Abstract
Although arthroscopic treatment is a minimally invasive surgery that effectively treats septic arthritis of the knee joint, it requires general or regional anesthesia. This study aimed to compare the clinical results of lavage after tube insertion versus arthroscopic treatment. Patients treated with arthroscopic treatment were included in group I (n = 76), while those treated with lavage by tube were included in group II (n = 34). We investigated the following in all patients: demographics, underlying disorders, initial serum white blood cell (WBC) count, C-reactive protein (CRP) level, synovial fluid WBC and polymorphonuclear cell counts, causative organism, initial Kellgren–Lawrence grade, lavage number, interventional delay, hospitalization days, CRP normalization time, and Western Ontario McMaster Universities Osteoarthritis index scores for clinical outcomes at 3 months postoperative. The mean interventional delay was significantly greater in group I (23.6 ± 15.6 h vs. 8.7 ± 9.3 h, p < 0.001). The lavage by tube featured a significantly shorter interventional delay time than arthroscopy, while the CRP decrease rate did not differ between groups. Moreover, lavage by tube showed no significant differences in outcomes, including laboratory results and functional outcomes at 3 months postoperative.
1. Introduction
Septic arthritis (SA) is an arthropathy caused by infection with microorganisms. SA may occur during hematogenous spreading, wounds, trauma, surgery, acupuncture, and injection. The knee joint, which is most commonly involved, has an increasing incidence [1,2]. SA can lead to sequelae such as joint structural changes and joint malfunction due to severe inflammatory reactions. Furthermore, it can result in a life-threatening condition with a mortality rate of approximately 3–29%. Thus, SA requires prompt treatment including drainage, lavage, and intravenous antibiotics [3,4].
The gold standard for diagnosing the septic origin is the isolation of pathogenic causative organisms; however, blood or synovial fluid Gram-staining and culturing have poor yields and take time. Moreover, if the culture fails, the initiation of antibiotics can negatively affect microbiological samples [5]. However, SA can be life-threatening and require prompt treatment. Thus, the diagnosis of SA was made comprehensively using clinical symptoms, laboratory results, and findings of pathogenic causative organisms [6]. This information is usually used for diagnosing SA based on laboratory findings such as white blood cell (WBC) count, erythrocyte sedimentation rate, C-reactive protein (CRP) level in the blood, WBC from the synovial fluid, and the rate of polymorphonuclear leukocytes from the synovial fluid [6]. Although the CRP response is nonspecific for diagnosing SA, it has never been used as a single diagnostic tool but is used to aid the diagnosis of SA. CRP testing is cost-effective and easily performed in most hospitals; therefore, CRP is a well-established marker of inflammation and infection. It is usually used to screen for infectious activity and antibiotic efficacy [7].
The treatment of SA requires intravenous antibiotics and prompt intervention, such as open arthrotomy, arthroscopic treatment, and drainage using a syringe. Several studies have reported the effectiveness of arthroscopic treatment. Arthroscopic treatment is minimally invasive versus open arthrotomy as an initial treatment and results in better postoperative rehabilitation [1,8,9]. Despite its advantages, infection recurrence or aggravation rates were 10–50% in previous studies [1,10,11]. However, it is difficult to repeat arthroscopic treatment in such cases since it requires general or regional anesthesia.
Old age, the patient’s general condition, a pre-existing joint disease, delayed treatment, and bacteria isolated from the knee are factors associated with a poor prognosis. Acute SA usually has one or more poor prognostic factors [12]. The confirmations of operability and fasting time are necessary for general or regional anesthesia. Therefore, arthroscopic treatment delays are inevitable for the confirmation of operability and fasting time and can contribute to the poor prognosis of SA [1,12].
The lavage method using a tube inserted under local anesthesia does not require further anesthesia after the tube insertion. Therefore, it is possible that lavage without delay is not influenced by the patient’s general condition. This study aimed to compare the clinical results of lavage after tube insertion and arthroscopic treatment. This study hypothesized the following: first, lavage by tube would involve a shorter interventional delay; and second, it would have comparable outcomes, including laboratory results.
2. Materials and Methods
Between January 2014 and December 2018, patients with SA were treated arthroscopically and included in group I (n = 76). Patients diagnosed with SA between January 2019 and April 2022 were treated with lavage tubes and included in group II (n = 16). The inclusion criterion was SA of the knee joint as confirmed by laboratory markers in the blood and joint fluid obtained by arthrocentesis. The diagnosis of SA was supported by the Newman criteria [6] and the patient’s clinical symptoms. The clinical symptoms were as follows: systemic fever, tenderness of the entire joint, swelling, effusion, joint irritability, heating sensation, erythema, and limited motion. An elevated CRP level, serum leukocytosis, and an elevated synovial fluid WBC count and rate of polymorphonuclear leukocytes were used to diagnose SA. According to Newman [6], we included patients who had positive causative organisms, positive culture-associated hematogenous, and negative synovial fluid and blood cultures; however, pus or turbid fluid was present or the histological and laboratory evidence of SA was noted. Our detailed laboratory diagnostic criteria for SA were as follows: elevated serum CRP with WBC > 50,000/µL, polymorphonuclear leukocyte rate <75%, and synovial fluid glucose/serum glucose < 50%.
Serum laboratory results were evaluated weekly. The last follow-up day was at 3 months postoperative to confirm the resolved laboratory results and clinical symptoms. This study was approved by our local institutional review board. The study participants provided informed consent for the use of their data.
2.1. Evaluation Methods
To test our hypothesis, we investigated the following variables in all patients: demographics, underlying disorders (hypertension and diabetes), initial serum WBC count and CRP levels, synovial fluid WBC and polymorphonuclear cells, causative organisms, initial Kellgren–Lawrence (K-L) grade, lavage number, time to intervention, hospitalization day (length of hospital stay), and CRP normalization time. Clinical outcomes of the range of motion (ROM) at 3 months postoperative were evaluated using flexion contracture (FC) and further flexion (FF). Clinical scores were evaluated using the Western Ontario and McMaster University pain, stiffness, and function scales preoperatively and 3 months postoperatively.
The K-L grade was assessed using anteroposterior knee radiography before the intervention. The lavage number was the total number of SA interventions required for treatment. The time to intervention was defined as the time that elapsed after the diagnosis of SA. The CRP normalization time was defined as the time required to obtain the first normalized result. The FC and FF were measured at the femoral epicondyle as the knee joint while lying on the bed using a goniometer at a unit of 10°. The fully extended knee was assumed parallel to the horizontal line. The FF was defined as limited when active flexion was >100° considering the daily activity.
2.2. Surgical Technique
All patients with SA underwent primary arthroscopic intervention performed by an arthroscopic surgeon. Anteromedial and anterolateral portals were used as the routine arthroscopic portals, and >3 L of normal saline was used for the lavage. We performed a synovectomy and debridement of the inflammatory tissue. Subsequently, a Hemovac was maintained at 4 days postoperative.
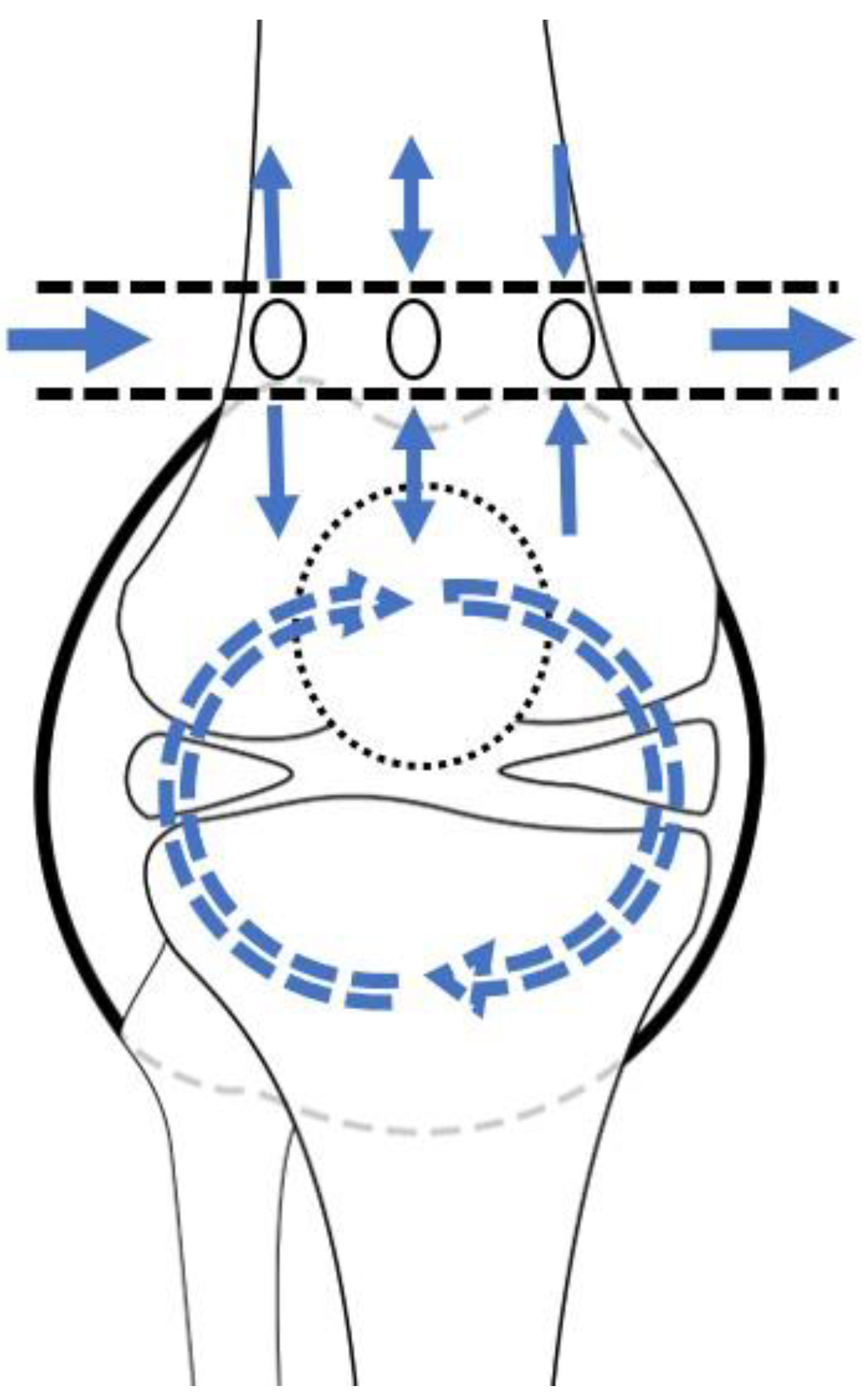
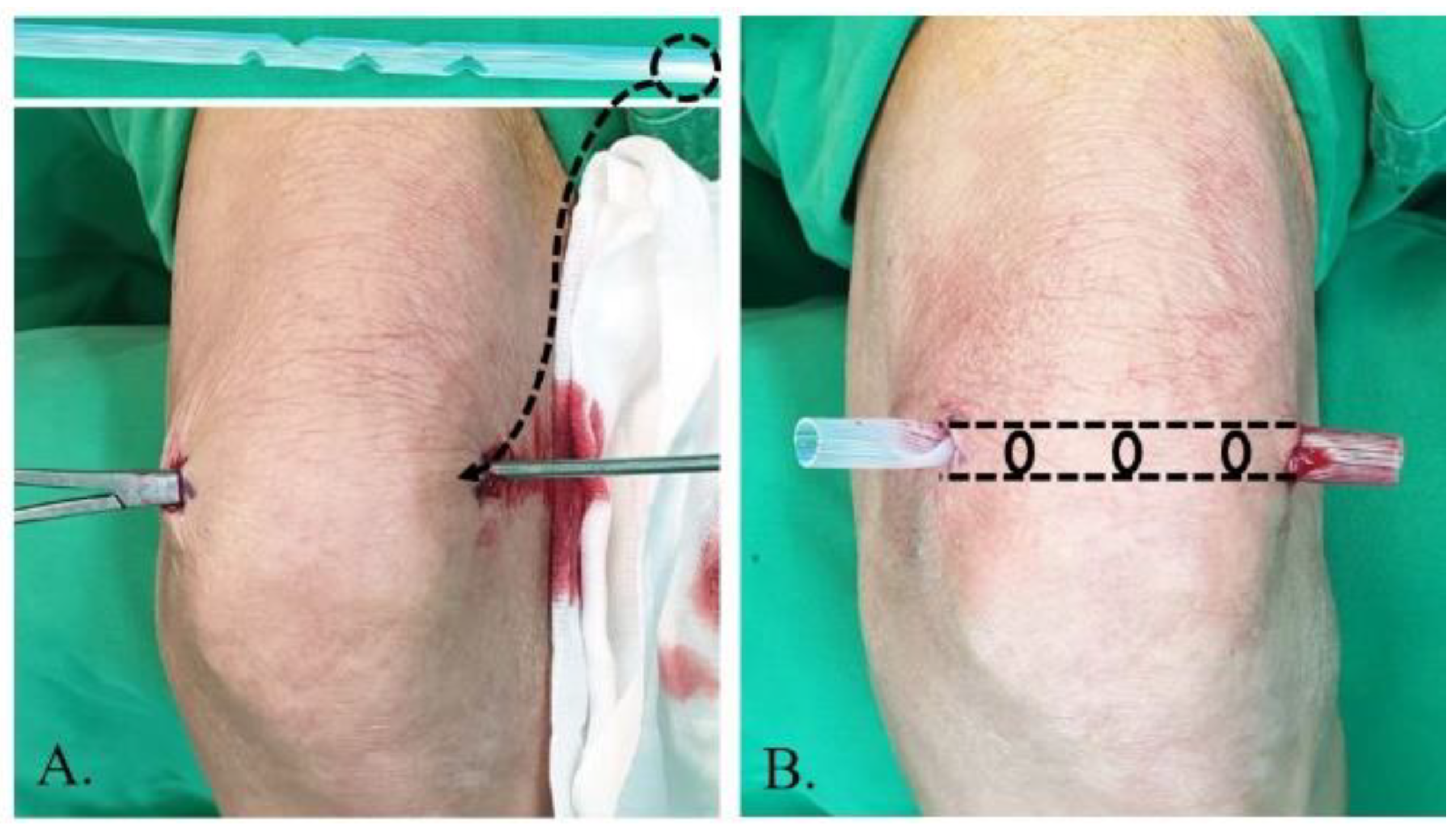
The principle of lavage using the tube is shown in Figure 1. Superomedial and superolateral portals were used for tube insertion under local anesthesia. First, a stab incision on the superolateral portal was made, mosquito forceps penetrating the joint capsule were inserted, and the portal was dilated. Second, a switching stick was inserted and a stab incision made on the superomedial portal. Third, the drain was retracted from the superomedial to superolateral portals using Kelly forceps or a beath pin. Finally, both sides of the drain were tagged to prevent falls (Figure 2). Once the tube was located, >3 L of normal saline was used for the lavage. The tube was maintained for approximately 4 days postoperative for natural drainage, and the dressing was changed daily. The tube was planned to be removed once the CRP level had decreased at approximately 4 days postoperative, but this did not occur, and an additional intervention was planned.
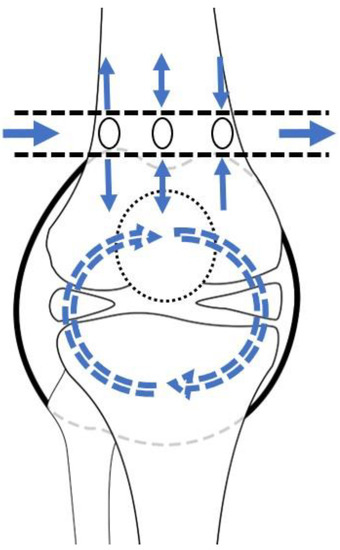
Figure 1.
Schematic diagram of lavage by tube. The arrows depict the saline flow.
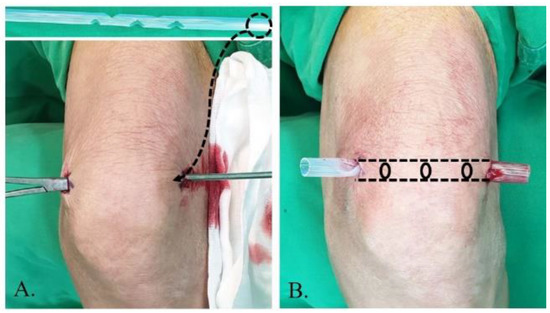
Figure 2.
Tube insertion strategy from the superolateral to superomedial portal performed under local anesthesia. (A) The tube is inserted using Kelly forceps and switching stick. (B) The tube is positioned by tagging.
The same antibiotic treatment regimen was used for the two treatment courses. A first-generation cephalosporin was used as the primary antibiotic until the organism was cultured. The antibiotic was changed appropriately once the causative organism was identified on the culture. Intravenous antibiotics were used for approximately 2–3 weeks and then changed to oral antibiotics.
2.3. Statistical Analysis
All statistical analyses were performed using SPSS (version 22.0; IBM Corp., Armonk, NY, USA). Intra- and inter-rater reliabilities of the measurement were assessed using an intra-class correlation coefficient (ICC) analysis. Data are reported as mean and standard deviation for continuous variables. The differences in quantitative variables (age, body mass index [BMI], serum and synovial fluid analysis, initial K-L grade, lavage number, time to intervention, hospitalization, CRP normalization time, and clinical scores) were analyzed using the Mann–Whitney test. Fisher’s exact test was used to compare qualitative variables (sex, right or left side, underlying disorders, causative organisms, ratio of initial K-L grade, and ROM). Statistical significance was set at p < 0.05.
3. Results
The ICC values were satisfactory for the radiographic measurements (inter-rater reliability, >0.81; intra-rater reliability, >0.80). The demographic characteristics of the patients by study group are presented in Table 1. There were more re-aggravation cases in group I than in group II, but the difference was not significant. No statistically significant differences were found between the groups in terms of age, sex ratio, site (right or left), BMI, underlying disorder, or follow-up period.

Table 1.
Patient demographics.
The initial laboratory results were not significantly different between the groups (Table 2). The mean CRP level was higher in group II (16.7 ± 9.1 mg/dL) than in group I (15.0 ± 8.2 mg/dL); however, the mean serum WBC count was higher in group I (10825 ± 3560/µL) than in group II (9787 ± 2339/µL). However, the differences were not statistically significant. The mean synovial fluid WBC count was higher in group II (71804 ± 41949/µL) than in group I (70934 ± 43322/µL) by 870, but the difference was not statistically significant. In both groups, the most common causative organism was Staphylococcus aureus (group I, 64% (49); group II: 60% (20)).

Table 2.
Laboratory results by study group.
Comparisons of patient characteristics are shown in Table 3. The initial K-L grades showed no statistically significant differences. There was no statistically significant difference in the number of lavages. The mean lavage number of group I (1.1 ± 0.3) was similar to that of group II (1.1 ± 0.4; p < 0.952). The time to the intervention was significantly delayed by approximately 15.9 h in group I (23.6 ± 15.6) compared to group II (6.7 ± 9.3, p < 0.001). There were no statistically significant differences in hospitalization or CRP normalization time. The CRP level and numerical rating scale (NRS) score change patterns are shown in Supplementary Figure S1, showing no significant differences.

Table 3.
Patients’ variables by study group.
A comparison of the clinical outcomes revealed no significant inter-group differences (Table 4). FC occurred more frequently than FF, but there were no significant inter-group differences of prevalence. There were no cases of FC > 10° and limited FF cases in group II, versus two cases of FC > 10° and five cases of limited FF in group I. However, this difference was not statistically significant. The preoperative clinical scores were similar between groups without significant differences. There were no significant differences in the clinical scores at 3 months postoperative. However, improved clinical scores at 3 months postoperative versus preoperative were observed in both groups.

Table 4.
Patients’ clinical outcomes by study group.
4. Discussion
The principal findings of this study were as follows. First, lavage by tube featured significantly less of an interventional delay as well as more opportunities for reintervention upon reaggravation. Second, lavage by tube showed no significant differences in outcomes, including laboratory results and functional outcomes at 3 months postoperative. Thus, our hypotheses were verified.
The prevalence of SA is increasing owing to societal aging, the use of intra-articular injections, and the inappropriate use of antibiotics [3,13,14,15,16]. The definitive diagnostic criteria for SA are controversial. However, SA can lead to sequelae and life-threatening conditions in a short period of time because of its aggressive progression. Therefore, if findings suggestive of SA are observed, rapid treatment significantly influences prognosis. Reducing the time to intervention and summarizing the procedure are important, especially for patients with general weakness [3,4].
Appropriate early intervention is important in patients with SA. Higher rates of mortality, osteomyelitis, osteonecrosis, severe articular destruction, and stiffness may occur if the intervention is delayed [17]. Nil per os (NPO) is one problem associated with arthroscopic treatment for SA in older patients or those with comorbidities. Furthermore, NPO for general or spinal anesthesia causes delayed intervention. Patients with SA usually have comorbidities such as immunodeficiency, diabetes mellitus, and pre-existing joint diseases. Interventions performed under local anesthesia do not require fasting, which is critical for older or high-risk patients [18,19]. In this study, when lavage under local anesthesia was used, no local or systemic complications occurred. Thus, interventions performed under local anesthesia can be considered advantageous for patients with various underlying diseases or known side effects of general anesthesia [1].
The treatment of SA with surgical drainage of the infection is highly controversial [20,21]. Mathews et al. [9] reported a lack of robust clinical evidence of SA. However, the removal of purulent material is essential for successful treatment, and washout is mainly performed during arthroscopy or arthrotomy with or without synovectomy and debridement. Tsumura et al. [22] treated knees with SA using arthrotomy. They also performed a synovectomy, debridement, and continuous irrigation. Nine of eleven patients were cured, and the average Japanese Orthopedic Association score was 84 ± 11. However, deteriorated osteoarthritis occurred in all but one patient. Stutz et al. [23] studied the arthroscopic treatment of SA using Gächter criteria [24]. Twenty-one of twenty-two stage I patients were treated with arthroscopic irrigation combined with antibiotics. However, 52% of stage II and 75% of stage III patients required additional arthroscopic irrigation procedures. Johns et al. [1] reported that arthroscopic treatment was more successful in irrigation procedures and boasted a better range of motion compared with arthrotomy. However, 50% (n = 60) of the 119 patients required additional arthroscopic procedures. Furthermore, 71% (n = 30) of the 42 patients who underwent arthrotomy required additional procedures. Most surgeons prefer arthroscopy for managing septic knees because they can be drained and the debridement defined by visualization [1]. However, consensus is lacking regarding the treatment of debridement using synovectomy [20]. In this study, drainage of SA using a tube was effective as evidenced by the decline in the CRP level and NRS score.
This study has some limitations. First, patients who underwent lavage by tube demonstrated a slower decrease in NRS scores than those who underwent general anesthesia. The patients were treated with local anesthesia when the tube was first inserted. Subsequently, the penetrated tube remained in the suprapatellar synovial cavity. This could have caused the pain in the early phase of the lavage. Second, a relatively small number of patients were treated using a lavage tube, and the statistical analyses were performed on a small number of samples. Furthermore, the delay to intervention was significantly different between groups; therefore, the results could be confirmed as statistically significant even with a relatively small number of patients. Finally, this study had a short-term follow-up period (approximately 3 months). However, considering the period required for antibiotic treatment, it was sufficient. Therefore, it was neither too soon nor too late to determine the treatment outcome.
5. Conclusions
The lavage by tube featured a significantly shorter interventional delay time than arthroscopy, while the CRP decrease rate did not differ between the groups. Moreover, lavage by tube showed no significant differences in outcomes, including laboratory results and functional outcomes at 3 months postoperative.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics13030371/s1.
Author Contributions
Conceptualization, B.H.O.; data curation, H.J.Y.; formal analysis, Y.M.H. and I.U.Y.; resources, W.J.S. and H.J.Y.; software, H.J.Y.; supervision, B.H.O.; writing—original draft, H.J.Y.; writing—review and editing, H.J.Y. and B.H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by Konyang University Myunggok Research Fund of 2016-08.
Institutional Review Board Statement
This study was approved by the Konyang University Hospital Institutional Review Board (IRB No: KYUH 2021-06-014).
Informed Consent Statement
The requirement for informed consent was waived owing to the retrospective nature of this study and its use of anonymized patient data.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johns, B.P.; Loewenthal, M.R.; Dewar, D.C. Open Compared with Arthroscopic Treatment of Acute Septic Arthritis of the Native Knee. J. Bone Jt. Surg. Am. 2017, 99, 499–505. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Liu, Y.; Chen, W.; Li, J.; Yuan, Z.; Yong, B.; Xu, H. Delayed treatment of septic arthritis in the neonate: A review of 52 cases. Medicine 2016, 95, e5682. [Google Scholar] [CrossRef]
- Dubost, J.J.; Soubrier, M.; Sauvezie, B. Pyogenic arthritis in adults. Jt. Bone Spine 2000, 67, 11–21. [Google Scholar]
- Jeon, Y.D.; Moon, J.Y.; Son, J.H.; Kim, J.M.; Choi, Y. The Efficacy of Arthroscopic Debridement with Continuous Irrigation in Failed Arthroscopic Debridement for Septic Arthritis of the Knee. J. Korean Orthop. Assoc. 2016, 51, 308–314. [Google Scholar] [CrossRef]
- Chouk, M.; Verhoeven, F.; Sondag, M.; Guillot, X.; Prati, C.; Wendling, D. Value of serum procalcitonin for the diagnosis of bacterial septic arthritis in daily practice in rheumatology. Clin. Rheumatol. 2019, 38, 2265–2273. [Google Scholar] [CrossRef]
- Newman, J.H. Review of septic arthritis throughout the antibiotic era. Ann. Rheum. Dis. 1976, 35, 198–205. [Google Scholar] [CrossRef]
- Talebi-Taher, M.; Shirani, F.; Nikanjam, N.; Shekarabi, M. Septic versus inflammatory arthritis: Discriminating the ability of serum inflammatory markers. Rheumatol. Int. 2013, 33, 319–324. [Google Scholar] [CrossRef]
- Flores-Robles, B.J.; Palop, M.J.; Sanchinel, A.A.S.; Andrus, R.F.; Vicente, A.R.; Pérez, M.I.S.; Malpartida, M.E.; Giráldez, C.R.; Argumanez, C.M.; Alcázar, L.F.V.; et al. Medical Versus Surgical Approach to Initial Treatment in Septic Arthritis: A Single Spanish Center’s 8-Year Experience. J. Clin. Rheumatol. 2019, 25, 4–8. [Google Scholar] [CrossRef]
- Mathews, C.J.; Kingsley, G.; Field, M.; Jones, A.; Weston, V.C.; Phillips, M.; Walker, D.; Coakley, G. Management of septic arthritis: A systematic review. Postgrad. Med. J. 2008, 84, 265–270. [Google Scholar] [CrossRef]
- Wirtz, D.C.; Marth, M.; Miltner, O.; Schneider, U.; Zilkens, K.W. Septic arthritis of the knee in adults: Treatment by arthroscopy or arthrotomy. Int. Orthop. 2001, 25, 239–241. [Google Scholar] [CrossRef]
- Agarwal, N.; Santra, S.; Sarkar, P.S.; Mandal, A. Arthroscopic drainage of acute septic arthritis of knee in adult. Int. J. Orthop. Sci. 2016, 2, 91–93. [Google Scholar] [CrossRef]
- Kang, T.; Lee, J.K. Host Factors Affect the Outcome of Arthroscopic Lavage Treatment of Septic Arthritis of the Knee. Orthopedics 2018, 41, e184–e188. [Google Scholar] [CrossRef]
- Lee, S.H.; Heo, H.Y.; Chang, J.S.; Song, H.Y.; Moon, J.Y. A Clinical Study Upon Acute Osteomyelitis and Pyogenic Arthritis. J. Korean Orthop. Assoc. 2018, 26, 1891–1897. [Google Scholar] [CrossRef]
- Soe, S.-S.; Ha, D.-J.; Kim, C.-W.; Kim, K.-W.; Seo, J.-H. Etiologic transition of septic arthritis of the knee. J. Korean Knee Soc. 2008, 20, 44–49. [Google Scholar]
- Dubost, J.J.; Soubrier, M.; De Champs, C.; Ristori, J.M.; Bussiére, J.L.; Sauvezie, B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann. Rheum. Dis. 2002, 61, 267. [Google Scholar] [CrossRef]
- Hindle, P.; Davidson, E.; Biant, L.C. Septic arthritis of the knee: The use and effect of antibiotics prior to diagnostic aspiration. Ann. R. Coll. Surg. Engl. 2012, 94, 351–355. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Schuur, J.D.; Everett, W.W.; Pines, J.M. Evidence-based diagnostics: Adult septic arthritis. Acad. Emerg. Med. 2011, 18, 781–796. [Google Scholar] [CrossRef]
- Yi, J.W.; Oh, B.H.; Heo, Y.M.; Jang, M.G.; Min, Y.K.; Seo, K.D. Effectiveness of Drain Insertion and Irrigation in the Treatment of Septic Arthritis of the Knee under Local Anesthesia. J. Korean Orthop. Assoc. 2021, 56, 310–316. [Google Scholar] [CrossRef]
- Scarlett, M.; Crawford-Sykes, A.; Nelson, M. Preoperative starvation and pulmonary aspiration. New perspectives and guidelines. West Indian Med. J. 2002, 51, 241–245. [Google Scholar]
- Jaffe, D.; Costales, T.; Jauregui, J.J.; Koenig, S.; Weir, T.B.; Greenwell, P.; Christian, M.; Henn, R.F. Current surgical practice for septic arthritis of the knee in the United States. J. Orthop. 2021, 25, 88–92. [Google Scholar] [CrossRef]
- Kortekangas, P. Bacterial Arthritis in the Elderly. Drugs Aging 1999, 14, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, H.; Ikeda, S.; Torisu, T. Debridement and continuous irrigation for the treatment of pyogenic arthritis caused by the use of intra-articular injection in the osteoarthritic knee: Indications and outcomes. J. Orthop. Surg. 2005, 13, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Stutz, G.; Kuster, M.S.; Kleinstuck, F.; Gachter, A. Arthroscopic management of septic arthritis: Stages of infection and results. Knee Surg. Sport. Traumatol. Arthrosc. 2000, 8, 270–274. [Google Scholar] [CrossRef]
- Gaechter, A. Arthroscopic lavage for joint infections. Orthop. Traumatol. 1993, 2, 104–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).