The Role of Nasal Cytology and Serum Atopic Biomarkers in Paediatric Rhinitis
Abstract
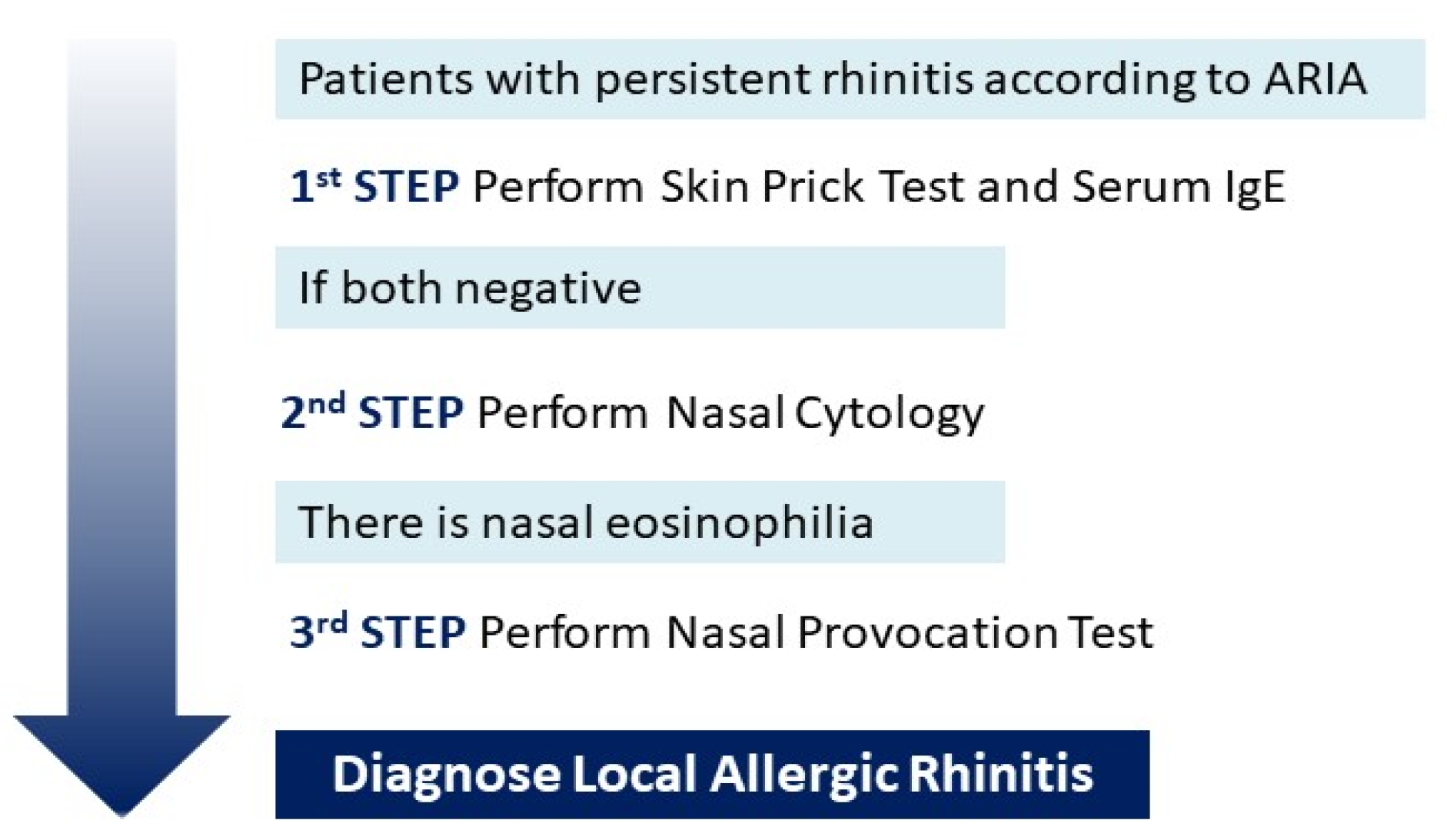
1. Introduction
2. Materials and Methods
- −
- mild symptoms: overall score from 0 to 5,
- −
- moderate symptoms: overall score from 6 to 15,
- −
- severe symptoms: overall score from 16 to 25.
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, C.H.M.; da Silva, T.E.; da Morales, N.M.O.; Fernandes, K.P.; Pinto, R.M.C. Quality of life in children and adolescents with allergic rhinitis. Braz. J. Otorhinolaryngol. 2009, 75, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Hellings, P.W.; Klimek, L.; Cingi, C.; Agache, I.; Akdis, C.; Bachert, C.; Bousquet, J.; Demoly, P.; Gevaert, P.; Hox, V.; et al. Non-allergic rhinitis: Position paper of the European Academy of Allergy and Clinical Immunology. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Xatzipsalti, M.; Borrego, L.M.; Custovic, A.; Halken, S.; Hellings, P.W. Paediatric rhinitis: Position paper of the European Academy of Allergy and Clinical Immunology. Allergy 2013, 68, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Gourgiotis, D.; Papadopoulos, N.G.; Bossios, A.; Zamanis, P.; Saxoni-Papageorgiou, P. Immune modulator pidotimod decreases the in vitro expression of CD30 in peripheral blood mononuclear cells of atopic asthmatic and normal children. J. Asthma 2004, 41, 285–287. [Google Scholar] [CrossRef]
- Augé, J.; Vent, J.; Agache, I.; Airaksinen, L.; Mozo, P.C.; Chaker, A.; Cingi, C.; Durham, S.; Fokkens, W.; Gevaert, P.; et al. EAACI Position paper on the standardization of nasal allergen challenges. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 1597–1608. [Google Scholar] [CrossRef]
- Greiner, A.N.; Hellings, P.W.; Rotiroti, G.; Scadding, G.K. Allergic rhinitis. Lancet 2011, 378, 2112–2122. [Google Scholar] [CrossRef]
- Eifan, A.O.; Durham, S.R. Pathogenesis of rhinitis. Clin. Exp. Allergy 2016, 46, 1139–1151. [Google Scholar] [CrossRef]
- Mierzejewska, A.; Jung, A.; Kalicki, B. Nasal Cytology as a Marker of Atopy in Children. Dis. Markers 2017, 2017, 4159251. [Google Scholar] [CrossRef]
- Pal, I.; Babu, A.S.; Halder, I.; Kumar, S. Nasal smear eosinophils and allergic rhinitis. Ear Nose Throat J. 2017, 96, 17–22. [Google Scholar] [CrossRef]
- Bousquet, J.J.; Schünemann, H.J.; Togias, A.; Erhola, M.; Hellings, P.W.; Zuberbier, T.; Agache, I.; Ansotegui, I.J.; Anto, J.M.; Bachert, C.; et al. Next-generation ARIA care pathways for rhinitis and asthma: A model for multimorbid chronic diseases. Clin. Transl. Allergy 2019, 9, 44. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Bernstein, J.A.; Demoly, P.; Dykewicz, M.; Fokkens, W.; Hellings, P.W.; Peters, A.T.; Rondon, C.; Togias, A.; Cox, L.S. Phenotypes and endotypes of rhinitis and their impact on management: A PRACTALL report. Allergy 2015, 70, 474–494. [Google Scholar] [CrossRef]
- Rondón, C.; Canto, G.; Blanca, M. Local allergic rhinitis: A new entity, characterization and further studies. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 1–7. [Google Scholar] [CrossRef]
- Heffler, E.; Landi, M.; Caruso, C.; Fichera, S.; Gani, F.; Guida, G.; Liuzzo, M.T.; Pistorio, M.P.; Pizzimenti, S.; Riccio, A.M.; et al. Nasal cytology: Methodology with application to clinical practice and research. Clin. Exp. Allergy 2018, 48, 1092–1106. [Google Scholar] [CrossRef]
- Tantilipikorn, P.; Vichyanond, P.; Lacroix, J.S. Nasal provocation test: How to maximize its clinical use? Asian Pac. J. Allergy Immunol. 2010, 28, 225–231. [Google Scholar]
- Colavita, L.; Catalano, N.; Sposito, G.; Loddo, S.; Galletti, B.; Salpietro, C.; Galletti, F.; Cuppari, C. Local Allergic Rhinitis in Pediatric Patients: Is IgE Dosage in Nasal Lavage Fluid a Useful Diagnostic Method in Children? Int. J. Mol. Cell Med. 2017, 6, 174–182. [Google Scholar] [CrossRef]
- Phothijindakul, N.; Chusakul, S.; Aeumjaturapat, S.; Snidvongs, K.; Kanjanaumporn, J.; Ruangritchankul, K.; Phannaso, C. Nasal Cytology as a Diagnostic Tool for Local Allergic Rhinitis. Am. J. Rhinol. Allergy 2019, 33, 540–544. [Google Scholar] [CrossRef]
- Pipolo, C.; Bianchini, S.; Barberi, S. Nasal cytology in children: Scraping or swabbing? Rhinology 2017, 55, 242–250. [Google Scholar] [CrossRef]
- Provero, M.C.; Macchi, A.; Antognazza, S.; Marinoni, M.; Nespoli, L. Allergic and nonallergic rhinitis in children: The role of nasal cytology. Open J. Pediatr. 2013, 2013, 133–138. [Google Scholar] [CrossRef]
- Gelardi, M.; Iannuzzi, L.; Quaranta, N.; Landi, M.; Passalacqua, G. NASAL cytology: Practical aspects and clinical relevance. Clin. Exp. Allergy. 2016, 46, 785–792. [Google Scholar] [CrossRef]
- Campo, P.; Salas, M. Local Allergic Rhinitis. Immunol. Allergy Clin. N. Am. 2016, 36, 321–332. [Google Scholar] [CrossRef]
- Rondón, C.; Campo, P.; Togias, A.; Fokkens, W.J.; Durham, S.R.; Powe, D.G.; Mullol, J.; Blanca, M. Local allergic rhinitis: Concept, pathophysiology, and management. J. Allergy Clin. Immunol. 2012, 129, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Del Giudice, A.M.; Fiorella, M.; Fiorella, R.; Russo, C.; Soleti, P.; Di Gioacchino, M.; Ciprandi, G. Non-allergic rhinitis with eosinophils and mast cells constitutes a new severe nasal disorder. Int. J. Immunopathol. Pharmacol. 2008, 21, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.K.; Keith, P.K. Nonallergic rhinitis with eosinophilia syndrome. Curr. Allergy Asthma Rep. 2006, 6, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Van Cauwenberge, P.; Khaltaev, N.; Aria Workshop Group; World Health Organization. Allergic rhinitis and its impact on asthma. J. Allergy Clin. Immunol. 2001, 108, S147–S334. [Google Scholar] [CrossRef] [PubMed]
- Wittig, H.J.; Belloit, J.; De Fillippi, I.; Royal, G. Age-related serum immunoglobulin E levels in healthy subjects and in patients with allergic disease. J. Allergy Clin. Immunol. 1980, 66, 305–313. [Google Scholar] [CrossRef]
- Kovalszki, A.; Weller, P.F. Eosinophilia. Prim. Care. 2016, 43, 607–617. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Blaiss, M.S.; Derebery, M.J.; Mahr, T.A.; Gordon, B.R.; Sheth, K.K.; Simmons, A.L.; Wingertzahn, M.A.; Boyle, J.M. Burden of allergic rhinitis: Results from the pediatric allergies in America survey. J. Allergy Clin. Immunol. 2009, 124, S43–S70. [Google Scholar] [CrossRef]
- Devillier, P.; Bousquet, P.J.; Grassin-Delyle, S.; Salvator, H.; Demoly, P.; Bousquet, J.; de Beaumont, O. Comparison of outcome measures in allergic rhinitis in children, adolescents and adults. Pediatr. Allergy Immunol. 2016, 27, 375–381. [Google Scholar] [CrossRef]
- Jáuregui, I.; Dávila, I.; Sastre, J.; Bartra, J.; del Cuvillo, A.; Ferrer, M.; Montoro, J.; Mullol, J.; Molina, X.; Valero, A. Validation of ARIA (Allergic Rhinitis and its Impact on Asthma) classification in a pediatric population: The PEDRIAL study. Pediatr. Allergy Immunol. 2011, 22, 388–392. [Google Scholar] [CrossRef]
- Campo, P.; Rondón, C.; Gould, H.J.; Barrionuevo, E.; Gevaert, P.; Blanca, M. Local IgE in nonallergic rhinitis. Clin. Exp. Allergy 2015, 45, 872–881. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Freedman, P.M.; Boswell, R.N. Nonallergic rhinitis with eosinophilia (NARES syndrome): Clinical and immunologic presentation. J. Allergy Clin. Immunol. 1981, 67, 253–262. [Google Scholar] [CrossRef]
- Bachert, C. Persistent rhinitis—Allergic or nonallergic? Allergy 2004, 59, 11–15. [Google Scholar] [CrossRef]
- Schroer, B.; Pien, L.C. Nonallergic rhinitis: Common problem, chronic symptoms. Cleve Clin. J. Med. 2012, 79, 285–293. [Google Scholar] [CrossRef]
- Settipane, R.A.; Lieberman, P. Update on nonallergic rhinitis. Ann. Allergy Asthma Immunol. 2001, 86, 494–507. [Google Scholar] [CrossRef]
- Bachert, C.; Von Bruaene, N.; Toskala, E.; Zhang, N.; Olze, H.; Scadding, G.; Van Drunen, C.M.; Mullol, J.; Cardell, L.; Gevaert, P.; et al. Important research questions in allergy and related diseases: 3-chronic rhinosinusitis and nasal polyposis—A GALEN study. Allergy 2009, 64, 520–533. [Google Scholar] [CrossRef]
- Moneret-Vautrin, D.A.; Hsieh, V.; Wayoff, M.; Guyot, J.L.; Mouton, C.; Maria, Y. Nonallergic rhinitis with eosinophilia syndrome a precursor of the triad: Nasal polyposis, intrinsic asthma, and intolerance to aspirin. Ann. Allergy 1990, 64, 513–518. [Google Scholar]
- Wallace, D.V.; Dykewicz, M.S.; Bernstein, D.I.; Blessing-Moore, J.; Cox, L.; Khan, D.A.; Lang, D.M.; Nicklas, R.A.; Oppenheimer, J.; Portnoy, J.M. The diagnosis and management of rhinitis: An updated practice parameter. J. Allergy Clin. Immunol. 2008, 122, S1–S84. [Google Scholar] [CrossRef]
- Rondón, C.; Fernández, J.; López, S.; Campo, P.; Doña, I.; Torres, M.J.; Mayorga, C.; Blanca, M. Nasal inflammatory mediators and spe cific IgE production after nasal challenge with grass pollen in local allergic rhinitis. J. Allergy Clin. Immunol. 2009, 124, 1005–1011.e1. [Google Scholar] [CrossRef]
- Nozad, C.H.; Michael, L.M.; Lew, D.B.; Michael, C.F. Non-allergic rhinitis: A case report and review. Clin. Mol. Allergy 2010, 8, 1. [Google Scholar] [CrossRef]
- Meng, Y.; Lou, H.; Wang, Y.; Wang, X.; Cao, F.; Wang, K.; Chu, X.; Wang, C.; Zhang, L. Endotypes of chronic rhinitis: A cluster analysis study. Allergy 2019, 74, 720–730. [Google Scholar] [CrossRef]
- Zidarn, M.; Zuberbier, T.; Schünemann, H.J. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar]
- Ruttanaphol, S. Clinical efficacy of nasal steroids on nonallergic rhinitis and the associated inflammatory cell phenotypes. Am. J. Rhinol. Allergy 2015, 29, 343–349. [Google Scholar] [CrossRef]
- Patrascu, E.; Posdru, S.; Sarafoleanu, C. Nasal cytology assessment after topical intranasal corticosteroids therapy in allergic rhinitis. Rom. J. Rhinol. 2014, 4, 191–199. [Google Scholar]
- Ellis, A.K.; Keith, P.K. Nonallergic rhinitis with eosinophilia syndrome and related disorders. Clin. Allergy Immunol. 2007, 19, 87–100. [Google Scholar]
- Sur, D.K.C.; Plesa, M.L. Chronic Nonallergic Rhinitis. Am. Fam Physician 2018, 98, 171–176. [Google Scholar]
- Fokkens, W.J. Thoughts on the pathophysiology of nonallergic rhinitis. Curr. Allergy Asthma Rep. 2002, 2, 203–209. [Google Scholar] [CrossRef]
- Purello-D’Ambrosio, F.; Isola, S.; Ricciardi, L.; Gangemi, S.; Barresi, L.; Bagnato, G.F. A controlled study on the effectiveness of loratadine in combination with flunisolide in the treatment of nonallergic rhinitis with eosinophilia (NARES). Clin. Exp. Allergy 1999, 29, 1143–1147. [Google Scholar] [CrossRef]
- Carosso, A.; Bugiani, M.; Migliore, E.; Antò, J.M.; DeMarco, R. Reference values of total serum IgE and their significance in the diagnosis of allergy in young European adults. Int. Arch. Allergy Immunol. 2007, 142, 230–238. [Google Scholar] [CrossRef]
- Sacco, C.; Perna, S.; Vicari, D.; Alfò, M.; Bauer, C.P.; Hoffman, U.; Forster, J.; Zepp, F.; Schuster, A.; Wahn, U.; et al. Growth curves of “normal” serum total IgE levels throughout childhood: A quantile analysis in a birth cohort. Pediatr. Allergy Immunol. 2017, 28, 525–534. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, S.; Liu, P.; Mu, Z.; Zhang, J. Clinical relevance of eosinophils, basophils, serum total IgE level, allergen-specific IgE, and clinical features in atopic dermatitis. J. Clin. Lab. Anal. 2020, 34, e23214. [Google Scholar] [CrossRef]
- Sharma, S.; Kathuria, P.C.; Gupta, C.K.; Nordling, K.; Ghosh, B.; Singh, A.B. Total serum immunoglobulin E levels in a case-control study in asthmatic/allergic patients, their family members, and healthy subjects from India. Clin. Exp. Allergy 2006, 36, 1019–1027. [Google Scholar] [CrossRef]
- Tu, Y.L.; Chang, S.W.; Tsai, H.J.; Chen, L.C.; Lee, W.I.; Hua, M.C.; Cheng, J.H.; Ou, L.S.; Yeh, K.W.; Huang, J.L.; et al. Total serum IgE in a population-based study of Asian children in Taiwan: Reference value and significance in the diagnosis of allergy. PLoS ONE 2013, 8, e80996. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Bockelbrink, A.; Grüber, C.; Keil, T.; Hamelmann, E.; Wahn, U.; Lau, S. Longitudinal trends of total and allergen-specific IgE throughout childhood. Allergy Eur. J. Allergy Clin. Immunol. 2009, 64, 1093–1098. [Google Scholar] [CrossRef]
- De Schryver, E.; Devuyst, L.; Derycke, L.; Dullaers, M.; Van Zele, T.; Bachert, C.; Gevaert, P. Local immunoglobulin e in the nasal mucosa: Clinical implications. Allergy Asthma Immunol. Res. 2015, 7, 321–331. [Google Scholar] [CrossRef]
- Ota, Y.; Ikemiyagi, Y.; Sato, T.; Funakoshi, T.; Hiruta, N.; Kitamura, M.; Bujo, H.; Suzuki, M. Measuring local immunoglobulin E in the inferior turbinate nasal mucosa in patients with allergic rhinitis. Allergol. Int. 2016, 65, 396–399. [Google Scholar] [CrossRef]
- Bachert, C.; Gevaert, P.; Holtappels, G.; Johansson, S.G.; van Cauwenberge, P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J. Allergy Clin. Immunol. 2001, 107, 607–614. [Google Scholar] [CrossRef]
- Badar, A.; Salem, A.M.; Bamosa, A.O.; Qutub, H.O.; Gupta, R.K.; Siddiqui, I.A. Association Between FeNO, Total Blood IgE, Peripheral Blood Eosinophil and Inflammatory Cytokines in Partly Controlled Asthma. J. Asthma Allergy 2020, 13, 533–543. [Google Scholar] [CrossRef]
- Small, P.; Kim, H. Allergic rhinitis. Allergy Asthma Clin. Immunol. 2011, 7, S3. [Google Scholar] [CrossRef]
- Sonawane, R.; Ahire, N.; Patil, S.; Korde, A. Study of eosinophil count in nasal and blood smear in allergic respiratory diseases. MVP J. Med. Sci. 2016, 3, 44–51. [Google Scholar] [CrossRef]
- Perić, A.; Vojvodić, D.; Vukomanović-Đurđević, B.; Baletić, N. Eosinophilic inflammation in allergic rhinitis and nasalpolyposis. Arh. Hig. Rada Tokiskol. 2011, 62, 341–348. [Google Scholar] [CrossRef]
- Demirjian, M.; Rumbyrt, J.S.; Gowda, V.C.; Klaustermeyer, W.B. Serum IgE and eosinophil count in allergic rhinitis—Analysis using a modified Bayes’ theorem. Allergol. Immunopathol. 2012, 40, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Brakhas, S.A.; Atia, M.R.; Aziz, Y.J.; Al-Sharqi, S.A.H. Study oftotal IgE levels and eosinophil count according to age and genderin patients with allergic rhinitis. World J. Pharm. Res. 2016, 4, 295–303. [Google Scholar]
- Ishitoya, J.; Sakuma, Y.; Tsukuda, M. Eosinophilic chronic rhinosinusitis in Japan. Allergol. Int. 2010, 59, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cao, P.P.; Liang, G.T.; Cui, Y.H.; Liu, Z. Diagnostic significance of blood eosinophil count in eosinophilic chronic rhinosinusitis with nasal polyps in Chinese adults. Laryngoscope 2012, 122, 498–503. [Google Scholar] [CrossRef]
- Ho, J.; Hamizan, A.W.; Alvarado, R.; Rimmer, J.; Sewell, W.A.; Harvey, R.J. Systemic predictors of eosinophilic chronic rhinosinusitis. Am. J. Rhinol. Allergy. 2018, 32, 252–257. [Google Scholar] [CrossRef]
- Wang, K.; Deng, J.; Yang, M.; Chen, Y.; Chen, F.; Gao, W.X.; Lai, Y.; Shi, J.; Sun, Y. Concordant systemic and local eosinophilia relates to poorer disease control in patients with nasal polyps. World Allergy Organ. J. 2019, 12, 100052. [Google Scholar] [CrossRef]
- Watts, A.M.; West, N.P.; Cripps, A.W.; Smith, P.K.; Cox, A.J. Distinct Gene Expression Patterns between Nasal Mucosal Cells and Blood Collected from Allergic Rhinitis Sufferers. Int. Arch. Allergy Immunol. 2018, 177, 29–34. [Google Scholar] [CrossRef]
- Occasi, F.; Duse, M.; Vittori, T.; Rugiano, A.; Tancredi, G.; De Castro, G.; Indinnimeo, L.; Zicari, A.M. Primary school children often underestimate their nasal obstruction. Rhinology 2016, 54, 164–169. [Google Scholar] [CrossRef]
- Poddighe, D.; Gelardi, M.; Licari, A.; Del Giudice, M.M.; Marseglia, G.L. Non-allergic rhinitis in children: Epidemiological aspects, pathological features, diagnostic methodology and clinical management. World J. Methodol. 2016, 6, 200–213. [Google Scholar] [CrossRef]

| n = 29 | |
|---|---|
| Male sex (n, %) | 15 (51.7) |
| Age (years) | 8.4 ± 2.8 |
| Height (cm) | 129.2 ± 15.9 |
| Weight (kg) | 29.4 ± 10.9 |
| Family history of atopy (n, %) | 16 (55.2) |
| Family history of asthma (n, %) | 3 (10.3) |
| Conjunctivitis (n, %) | 8 (27.6) |
| Sinusitis (n, %) | 8 (27.6) |
| OSAS (n, %) | 2 (6.9) |
| n = 29 | |
|---|---|
| Serum results | |
| Eosinophilia (n, %) | 2 (6.9) |
| Increased total IgE values (n, %) | 8 (27.9) |
| ECP values (n, %) | 25 (86.9) |
| Basophilia (n, %) | 0 (0) |
| Cytology results | |
| Nasal eosinophils (n, %) | 10 (34.5) |
| Mild (n, %) | 4 (40.0) |
| Moderate (n, %) | 5 (50.0) |
| Severe (n, %) | 1 (10.0) |
| Nasal neutrophils & Bacterial biofilm (n, %) | 19 (65.5) |
| OR | CI | p-Value | |
|---|---|---|---|
| Total IgE | 2.24 | 1.08–4.65 | 0.031 |
| Serum eosinophils | 1.35 | 0.57–3.22 | 0.495 |
| Serum basophils | 1.13 | 0.41–3.09 | 0.813 |
| ECP | 1.01 | 0.28–3.67 | 0.991 |
| Clinical Score | 0.96 | 0.81–1.16 | 0.651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodi, G.; Di Filippo, P.; Ciarelli, F.; Porreca, A.; Cazzato, F.; Matonti, L.; Di Pillo, S.; Neri, G.; Chiarelli, F.; Attanasi, M. The Role of Nasal Cytology and Serum Atopic Biomarkers in Paediatric Rhinitis. Diagnostics 2023, 13, 555. https://doi.org/10.3390/diagnostics13030555
Dodi G, Di Filippo P, Ciarelli F, Porreca A, Cazzato F, Matonti L, Di Pillo S, Neri G, Chiarelli F, Attanasi M. The Role of Nasal Cytology and Serum Atopic Biomarkers in Paediatric Rhinitis. Diagnostics. 2023; 13(3):555. https://doi.org/10.3390/diagnostics13030555
Chicago/Turabian StyleDodi, Giulia, Paola Di Filippo, Francesca Ciarelli, Annamaria Porreca, Fiorella Cazzato, Lorena Matonti, Sabrina Di Pillo, Giampiero Neri, Francesco Chiarelli, and Marina Attanasi. 2023. "The Role of Nasal Cytology and Serum Atopic Biomarkers in Paediatric Rhinitis" Diagnostics 13, no. 3: 555. https://doi.org/10.3390/diagnostics13030555
APA StyleDodi, G., Di Filippo, P., Ciarelli, F., Porreca, A., Cazzato, F., Matonti, L., Di Pillo, S., Neri, G., Chiarelli, F., & Attanasi, M. (2023). The Role of Nasal Cytology and Serum Atopic Biomarkers in Paediatric Rhinitis. Diagnostics, 13(3), 555. https://doi.org/10.3390/diagnostics13030555









