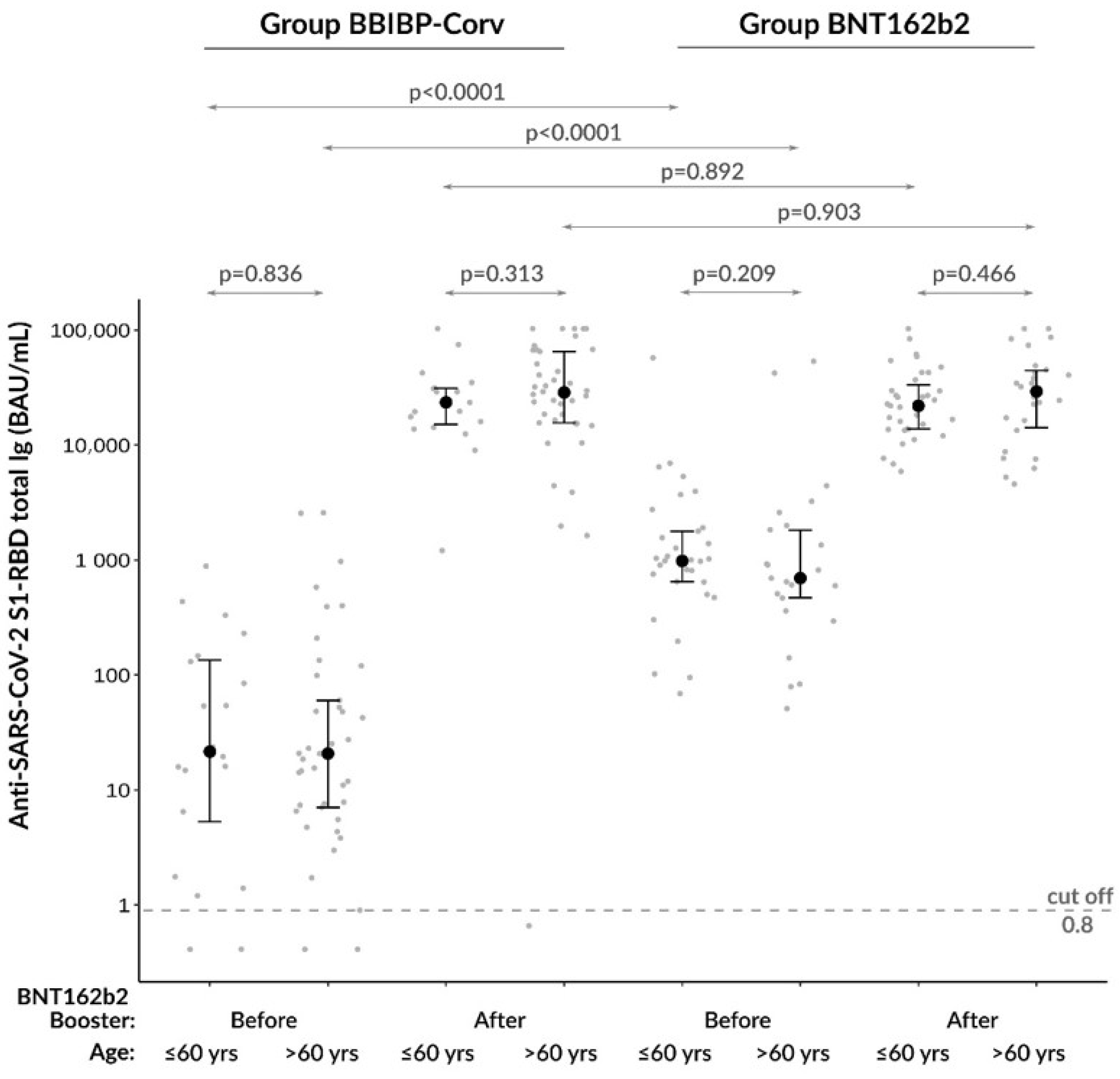
3.2. Comparison of Anti-SARS-CoV−2 Total Ig Levels between Age Groups
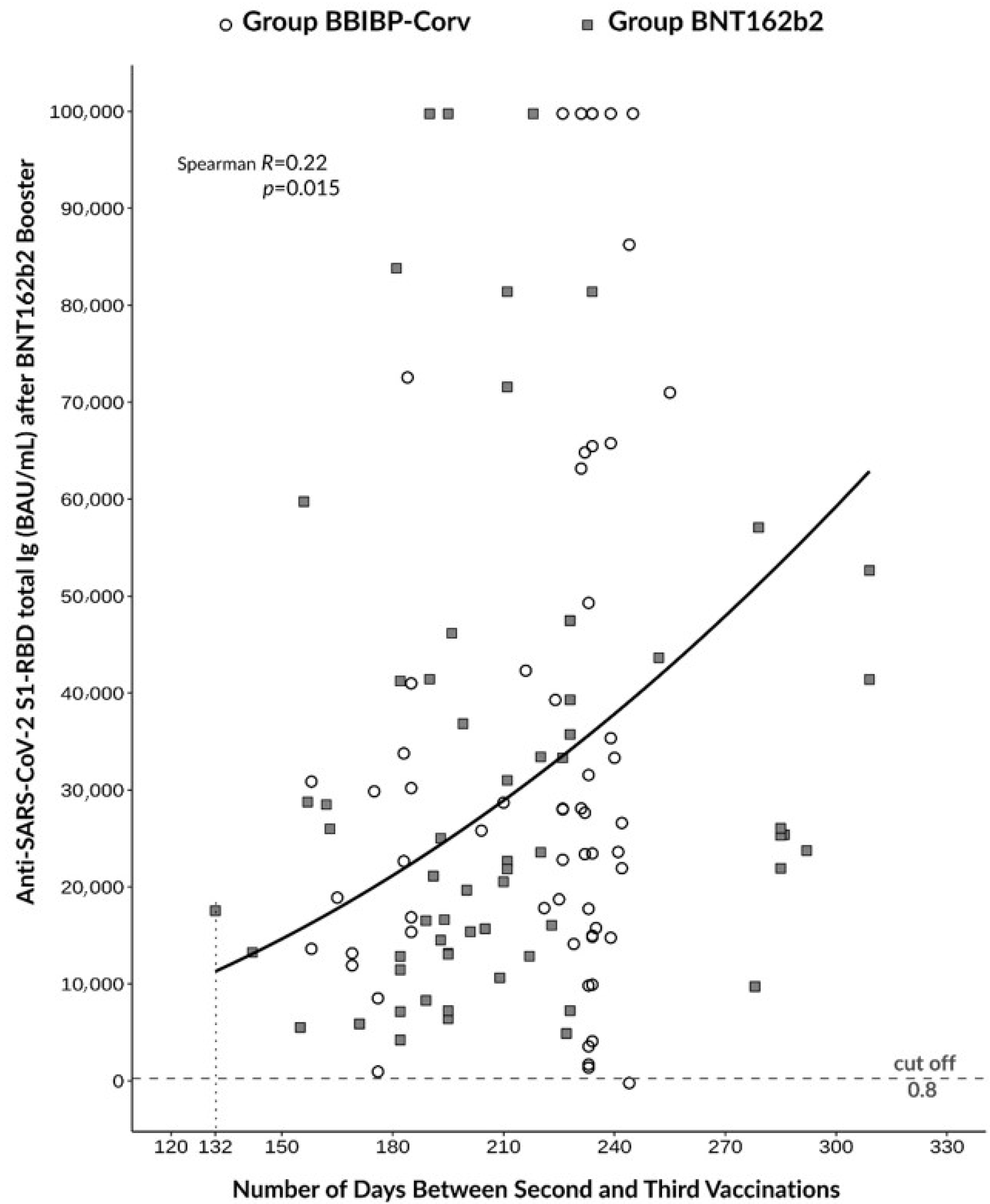
3.3. Correlation between Antibody Response and the Timing of Booster Immunization
3.4. Clinical Course of Vaccinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Coronavirus Pandemic Weekly Trends. 2022. Available online: https://www.worldometers.info/coronavirus/ (accessed on 5 January 2023).
- Plan of Tasks Related to COVID−19 Vaccination. 2021. Available online: http://koronavirus.gov.hu (accessed on 5 January 2023).
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV−2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Rearte, A.; Castelli, J.; Rearte, R.; Fuentes, N.; Pennini, V.; Pesce, M.; Barbeira, P.; Iummato, L.; Laurora, M.; Bartolomeu, M.; et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV−19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV−2 and death due to COVID−19 in people older than 60 years in Argentina: A test-negative, case-control, and retrospective longitudinal study. Lancet 2022, 399, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Alqassieh, R.; Suleiman, A.; Abu-Halaweh, S.; Santarisi, A.; Shatnawi, O.; Shdaifat, L.; Tarifi, A.; Al-Tamimi, M.; Al-Shudifat, A.; Alsmadi, H.; et al. Pfizer-BioNTech and Sinopharm: A Comparative Study on Post-Vaccination Antibody Titers. Vaccines 2021, 9, 1223. [Google Scholar] [CrossRef]
- Moghnieh, R.; Mekdashi, R.; El-Hassan, S.; Abdallah, D.; Jisr, T.; Bader, M.; Jizi, I.; Sayegh, M.; Bizri, A.R. Immunogenicity and reactogenicity of BNT162b2 booster in BBIBP-CorV-vaccinated individuals compared with homologous BNT162b2 vaccination: Results of a pilot prospective cohort study from Lebanon. Vaccine 2021, 39, 6713–6719. [Google Scholar] [CrossRef]
- Farid, E.; Herrera-Uribe, J.; Stevenson, N. The Effect of Age, Gender and Comorbidities Upon SARS-CoV−2 Spike Antibody Induction After Two Doses of Sinopharm Vaccine and the Effect of a Pfizer/BioNtech Booster Vaccine. Front. Immunol. 2022, 13, 817597. [Google Scholar] [CrossRef]
- Hueda-Zavaleta, M.; de la Torre, J.G.; Aguila, J.C.-D.; Muro-Rojo, C.; De La Cruz-Escurra, N.; Siles, D.A.; Minchon-Vizconde, D.; Copaja-Corzo, C.; Bardales-Silva, F.; Benites-Zapata, V.; et al. Evaluation of the Humoral Immune Response of a Heterologous Vaccination between BBIBP-CorV and BNT162b2 with a Temporal Separation of 7 Months, in Peruvian Healthcare Workers with and without a History of SARS-CoV−2 Infection. Vaccines 2022, 10, 502. [Google Scholar] [CrossRef]
- Park, S.; Gatchalian, K.; Oh, H. Association of Homologous and Heterologous Vaccine Boosters With SARS-CoV−2 Infection in BBIBP-CorV Vaccinated Healthcare Personnel. Cureus 2022, 14, e27323. [Google Scholar] [CrossRef]
- Vargas-Herrera, N.; Fernandez-Navarro, M.; Cabezudo, N.; Soto-Becerra, P.; Solis-Sanchez, G.; Escobar-Agreda, S.; Silva-Valencia, J.; Pampa-Espinoza, L.; Bado-Perez, R.; Solari, L.; et al. Immunogenicity and reactogenicity of a third dose of BNT162b2 vaccine for COVID−19 after a primary regimen with BBIBP-CorV or BNT162b2 vaccines in Lima, Peru. PLoS ONE 2022, 17, e0268419. [Google Scholar] [CrossRef]
- Kanokudom, S.; Chansaenroj, J.; Suntronwong, N.; Assawakosri, S.; Yorsaeng, R.; Nilyanimit, P.; Aeemjinda, R.; Khanarat, N.; Vichaiwattana, P.; Klinfueng, S.; et al. Safety and immunogenicity of a third dose of COVID−19 protein subunit vaccine (Covovax(TM)) after homologous and heterologous two-dose regimens. Int. J. Infect. Dis. 2022, 126, 64–72. [Google Scholar] [CrossRef]
- Pascuale, C.; Varese, A.; Ojeda, D.S.; Pasinovich, M.E.; Lopez, L.; Rossi, A.H.; Rodriguez, P.E.; Miglietta, E.A.; Laboratorio SeVa Group; Mazzitelli, I.; et al. Immunogenicity and reactogenicity of heterologous immunization against SARS CoV−2 using Sputnik V, ChAdOx1-S, BBIBP-CorV, Ad5-nCoV, and mRNA−1273. Cell Rep. Med. 2022, 3, 100706. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 5 January 2023).
- Nagy, B., Jr. Foreword: Current Laboratory Aspects of COVID−19. EJIFCC 2022, 33, 75–78. [Google Scholar]
- Muller, J.; Szucs-Farkas, D.; Szegedi, I.; Csoka, M.; Garami, M.; Tiszlavicz, L.; Hauser, P.; Krivan, G.; Csanadi, K.; Ottoffy, G.; et al. Clinical Course of COVID−19 Disease in Children Treated with Neoplastic Diseases in Hungary. Pathol. Oncol. Res. 2022, 28, 1610261. [Google Scholar] [CrossRef]
- Ali, H.; Alahmad, B.; Al-Shammari, A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.; Mohammad, A.; et al. Previous COVID−19 Infection and Antibody Levels After Vaccination. Front. Public Health 2021, 9, 778243. [Google Scholar] [CrossRef]
- Salvagno, G.; Henry, B.; di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.; Lippi, G. Anti-SARS-CoV−2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID−19 mRNA BNT162b2 Vaccination. Diagnostics 2021, 11, 832. [Google Scholar] [CrossRef]
- Salvagno, G.; Henry, B.; Pighi, L.; De Nitto, S.; Lippi, G. Variation of Total Anti-SARS-CoV−2 Antibodies After Primary BNT162b2 Vaccination and Homologous Booster. EJIFCC 2022, 33, 166–174. [Google Scholar]
- Urlaub, D.; Wolfsdorff, N.; Durak, D.; Renken, F.; Watzl, C. SARS-CoV−2 infection shortly after BNT162b2 vaccination results in high anti-spike antibody levels in nursing home residents and staff. Immun. Inflamm. Dis. 2021, 9, 1702–1706. [Google Scholar] [CrossRef]
- Boshku, A.; Aleksovski, V.; Tofoski, G.; Spasova, R. Kinetics of Antibody Response to Repeated Vaccination with Sputnik V: A Pilot Study with a Series of Five Cases. EJIFCC 2022, 33, 175–186. [Google Scholar]
- Fodor, E.; Olmos Calvo, I.; Kuten-Pella, O.; Hamar, E.; Bukva, M.; Madár, Á.; Hornyák, I.; Hinsenkamp, A.; Hetényi, R.; Földes, F.; et al. Comparison of immune activation of the COVID vaccines: ChAdOx1, BNT162b2, mRNA−1273, BBIBP-CorV, and Gam-COVID-Vac from serological human samples in Hungary showed higher protection after mRNA-based immunization. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5297–5306. [Google Scholar]
- Aijaz, J.; Hussain, S.; Naseer, F.; Kanani, F.; Anis, S.; Sarfaraz, S.; Saeed, S.; Farooq, H.; Jamal, S. Neutralizing Antibody Response to BBIBP-CorV in Comparison with COVID−19 Recovered, Unvaccinated Individuals in a Sample of the Pakistani Population. Vaccines 2022, 10, 692. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Oulhaj, A.; Ganesan, S.; Al Hosani, F.; Najim, O.; Ibrahim, H.; Acuna, J.; Alsuwaidi, A.; Kamour, A.; Alzaabi, A.; et al. Effectiveness of BBIBP-CorV vaccine against severe outcomes of COVID−19 in Abu Dhabi, United Arab Emirates. Nat. Commun. 2022, 13, 3215. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Suntronwong, N.; Kanokudom, S.; Assawakosri, S.; Yorsaeng, R.; Vichaiwattana, P.; Klinfueng, S.; Wongsrisang, L.; Srimuan, D.; Thatsanatorn, T.; et al. Immunogenicity Following Two Doses of the BBIBP-CorV Vaccine and a Third Booster Dose with a Viral Vector and mRNA COVID−19 Vaccines against Delta and Omicron Variants in Prime Immunized Adults with Two Doses of the BBIBP-CorV Vaccine. Vaccines 2022, 10, 1071. [Google Scholar] [CrossRef]
- Gonzalez, S.; Olszevicki, S.; Gaiano, A.; Baino, A.; Regairaz, L.; Salazar, M.; Pesci, S.; Marin, L.; Martinez, V.; Varela, T.; et al. Effectiveness of BBIBP-CorV, BNT162b2 and mRNA−1273 vaccines against hospitalisations among children and adolescents during the Omicron outbreak in Argentina: A retrospective cohort study. Lancet Reg. Health Am. 2022, 13, 100316. [Google Scholar] [CrossRef] [PubMed]
- Kiss, Z.; Wittmann, I.; Polivka, L.; Surjan, G.; Surjan, O.; Barcza, Z.; Molnar, G.; Nagy, D.; Muller, V.; Bogos, K.; et al. Nationwide Effectiveness of First and Second SARS-CoV2 Booster Vaccines During the Delta and Omicron Pandemic Waves in Hungary (HUN-VE 2 Study). Front. Immunol. 2022, 13, 905585. [Google Scholar] [CrossRef] [PubMed]
- Voko, Z.; Kiss, Z.; Surjan, G.; Surjan, O.; Barcza, Z.; Wittmann, I.; Molnar, G.; Nagy, D.; Muller, V.; Bogos, K.; et al. Effectiveness and Waning of Protection with Different SARS-CoV−2 Primary and Booster Vaccines During the Delta Pandemic Wave in 2021 in Hungary (HUN-VE 3 Study). Front. Immunol. 2022, 13, 919408. [Google Scholar] [CrossRef] [PubMed]
- Voko, Z.; Kiss, Z.; Surjan, G.; Surjan, O.; Barcza, Z.; Palyi, B.; Formanek-Balku, E.; Molnar, G.; Herczeg, R.; Gyenesei, A.; et al. Nationwide effectiveness of five SARS-CoV−2 vaccines in Hungary-the HUN-VE study. Clin. Microbiol. Infect. 2022, 28, 398–404. [Google Scholar] [CrossRef]
- Annexes to WHO Interim Recommendations for Use of the COVID−19 Vaccine BIBP. 2022. Available online: https://www.who.int/publications/i/item/WHO−2019-nCoV-vaccines-SAGE-recommendation-BIBP-annexes (accessed on 5 January 2023).
- Horváth, J.; Ferenci, T.; Ferenczi, A.; Túri, G.; Röst, G.; Oroszi, B. Real-time monitoring of the effectiveness of six COVID−19 vaccines in Hungary in 2021 using the screening method. BMC Med. 2022, 20, 312. [Google Scholar]
- Blanco, S.; Spinsanti, L.; Aguilar, J.J.; Diaz, A.; Rivarola, M.E.; Beranek, M.; Fernandez, E.; Mangeaud, A.; Konigheim, B.S.; Gallego, S.V. Neutralizing response elicited by homologous and heterologous prime booster vaccination against ancestral SARS-CoV−2 B.1, P.1, C.37 and B.1.617.2 variants. Vaccine 2022, 40, 6706–6710. [Google Scholar] [CrossRef]
- Ali, H.; Alterki, A.; Sindhu, S.; Alahmad, B.; Hammad, M.; Al-Sabah, S.; Alghounaim, M.; Jamal, M.; Aldei, A.; Mairza, M.; et al. Robust Antibody Levels in Both Diabetic and Non-Diabetic Individuals After BNT162b2 mRNA COVID−19 Vaccination. Front. Immunol. 2021, 12, 752233. [Google Scholar] [CrossRef]
- Barriere, J.; Carles, M.; Audigier-Valette, C.; Re, D.; Adjtoutah, Z.; Seitz-Polski, B.; Gounant, V.; Descamps, D.; Zalcman, G. Third dose of anti-SARS-CoV−2 vaccine for patients with cancer: Should humoral responses be monitored? A position article. Eur. J. Cancer 2022, 162, 182–193. [Google Scholar] [CrossRef]
- Etemadifar, M.; Sedaghat, N.; Nouri, H.; Lotfi, N.; Chitsaz, A.; Khorvash, R.; Zolfaghari, H.; Movaghar, A.G.; Pourabbas, M.; Salari, M. SARS-CoV−2 serology among people with multiple sclerosis on disease-modifying therapies after BBIBP-CorV (Sinopharm) inactivated virus vaccination: Same story, different vaccine. Mult. Scler. Relat. Disord. 2022, 57, 103417. [Google Scholar] [CrossRef]

| All Patients | BBIBP-CorV Group | BNT162b2 Group | p | |
|---|---|---|---|---|
| All patients (n) | 122 | 61 | 61 | |
| Age [year] [mean (SD)] | 61.9 (12.87) | 63.9 (12.61) | 59.9 (12.92) | 0.090 |
| Gender [female (%)] | 80 (65.6) | 39 (62.9) | 41 (67.2) | 0.849 |
| All comorbidities [n (%)] | 90 (73.8) | 45 (73.8) | 45 (73.8) | ~1.0 |
| Cardiovascular [n (%)] | 86 (70.5) | 43 (70.5) | 43 (70.5) | ~1.0 |
| Autoimmune [n (%)] | 18 (14.8) | 9 (14.8) | 9 (14.8) | ~1.0 |
| Respiratory [n (%)] | 13 (10.7) | 7 (11.5) | 6 (9.8) | ~1.0 |
| Renal [n (%)] | 6 (4.9) | 3 (4.9) | 3 (4.9) | ~1.0 |
| Diabetes mellitus [n (%)] | 23 (18.9) | 13 (21.3) | 10 (16.4) | 0.643 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rákóczi, É.; Magócs, G.; Kovács, S.; Nagy, B., Jr.; Szűcs, G.; Szekanecz, Z. Evaluation of the Efficacy of BBIBP-CorV Inactivated Vaccine Combined with BNT62b2 mRNA Booster Vaccine. Diagnostics 2023, 13, 556. https://doi.org/10.3390/diagnostics13030556
Rákóczi É, Magócs G, Kovács S, Nagy B Jr., Szűcs G, Szekanecz Z. Evaluation of the Efficacy of BBIBP-CorV Inactivated Vaccine Combined with BNT62b2 mRNA Booster Vaccine. Diagnostics. 2023; 13(3):556. https://doi.org/10.3390/diagnostics13030556
Chicago/Turabian StyleRákóczi, Éva, Gusztáv Magócs, Sára Kovács, Béla Nagy, Jr., Gabriella Szűcs, and Zoltán Szekanecz. 2023. "Evaluation of the Efficacy of BBIBP-CorV Inactivated Vaccine Combined with BNT62b2 mRNA Booster Vaccine" Diagnostics 13, no. 3: 556. https://doi.org/10.3390/diagnostics13030556
APA StyleRákóczi, É., Magócs, G., Kovács, S., Nagy, B., Jr., Szűcs, G., & Szekanecz, Z. (2023). Evaluation of the Efficacy of BBIBP-CorV Inactivated Vaccine Combined with BNT62b2 mRNA Booster Vaccine. Diagnostics, 13(3), 556. https://doi.org/10.3390/diagnostics13030556







