Evaluation of Blood Biomarkers and Parameters for the Prediction of Stroke Survivors’ Functional Outcome upon Discharge Utilizing Explainable Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Description
2.3. Problem Definition
- Class A: involves 219 patients with 0, 1 and 2 mRS grades at discharge and
- Class B: comprises 247 patients with 3, 4, 5 and 6 mRS grades at discharge.
- Class A: involves 153 patients with 0 and 1 mRS grades at discharge and
- Class B: comprises 313 patients with 2, 3, 4, 5 and 6 mRS grades at discharge.
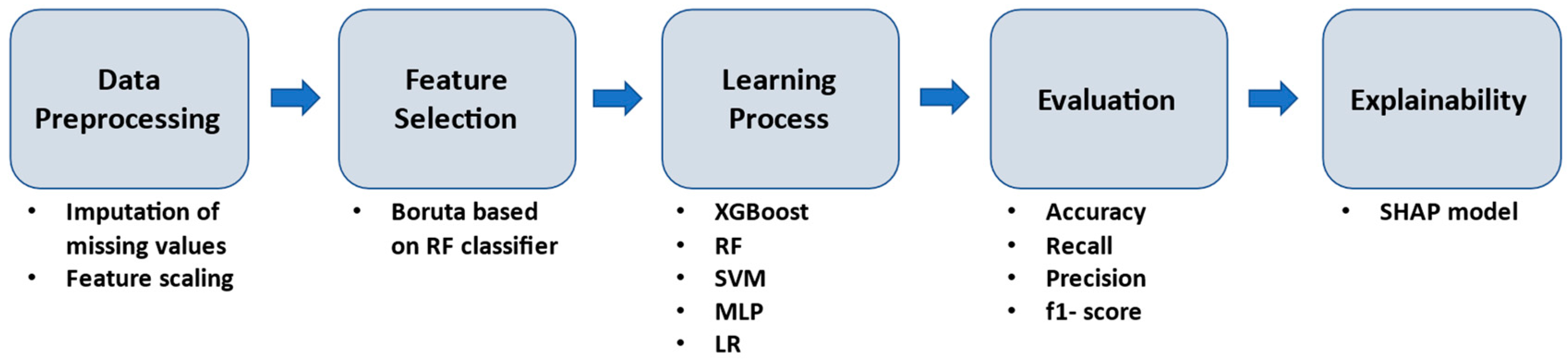
2.4. Machine Learning Workflow Methodology
3. Results
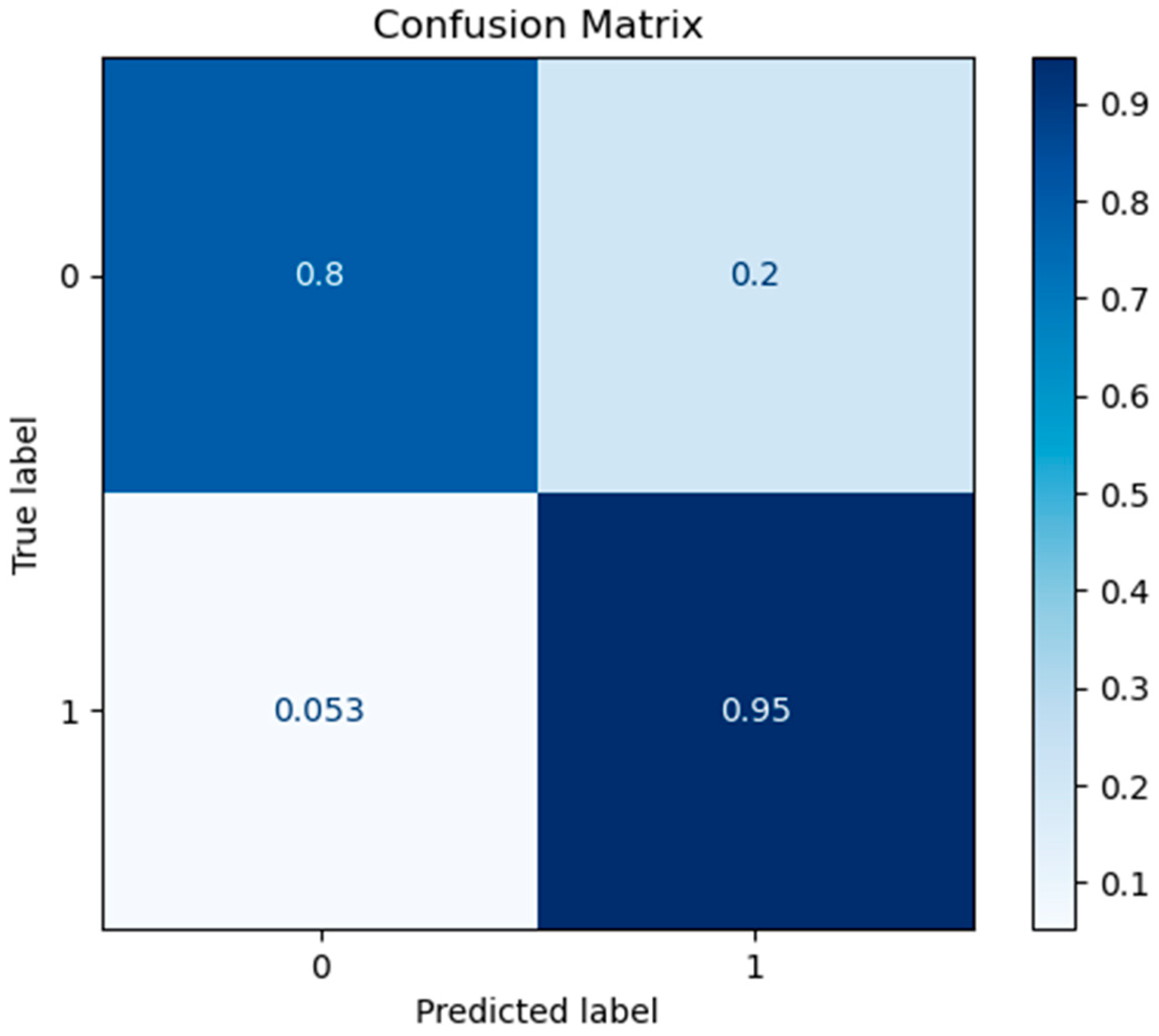
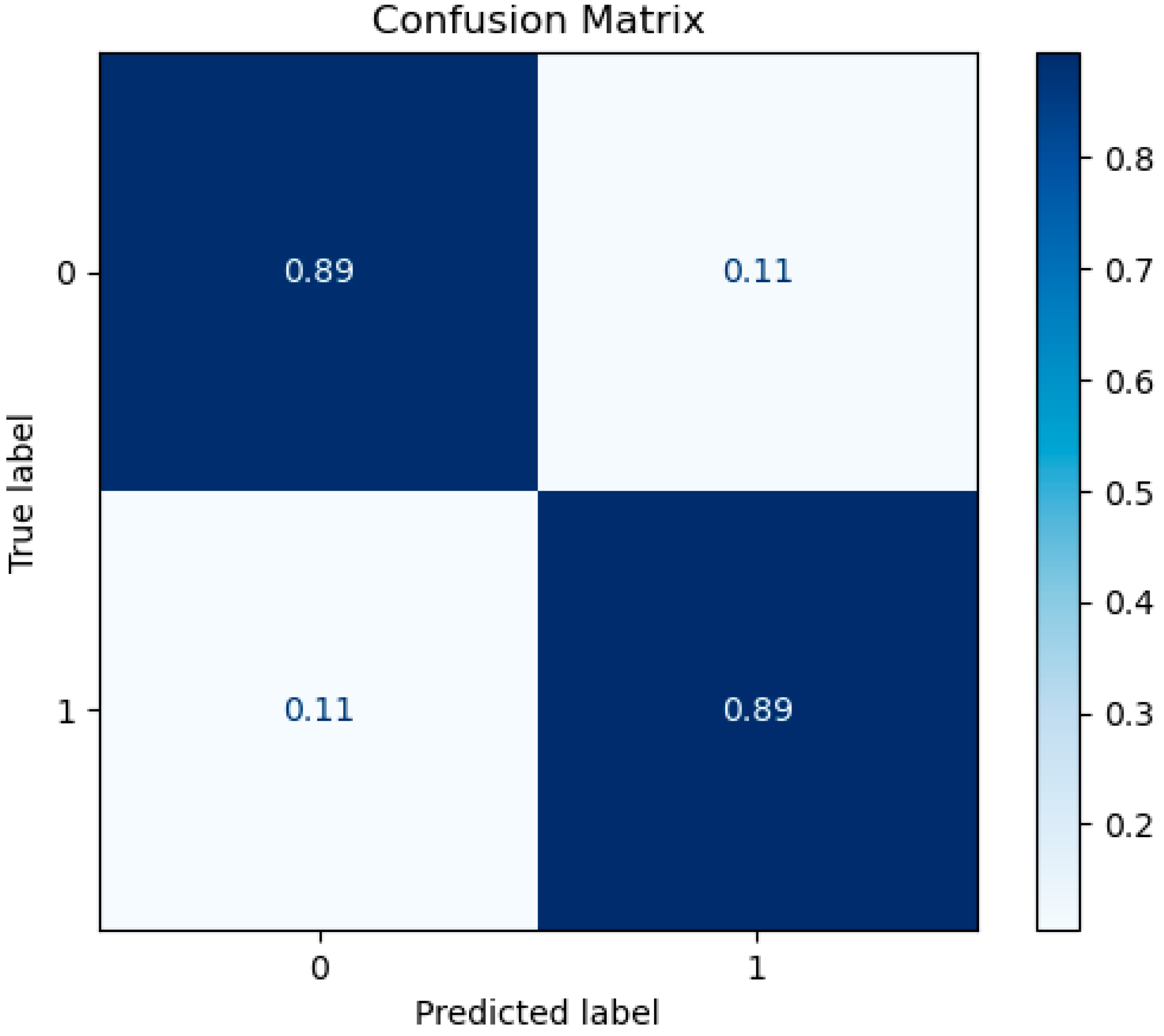
3.1. Prediction Performance
- First Approach: Independent and Non-Independent Categorization
- Second Approach: Disability and Non-Disability Categorization
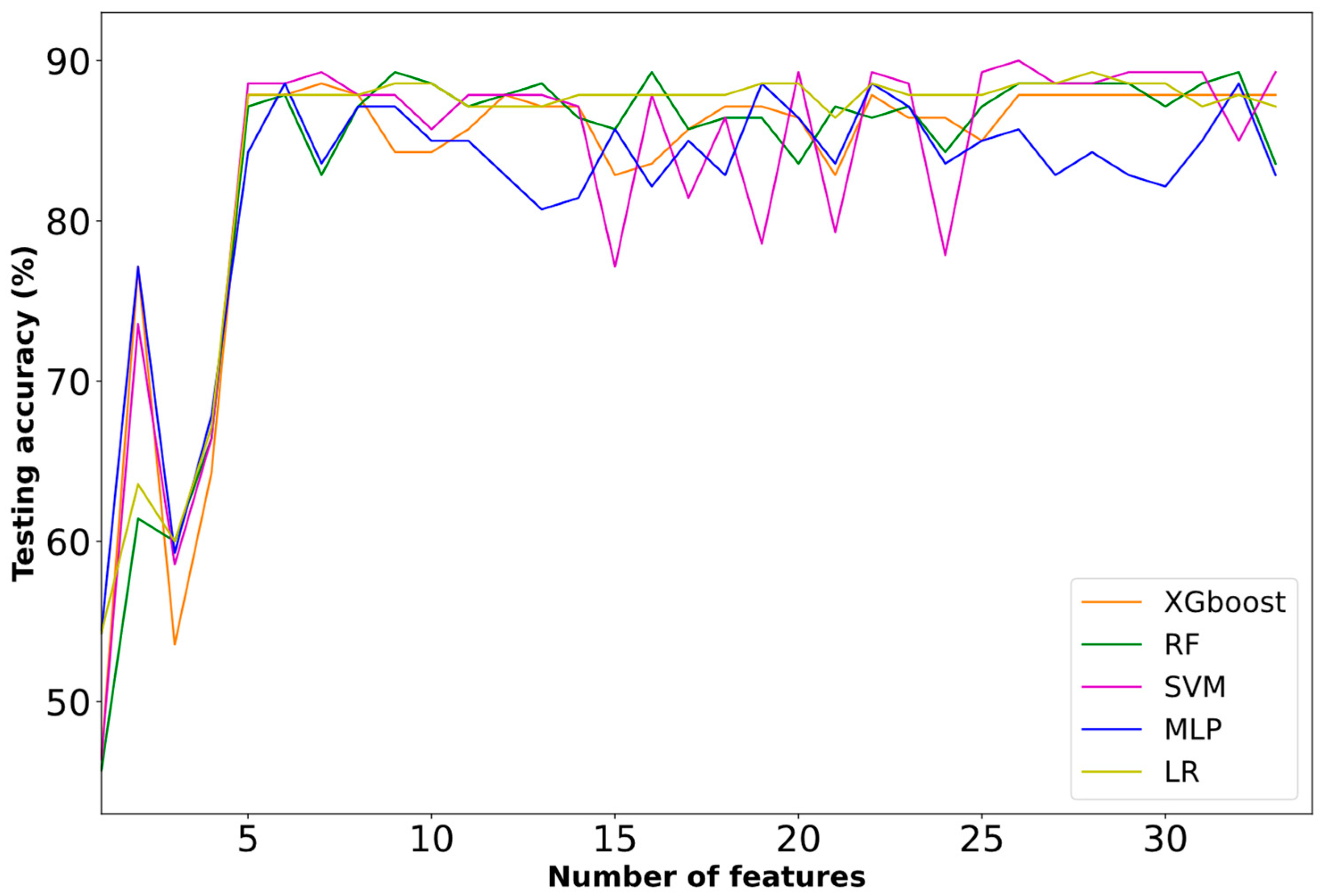
3.2. Selected Features
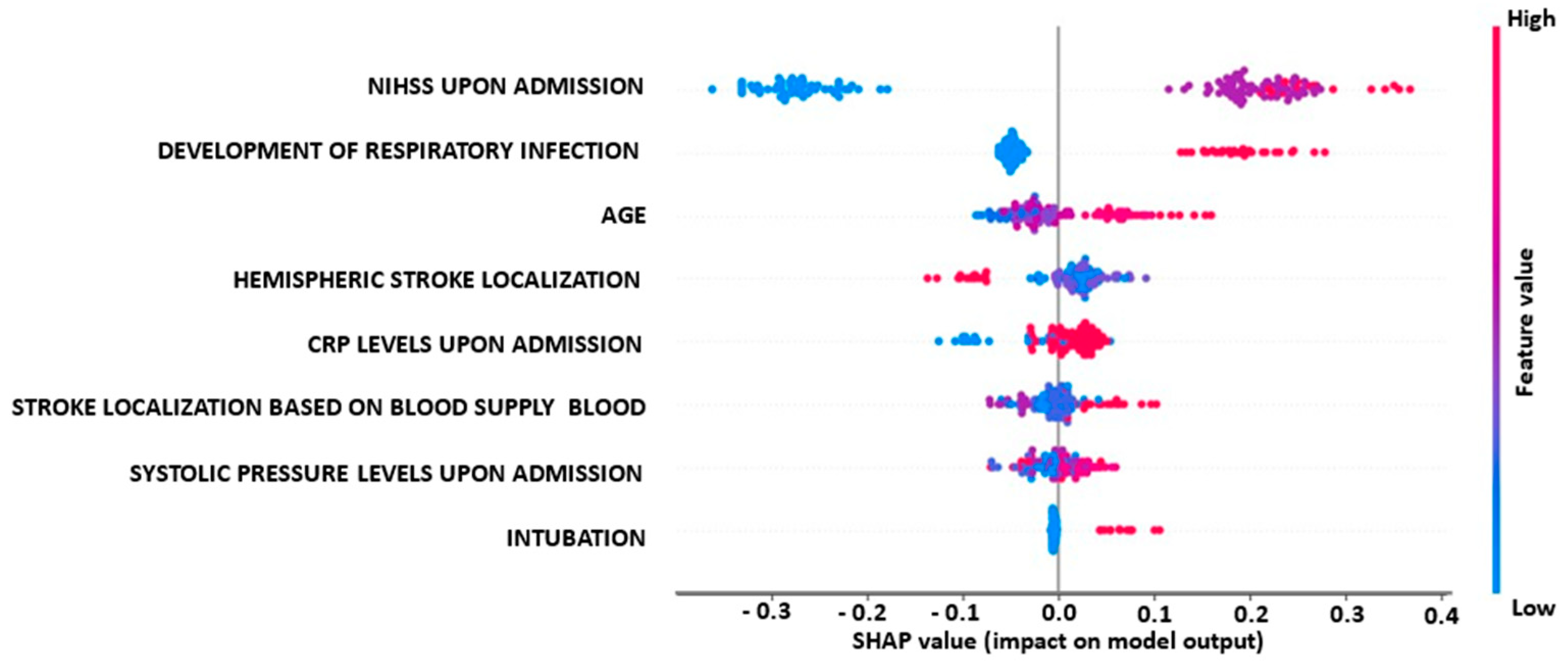
3.3. Explainability Analysis
4. Discussion
4.1. Limitations
4.2. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donkor, E.S. Stroke in the 21st century: A snapshot of the burden, epidemiology, and quality of life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar]
- Christidi, F.; Tsiptsios, D.; Sousanidou, A.; Karamanidis, S.; Kitmeridou, S.; Karatzetzou, S.; Aitsidou, S.; Tsamakis, K.; Psatha, E.A.; Karavasilis, E.; et al. The Clinical Utility of Leukoaraiosis as a Prognostic Indicator in Ischemic Stroke Patients. Neurol. Int. 2022, 14, 952–980. [Google Scholar] [CrossRef]
- Xu, M.; Amarilla Vallejo, A.; Cantalapiedra Calvete, C.; Rudd, A.; Wolfe, C.; O’Connell, M.D.L.; Douiri, A. Stroke Outcomes in Women: A Population-Based Cohort Study. Stroke 2022, 53, 3072–3081. [Google Scholar] [CrossRef]
- Wańkowicz, P.; Nowacki, P.; Gołąb-Janowska, M. Risk factors for ischemic stroke in patients with non-valvular atrial fibrillation and therapeutic international normalized ratio range. Arch. Med. Sci. 2019, 15, 1217–1222. [Google Scholar] [CrossRef]
- Wańkowicz, P.; Gołąb-Janowska, M.; Nowacki, P. Risk factors for death by acute ischaemic stroke in patients from West-Pomerania, Poland. Neurol. Neurochir. Pol. 2020, 54, 150–155. [Google Scholar] [CrossRef]
- Ekker, M.S.; Verhoeven, J.I.; Schellekens, M.M.I.; Boot, E.M.; van Alebeek, M.E.; Brouwers, P.J.A.M.; Arntz, R.M.; van Dijk, G.W.; Gons, R.A.R.; van Uden, I.W.M.; et al. Risk Factors and Causes of Ischemic Stroke in 1322 Young Adults. Stroke 2022, 54, 439–447. [Google Scholar] [CrossRef]
- Xu, J.; Yalkun, G.; Wang, M.; Wang, A.; Wangqin, R.; Zhang, X.; Chen, Z.; Mo, J.; Meng, X.; Li, H.; et al. Impact of Infection on the Risk of Recurrent Stroke Among Patients With Acute Ischemic Stroke. Stroke 2020, 51, 2395–2403. [Google Scholar] [CrossRef]
- Li, J.; Pan, Y.; Xu, J.; Li, S.; Wang, M.; Quan, K.; Meng, X.; Li, H.; Lin, J.; Wang, Y.; et al. Residual Inflammatory Risk Predicts Poor Prognosis in Acute Ischemic Stroke or Transient Ischemic Attack Patients. Stroke 2021, 52, 2827–2836. [Google Scholar] [CrossRef]
- Gynnild, M.N.; Hageman, S.H.J.; Dorresteijn, J.A.N.; Spigset, O.; Lydersen, S.; Wethal, T.; Saltvedt, I.; Visseren, F.L.J.; Ellekjær, H. Risk Stratification in Patients with Ischemic Stroke and Residual Cardiovascular Risk with Current Secondary Prevention. Clin. Epidemiol. 2021, 13, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Cummock, J.S.; Li, G.; Ghosh, R.; Xu, P.; Volpi, J.J.; Wong, S.T.C. Automatic Segmentation in Acute Ischemic Stroke: Prognostic Significance of Topological Stroke Volumes on Stroke Outcome. Stroke 2022, 53, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Grefkes, C.; Fink, G.R. Recovery from stroke: Current concepts and future perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Campagnini, S.; Liuzzi, P.; Mannini, A.; Basagni, B.; Macchi, C.; Carrozza, M.C.; Cecchi, F. Cross-validation of predictive models for functional recovery after post-stroke rehabilitation. J. Neuroeng. Rehabil. 2022, 19, 96. [Google Scholar] [CrossRef]
- Gkantzios, A.; Tsiptsios, D.; Karatzetzou, S.; Kitmeridou, S.; Karapepera, V.; Giannakou, E.; Vlotinou, P.; Aggelousis, N.; Vadikolias, K. Stroke and Emerging Blood Biomarkers: A Clinical Prospective. Neurol. Int. 2022, 14, 784–803. [Google Scholar] [CrossRef]
- GBD 2016 Lifetime Risk of Stroke Collaborators. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Drozdowska, B.A.; Singh, S.; Quinn, T.J. Thinking about the future: A review of prognostic scales used in acute stroke. Front. Neurol. 2019, 10, 274. [Google Scholar] [CrossRef]
- Sung, S.F.; Chen, C.H.; Pan, R.C.; Hu, Y.H.; Jeng, J.S. Natural Language Processing Enhances Prediction of Functional Outcome After Acute Ischemic Stroke. J. Am. Heart Assoc. 2021, 10, e023486. [Google Scholar] [CrossRef]
- Winters, C.; Kwakkel, G.; van Wegen, E.E.H.; Nijland, R.H.M.; Veerbeek, J.M.; Meskers, C.G.M. Moving stroke rehabilitation forward: The need to change research. NeuroRehabilitation 2018, 43, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Campagnini, S.; Arienti, C.; Patrini, M.; Liuzzi, P.; Mannini, A.; Carrozza, M.C. Machine learning methods for functional recovery prediction and prognosis in post-stroke rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2022, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Hu, Z.; Fell, N.; Heath, G.W.; Qayyum, R.; Sartipi, M. Hospital Discharge Disposition of Stroke Patients in Tennessee. South Med. J. 2017, 110, 594–600. [Google Scholar] [CrossRef]
- Luker, J.A.; Bernhardt, J.; Grimmer, K.A.; Edwards, I. A qualitative exploration of discharge destination as an outcome or a driver of acute stroke care. BMC Health Serv. Res. 2014, 14, 193. [Google Scholar] [CrossRef]
- Bacchi, S.; Oakden-Rayner, L.; Menon, D.K.; Jannes, J.; Kleinig, T.; Koblar, S. Stroke prognostication for discharge planning with machine learning: A derivation study. J. Clin. Neurosci. 2020, 79, 100–103. [Google Scholar] [CrossRef]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef]
- Senders, J.T.; Staples, P.C.; Karhade, A.V.; Zaki, M.M.; Gormley, W.B.; Broekman, M.L.D.; Smith, T.R.; Arnaout, O. Machine Learning and Neurosurgical Outcome Prediction: A Systematic Review. World Neurosurg. 2018, 109, 476–486.e1. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Chen, C.-H.; Tseng, Y.-J.; Tsai, Y.-T.; Chang, C.-Y.; Wang, H.-Y.; Chen, C.-K. Predicting post-stroke Activities of Daily Living through a Machine Learning-Based Approach on Initiating Rehabilitation. Int. J. Med. Inform. 2018, 111, 159–164. [Google Scholar] [CrossRef]
- Van Os, H.J.A.; Ramos, L.A.; Hilbert, A.; van Leeuwen, M.; van Walderveen, M.A.A.; Kruyt, N.D.; Dippel, D.W.J.; Steyerberg, E.W.; van der Schaaf, I.C.; Lingsma, H.F.; et al. Predicting Outcome of Endovascular Treatment for Acute Ischemic Stroke: Potential Value of Machine Learning Algorithms. Front. Neurol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Debs, N.; Rasti, P.; Victor, L.; Cho, T.-H.; Frindel, C.; Rousseau, D. Simulated Perfusion MRI Data to Boost Training of Convolutional Neural Networks for Lesion Fate Prediction in Acute Stroke. Comput. Biol. Med. 2020, 116, 103579. [Google Scholar] [CrossRef]
- Fang, G.; Liu, W.; Wang, L. A Machine Learning Approach to Select Features Important to Stroke Prognosis. Comput. Biol. Chem. 2020, 88, 107316. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Huang, Z.; Wang, Z. Predicting Ischemic Stroke Outcome Using Deep Learning Approaches. Front. Genet. 2022, 12, 827522. [Google Scholar] [CrossRef]
- Hofer, I.S.; Burns, M.; Kendale, S.; Wanderer, J.P. Realistically Integrating Machine Learning Into Clinical Practice: A Road Map of Opportunities, Challenges, and a Potential Future. Anesth. Analg. 2020, 130, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.A.; Murray, J.; Greiner, R.; Cohen, J.P.; Shojania, K.G.; Ghassemi, M.; Straus, S.E.; Pou-Prom, C.; Mamdani, M. Implementing machine learning in medicine. CMAJ 2021, 193, E1351–E1357. [Google Scholar] [CrossRef] [PubMed]
- Kokkotis, C.; Moustakidis, S.; Papageorgiou, E.; Giakas, G.; Tsaopoulos, D.E. Machine learning in knee osteoarthritis: A review. Osteoarthr. Cartil. Open 2020, 2, 100069. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Hügle, M.; Omoumi, P.; van Laar, J.M.; Boedecker, J.; Hügle, T. Applied machine learning and artificial intelligence in rheumatology. Rheumatol. Adv. Pract. 2020, 4, rkaa005. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, J.G.; Park, H.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Machine Learning-Based Model for Prediction of Outcomes in Acute Stroke. Stroke 2019, 50, 1263–1265. [Google Scholar] [CrossRef]
- Jang, S.K.; Chang, J.Y.; Lee, J.S.; Lee, E.J.; Kim, Y.H.; Han, J.H.; Chang, D.I.; Cho, H.J.; Cha, J.K.; Yu, K.H.; et al. Reliability and Clinical Utility of Machine Learning to Predict Stroke Prognosis: Comparison with Logistic Regression. J. Stroke 2020, 22, 403–406. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsu, K.C.; Johnson, K.R.; Fann, Y.C.; Tsai, C.H.; Sun, Y.; Lien, L.M.; Chang, W.L.; Chen, P.L.; Lin, C.L.; et al. Evaluation of machine learning methods to stroke outcome prediction using a nationwide disease registry. Comput. Methods Programs Biomed. 2020, 190, 105381. [Google Scholar] [CrossRef]
- Murray, N.M.; Unberath, M.; Hager, G.D.; Hui, F.K. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: A systematic review. J. Neurointerv. Surg. 2020, 12, 156–164. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, S.; Bielinski, S.J.; Decker, P.A.; Chamberlain, A.M.; Roger, V.L.; Liu, H.; Larson, N.B. Natural Language Processing and Machine Learning for Identifying Incident Stroke From Electronic Health Records: Algorithm Development and Validation. J. Med. Internet Res. 2021, 23, e22951. [Google Scholar] [CrossRef]
- McDermott, B.; Elahi, A.; Santorelli, A.; O’Halloran, M.; Avery, J.; Porter, E. Multi-frequency symmetry difference electrical impedance tomography with machine learning for human stroke diagnosis. Physiol. Meas. 2020, 41, 075010. [Google Scholar] [CrossRef]
- Bivard, A.; Churilov, L.; Parsons, M. Artificial intelligence for decision support in acute stroke—Current roles and potential. Nat. Rev. Neurol. 2020, 16, 575–585. [Google Scholar] [CrossRef]
- Wang, W.; Kiik, M.; Peek, N.; Curcin, V.; Marshall, I.J.; Rudd, A.G.; Wang, Y.; Douiri, A.; Wolfe, C.D.; Bray, B. A systematic review of machine learning models for predicting outcomes of stroke with structured data. PLoS ONE 2020, 15, e0234722. [Google Scholar] [CrossRef]
- Sirsat, M.S.; Fermé, E.; Câmara, J. Machine Learning for Brain Stroke: A Review. J. Stroke Cerebrovasc. Dis. 2020, 29, 105162. [Google Scholar] [CrossRef]
- Kokkotis, C.; Giarmatzis, G.; Giannakou, E.; Moustakidis, S.; Tsatalas, T.; Tsiptsios, D.; Vadikolias, K.; Aggelousis, N. An Explainable Machine Learning Pipeline for Stroke Prediction on Imbalanced Data. Diagnostics 2022, 12, 2392. [Google Scholar] [CrossRef]
- Ding, L.; Liu, C.; Li, Z.; Wang, Y. Incorporating artificial intelligence into stroke care and research. Stroke 2020, 51, e351–e354. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nohara, Y.; Soejima, H.; Yonehara, T.; Nakashima, N.; Kamouchi, M. Stroke prognostic scores and data-driven prediction of clinical outcomes after acute ischemic stroke. Stroke 2020, 51, 1477–1483. [Google Scholar] [CrossRef]
- Harrison, J.K.; McArthur, K.S.; Quinn, T.J. Assessment scales in stroke: Clinimetric and clinical considerations. Clin. Interv. Aging 2013, 8, 201–211. [Google Scholar] [CrossRef]
- Broderick, J.P.; Adeoye, O.; Elm, J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef]
- Siniscalchi, A. Use of stroke scales in clinical practice: Current concepts. Turk. J. Emerg. Med. 2022, 22, 119–124. [Google Scholar] [CrossRef]
- Alijanpour, S.; Mostafazdeh-Bora, M.; Ahmadi Ahangar, A. Different Stroke Scales; Which Scale or Scales Should Be Used? Casp. J. Intern. Med. 2021, 12, 1–21. [Google Scholar] [CrossRef]
- De Haan, R.; Horn, J.; Limburg, M.; Van Der Meulen, J.; Bossuyt, P. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke 1993, 24, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Iman, A.N.; Ahmad, T. Improving Intrusion Detection System by Estimating Parameters of Random Forest in Boruta. In Proceedings of the 2020 International Conference on Smart Technology and Applications (ICoSTA), Surabaya, Indonesia, 20 February 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Poona, N.K.; van Niekerk, A.; Nadel, R.L.; Ismail, R. Random Forest (RF) Wrappers for Waveband Selection and Classification of Hyperspectral Data. Appl. Spectrosc. 2016, 70, 322–333. [Google Scholar] [CrossRef]
- Fernandez-Lozano, C.; Hervella, P.; Mato-Abad, V.; Rodríguez-Yáñez, M.; Suárez-Garaboa, S.; López-Dequidt, I.; Estany-Gestal, A.; Sobrino, T.; Campos, F.; Castillo, J.; et al. Random forest-based prediction of stroke outcome. Sci. Rep. 2021, 11, 10071. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Z.; Su, C.; Han, Y.; Duan, X.; Zhang, R.; Liu, X.; Yang, Y.; Xu, S. Copy number variation in plasma as a tool for lung cancer prediction using Extreme Gradient Boosting (XGBoost) classifier. Thorac. Cancer 2020, 11, 95–102. [Google Scholar] [CrossRef]
- Chan, L.C.; Li, H.H.T.; Chan, P.K.; Wen, C. A machine learning-based approach to decipher multi-etiology of knee osteoarthritis onset and deterioration. Osteoarthr. Cartil. Open 2021, 3, 100135. [Google Scholar] [CrossRef]
- Mohr, M.; von Tscharner, V.; Emery, C.A.; Nigg, B.M. Classification of gait muscle activation patterns according to knee injury history using a support vector machine approach. Hum. Mov. Sci. 2019, 66, 335–346. [Google Scholar] [CrossRef]
- Ali, A.A. Stroke Prediction using Distributed Machine Learning Based on Apache Spark. Stroke 2019, 28, 89–97. [Google Scholar]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. In Advances in Neural Information Processing Systems 30, Proceedings of the 31st Annual Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017; Curran Associates Inc.: Red Hook, NY, USA, 2017. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Janzing, D.; Minorics, L.; Blöbaum, P. Feature relevance quantification in explainable AI: A causal problem. In Proceedings of the 23th International Conference on Artificial Intelligence and Statistics, PMLR Means Proceedings of Machine Learning Research, Online, 26–28 August 2020. [Google Scholar]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Sharrief, A.; Grotta, J.C. Stroke in the elderly. Handb. Clin. Neurol. 2019, 167, 393–418. [Google Scholar] [CrossRef]
- Porcello Marrone, L.C.; Diogo, L.P.; de Oliveira, F.M.; Trentin, S.; Scalco, R.S.; de Almeida, A.G.; Gutierres Ldel, C.; Marrone, A.C.; da Costa, J.C. Risk factors among stroke subtypes in Brazil. J. Stroke Cerebrovasc. Dis. 2013, 22, 32–35. [Google Scholar] [CrossRef]
- Bhaskar, S.; Stanwell, P.; Bivard, A.; Spratt, N.; Walker, R.; Kitsos, G.H.; Parsons, M.W.; Evans, M.; Jordan, L.; Nilsson, M.; et al. The influence of initial stroke severity on mortality, overall functional outcome and in-hospital placement at 90 days following acute ischemic stroke: A tertiary hospital stroke register study. Neurol. India 2017, 65, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Reeves, M.J.; Zhao, X.; Olson, D.M.; Smith, E.E.; Saver, J.L.; Schwamm, L.H.; Get With the Guidelines-Stroke Steering Committee and Investigators. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation 2010, 121, 879–891. [Google Scholar] [CrossRef]
- Furlan, N.E.; Luvizutto, G.J.; Hamamoto Filho, P.T.; Zanati Bazan, S.G.; Modolo, G.P.; Ferreira, N.C.; Miranda, L.A.; de Souza, J.T.; Winckler, F.C.; Vidal, E.I.O.; et al. The Impact of Age on Mortality and Disability in Patients With Ischemic Stroke Who Underwent Cerebral Reperfusion Therapy: A Brazilian Cohort Study. Front. Aging Neurosci. 2021, 13, 649902. [Google Scholar] [CrossRef]
- Denes, G.; Semenza, C.; Stoppa, E.; Lis, A. Unilateral spatial neglect and recovery from hemiplegia: A follow-up study. Brain 1982, 105 Pt 3, 543–552. [Google Scholar] [CrossRef]
- Yoo, A.J.; Romero, J.; Hakimelahi, R.; Nogueira, R.G.; Rabinov, J.D.; Pryor, J.C.; González, R.G.; Hirsch, J.A.; Schaefer, P.W. Predictors of functional outcome vary by the hemisphere of involvement in major ischemic stroke treated with intra-arterial therapy: A retrospective cohort study. BMC Neurol. 2010, 10, 25. [Google Scholar] [CrossRef]
- Etherton, M.R.; Rost, N.S.; Wu, O. Infarct topography and functional outcomes. J. Cereb. Blood Flow Metab. 2018, 38, 1517–1532. [Google Scholar] [CrossRef]
- Cheng, B.; Forkert, N.D.; Zavaglia, M.; Hilgetag, C.C.; Golsari, A.; Siemonsen, S.; Fiehler, J.; Pedraza, S.; Puig, J.; Cho, T.H.; et al. Influence of stroke infarct location on functional outcome measured by the modified rankin scale. Stroke 2014, 45, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.B.; Jadbäck, G.; Norrving, B.; Widner, H.; Wiklund, I. Evaluation of long-term functional status in first-ever stroke patients in a defined population. Scand J. Rehabil. Med. Suppl. 1992, 26, 105–114. [Google Scholar] [PubMed]
- Laufer, Y.; Sivan, D.; Schwarzmann, R.; Sprecher, E. Standing balance and functional recovery of patients with right and left hemiparesis in the early stages of rehabilitation. Neurorehabil. Neural Repair 2003, 17, 207–213. [Google Scholar] [CrossRef]
- Kalra, L.; Smith, D.H.; Crome, P. Stroke in patients aged over 75 years: Outcome and predictors. Postgrad. Med. J. 1993, 69, 33–36. [Google Scholar] [CrossRef]
- Ween, J.E.; Alexander, M.P.; D’Esposito, M.; Roberts, M. Factors predictive of stroke outcome in a rehabilitation setting. Neurology 1996, 47, 388–392. [Google Scholar] [CrossRef]
- Wu, O.; Cloonan, L.; Mocking, S.J.; Bouts, M.J.; Copen, W.A.; Cougo-Pinto, P.T.; Fitzpatrick, K.; Kanakis, A.; Schaefer, P.W.; Rosand, J.; et al. Role of Acute Lesion Topography in Initial Ischemic Stroke Severity and Long-Term Functional Outcomes. Stroke 2015, 46, 2438–2444. [Google Scholar] [CrossRef]
- Rangaraju, S.; Streib, C.; Aghaebrahim, A.; Jadhav, A.; Frankel, M.; Jovin, T.G. Relationship Between Lesion Topology and Clinical Outcome in Anterior Circulation Large Vessel Occlusions. Stroke 2015, 46, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Menezes, N.M.; Ay, H.; Zhu, M.W.; Lopez, C.J.; Singhal, A.B.; Karonen, J.O.; Aronen, H.J.; Liu, Y.; Nuutinen, J.; Koroshetz, W.J.; et al. The real estate factor: Quantifying the impact of infarct location on stroke severity. Stroke 2007, 38, 194–197. [Google Scholar] [CrossRef]
- Königsberg, A.; DeMarco, A.T.; Mayer, C.; Wouters, A.; Schlemm, E.; Ebinger, M.; Cho, T.H.; Endres, M.; Fiebach, J.B.; Fiehler, J.; et al. Influence of stroke infarct location on quality of life assessed in a multivariate lesion-symptom mapping study. Sci. Rep. 2021, 11, 13490. [Google Scholar] [CrossRef]
- Paolucci, S.; Antonucci, G.; Pratesi, L.; Traballesi, M.; Lubich, S.; Grasso, M.G. Functional outcome in stroke inpatient rehabilitation: Predicting no, low and high response patients. Cerebrovasc. Dis. 1998, 8, 228–234. [Google Scholar] [CrossRef]
- Ring, H.; Feder, M.; Schwartz, J.; Samuels, G. Functional measures of first-stroke rehabilitation inpatients: Usefulness of the Functional Independence Measure total score with a clinical rationale. Arch. Phys. Med. Rehabil. 1997, 78, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Boers, A.M.M.; Forkert, N.D.; Berkhemer, O.A.; Roos, Y.B.; Dippel, D.W.J.; van der Lugt, A.; van Oostenbrugge, R.J.; van Zwam, W.H.; Vettorazzi, E.; et al. Impact of Ischemic Lesion Location on the mRS Score in Patients with Ischemic Stroke: A Voxel-Based Approach. AJNR Am. J. Neuroradiol. 2018, 39, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, J.C.; van de Beek, D.; Lyden, P.; Brady, M.C.; Bath, P.M.; van der Worp, H.B. Temporal Profile of Pneumonia After Stroke. Stroke 2022, 53, 53–60, Erratum in Stroke 2022, 53, e129. [Google Scholar] [CrossRef]
- Heuschmann, P.U.; Kolominsky-Rabas, P.L.; Misselwitz, B.; Hermanek, P.; Leffmann, C.; Janzen, R.W.; Rother, J.; Buecker-Nott, H.J.; Berger, K. German Stroke Registers Study Group. Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: The German Stroke Registers Study Group. Arch. Intern. Med. 2004, 164, 1761–1768. [Google Scholar] [CrossRef]
- Sellars, C.; Bowie, L.; Bagg, J.; Sweeney, M.P.; Miller, H.; Tilston, J.; Langhorne, P.; Stott, D.J. Risk factors for chest infection in acute stroke: A prospective cohort study. Stroke 2007, 38, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Gujjar, A.R.; Deibert, E.; Manno, E.M.; Duff, S.; Diringer, M.N. Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: Indications, timing, and outcome. Neurology 1998, 51, 447–451. [Google Scholar] [CrossRef]
- Armstrong, J.R.; Mosher, B.D. Aspiration pneumonia after stroke: Intervention and prevention. Neurohospitalist 2011, 1, 85–93. [Google Scholar] [CrossRef]
- Bustamante, A.; Giralt, D.; García-Berrocoso, T.; Rubiera, M.; Álvarez-Sabín, J.; Molina, C.; Serena, J.; Montaner, J. The impact of post-stroke complications on in-hospital mortality depends on stroke severity. Eur. Stroke J. 2017, 2, 54–63. [Google Scholar] [CrossRef]
- Kazi, S.; Siddiqui, M.; Majid, S. Stroke Outcome Prediction Using Admission Nihss In Anterior And Posterior Circulation Stroke. J. Ayub Med. Coll. Abbottabad 2021, 33, 274–278. [Google Scholar]
- Wouters, A.; Nysten, C.; Thijs, V.; Lemmens, R. Prediction of Outcome in Patients With Acute Ischemic Stroke Based on Initial Severity and Improvement in the First 24 h. Front. Neurol. 2018, 9, 308. [Google Scholar] [CrossRef]
- Sablot, D.; Belahsen, F.; Vuillier, F.; Cassarini, J.F.; Decavel, P.; Tatu, L.; Moulin, T.; Medeiros de Bustos, E. Predicting acute ischaemic stroke outcome using clinical and temporal thresholds. ISRN Neurol. 2011, 2011, 354642. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.E.; Ghonimi, N.A.; Elserafy, T.S.; Mahmoud, W. The NIHSS score can predict the outcome of patients with primary intracerebral hemorrhage. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 21. [Google Scholar] [CrossRef]
- Mazaheri, S.; Reisi, E.; Poorolajal, J.; Ghiasian, M. C-Reactive Protein Levels and Clinical Outcomes in Stroke Patients: A Prospective Cohort Study. Arch. Iran. Med. 2018, 21, 8–12. [Google Scholar]
- Idicula, T.T.; Brogger, J.; Naess, H.; Waje-Andreassen, U.; Thomassen, L. Admission C—Reactive protein after acute ischemic stroke is associated with stroke severity and mortality: The ‘Bergen stroke study’. BMC Neurol. 2009, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Bager, J.E.; Hjalmarsson, C.; Manhem, K.; Andersson, B. Acute blood pressure levels and long-term outcome in ischemic stroke. Brain Behav. 2018, 8, e00992. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, S.A.; Uniken Venema, S.M.; Mulder, M.J.H.L.; Treurniet, K.M.; Samuels, N.; Lingsma, H.F.; Goldhoorn, R.B.; Jansen, I.G.H.; Coutinho, J.M.; Roozenbeek, B.; et al. Admission Blood Pressure in Relation to Clinical Outcomes and Successful Reperfusion After Endovascular Stroke Treatment. Stroke 2020, 51, 3205–3214. [Google Scholar] [CrossRef]
- Verschoof, M.A.; Groot, A.E.; Vermeij, J.D.; Westendorp, W.F.; van den Berg, S.A.; Nederkoorn, P.J.; van de Beek, D.; Coutinho, J.M. Association Between Low Blood Pressure and Clinical Outcomes in Patients With Acute Ischemic Stroke. Stroke 2020, 51, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Maïer, B.; Gory, B.; Taylor, G.; Labreuche, J.; Blanc, R.; Obadia, M.; Abrivard, M.; Smajda, S.; Desilles, J.P.; Redjem, H.; et al. Mortality and Disability According to Baseline Blood Pressure in Acute Ischemic Stroke Patients Treated by Thrombectomy: A Collaborative Pooled Analysis. J. Am. Heart Assoc. 2017, 6, e006484, Erratum in J. Am. Heart Assoc. 2017, 6, e004193. [Google Scholar] [CrossRef]
- Mulder, M.J.H.L.; Ergezen, S.; Lingsma, H.F.; Berkhemer, O.A.; Fransen, P.S.S.; Beumer, D.; van den Berg, L.A.; Lycklama, À.; Nijeholt, G.; Emmer, B.J.; et al. Baseline Blood Pressure Effect on the Benefit and Safety of Intra-Arterial Treatment in MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands). Stroke 2017, 48, 1869–1876, Erratum in Stroke 2017, 48, e187. [Google Scholar] [CrossRef]
- Goyal, N.; Tsivgoulis, G.; Iftikhar, S.; Khorchid, Y.; Fawad Ishfaq, M.; Doss, V.T.; Zand, R.; Chang, J.; Alsherbini, K.; Choudhri, A.; et al. Admission systolic blood pressure and outcomes in large vessel occlusion strokes treated with endovascular treatment. J. Neurointerv. Surg. 2017, 9, 451–454. [Google Scholar] [CrossRef]
- Tziomalos, K.; Giampatzis, V.; Bouziana, S.D.; Spanou, M.; Papadopoulou, M.; Kostaki, S.; Dourliou, V.; Papagianni, M.; Savopoulos, C.; Hatzitolios, A.I. Elevated diastolic but not systolic blood pressure increases mortality risk in hypertensive but not normotensive patients with acute ischemic stroke. Am. J. Hypertens. 2015, 28, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.P.; Ovbiagele, B.; Markovic, D.; Towfighi, A. Systolic blood pressure and mortality after stroke: Too low, no go? Stroke 2015, 46, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, R.; Jiang, S.; Guo, J.; Luo, X.; Miao, J.; Liu, J.; Zheng, B.; Du, J.; Zhang, Y.; et al. The Relationship Between Admission Blood Pressure and Clinical Outcomes for Acute Basilar Artery Occlusion. Front. Neurosci. 2022, 16, 900868. [Google Scholar] [CrossRef] [PubMed]
- Abou-Chebl, A.; Lin, R.; Hussain, M.S.; Jovin, T.G.; Levy, E.I.; Liebeskind, D.S.; Yoo, A.J.; Hsu, D.P.; Rymer, M.M.; Tayal, A.H.; et al. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: Preliminary results from a retrospective, multicenter study. Stroke 2010, 41, 1175–1179. [Google Scholar] [CrossRef]
- Jumaa, M.A.; Zhang, F.; Ruiz-Ares, G.; Gelzinis, T.; Malik, A.M.; Aleu, A.; Oakley, J.I.; Jankowitz, B.; Lin, R.; Reddy, V.; et al. Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke 2010, 41, 1180–1184. [Google Scholar] [CrossRef]
- Nichols, C.; Carrozzella, J.; Yeatts, S.; Tomsick, T.; Broderick, J.; Khatri, P. Is periprocedural sedation during acute stroke therapy associated with poorer functional outcomes? J. Neurointerv. Surg. 2010, 2, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Liang, C.W.; Liebeskind, D.S.; Hinman, J.D. To Tube or Not to Tube? The Role of Intubation during Stroke Thrombectomy. Front. Neurol. 2014, 5, 170. [Google Scholar] [CrossRef]
- Hassan, A.E.; Chaudhry, S.A.; Zacharatos, H.; Khatri, R.; Akbar, U.; Suri, M.F.; Qureshi, A.I. Increased rate of aspiration pneumonia and poor discharge outcome among acute ischemic stroke patients following intubation for endovascular treatment. Neurocrit. Care 2012, 16, 246–250. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, A.; Kaur, A. Erythrocyte Sedimentation Rate: Its Determinants and Relationship with Risk Factors Involved in Ischemic Stroke. Korean J. Clin. Lab. Sci. 2022, 54, 1–8. [Google Scholar] [CrossRef]
- Jabal, M.S.; Joly, O.; Kallmes, D.; Harston, G.; Rabinstein, A.; Huynh, T.; Brinjikji, W. Interpretable Machine Learning Modeling for Ischemic Stroke Outcome Prediction. Front. Neurol. 2022, 13, 884693. [Google Scholar] [CrossRef]
- Brugnara, G.; Neuberger, U.; Mahmutoglu, M.A.; Foltyn, M.; Herweh, C.; Nagel, S.; Schönenberger, S.; Heiland, S.; Ulfert, C.; Ringleb, P.A.; et al. Multimodal Predictive Modeling of Endovascular Treatment Outcome for Acute Ischemic Stroke Using Machine-Learning. Stroke 2020, 51, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhu, G.; Xie, Y.; Heit, J.J.; Chen, H.; Li, Y.; Ding, V.; Eskandari, A.; Michel, P.; Zaharchuk, G.; et al. Prediction of Clinical Outcome in Patients with Large-Vessel Acute Ischemic Stroke: Performance of Machine Learning versus SPAN-100. AJNR Am. J. Neuroradiol. 2021, 42, 240–246. [Google Scholar] [CrossRef] [PubMed]


| Classifiers | Accuracy (%) | Recall | Precision | f1-Score | Num of Features |
|---|---|---|---|---|---|
| XGboost | 87.14 | 96.05 | 82.96 | 89.02 | 22 |
| RF | 88.57 | 94.74 | 85.71 | 90.00 | 8 |
| SVM | 85.71 | 93.42 | 82.56 | 87.65 | 5 |
| MLP | 87.86 | 96.05 | 83.91 | 89.57 | 6 |
| LR | 87.86 | 93.42 | 85.54 | 89.03 | 6 |
| Classifiers | Accuracy | Recall | Precision | F1-Score | Num of Features |
|---|---|---|---|---|---|
| XGBoost | 88.59 | 87.35 | 95.41 | 91.21 | 7 |
| RF | 89.27 | 89.45 | 94.43 | 91.88 | 9 |
| SVM | 89.29 | 89.47 | 94.44 | 91.89 | 7 |
| MLP | 88.57 | 87.37 | 95.42 | 91.22 | 6 |
| LR | 88.56 | 87.36 | 95.40 | 91.23 | 9 |
| Ranking | Features | Type |
|---|---|---|
| 1 | Age | Categorical |
| 2 | Hemispheric stroke localization | Categorical |
| 3 | Stroke localization based on blood supply | Categorical |
| 4 | Development of respiratory infection | Categorical |
| 5 | NIHSS upon admission | Categorical |
| 6 | CRP levels upon admission | Categorical |
| 7 | Systolic blood pressure levels upon admission | Categorical |
| 8 | Intubation | Categorical |
| Ranking | Features | Type |
|---|---|---|
| 1 | Age | Categorical |
| 2 | Hemispheric stroke localization | Categorical |
| 3 | Stroke localization based on blood supply | Categorical |
| 4 | Development of respiratory infection | Categorical |
| 5 | NIHSS upon admission | Categorical |
| 6 | Systolic blood pressure levels upon admission | Categorical |
| 7 | ESR levels upon admission | Categorical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkantzios, A.; Kokkotis, C.; Tsiptsios, D.; Moustakidis, S.; Gkartzonika, E.; Avramidis, T.; Aggelousis, N.; Vadikolias, K. Evaluation of Blood Biomarkers and Parameters for the Prediction of Stroke Survivors’ Functional Outcome upon Discharge Utilizing Explainable Machine Learning. Diagnostics 2023, 13, 532. https://doi.org/10.3390/diagnostics13030532
Gkantzios A, Kokkotis C, Tsiptsios D, Moustakidis S, Gkartzonika E, Avramidis T, Aggelousis N, Vadikolias K. Evaluation of Blood Biomarkers and Parameters for the Prediction of Stroke Survivors’ Functional Outcome upon Discharge Utilizing Explainable Machine Learning. Diagnostics. 2023; 13(3):532. https://doi.org/10.3390/diagnostics13030532
Chicago/Turabian StyleGkantzios, Aimilios, Christos Kokkotis, Dimitrios Tsiptsios, Serafeim Moustakidis, Elena Gkartzonika, Theodoros Avramidis, Nikolaos Aggelousis, and Konstantinos Vadikolias. 2023. "Evaluation of Blood Biomarkers and Parameters for the Prediction of Stroke Survivors’ Functional Outcome upon Discharge Utilizing Explainable Machine Learning" Diagnostics 13, no. 3: 532. https://doi.org/10.3390/diagnostics13030532
APA StyleGkantzios, A., Kokkotis, C., Tsiptsios, D., Moustakidis, S., Gkartzonika, E., Avramidis, T., Aggelousis, N., & Vadikolias, K. (2023). Evaluation of Blood Biomarkers and Parameters for the Prediction of Stroke Survivors’ Functional Outcome upon Discharge Utilizing Explainable Machine Learning. Diagnostics, 13(3), 532. https://doi.org/10.3390/diagnostics13030532










