Primary Effusion Lymphoma: A Rare and Challenging Diagnosis for Recurrent Pleural Effusion
Abstract
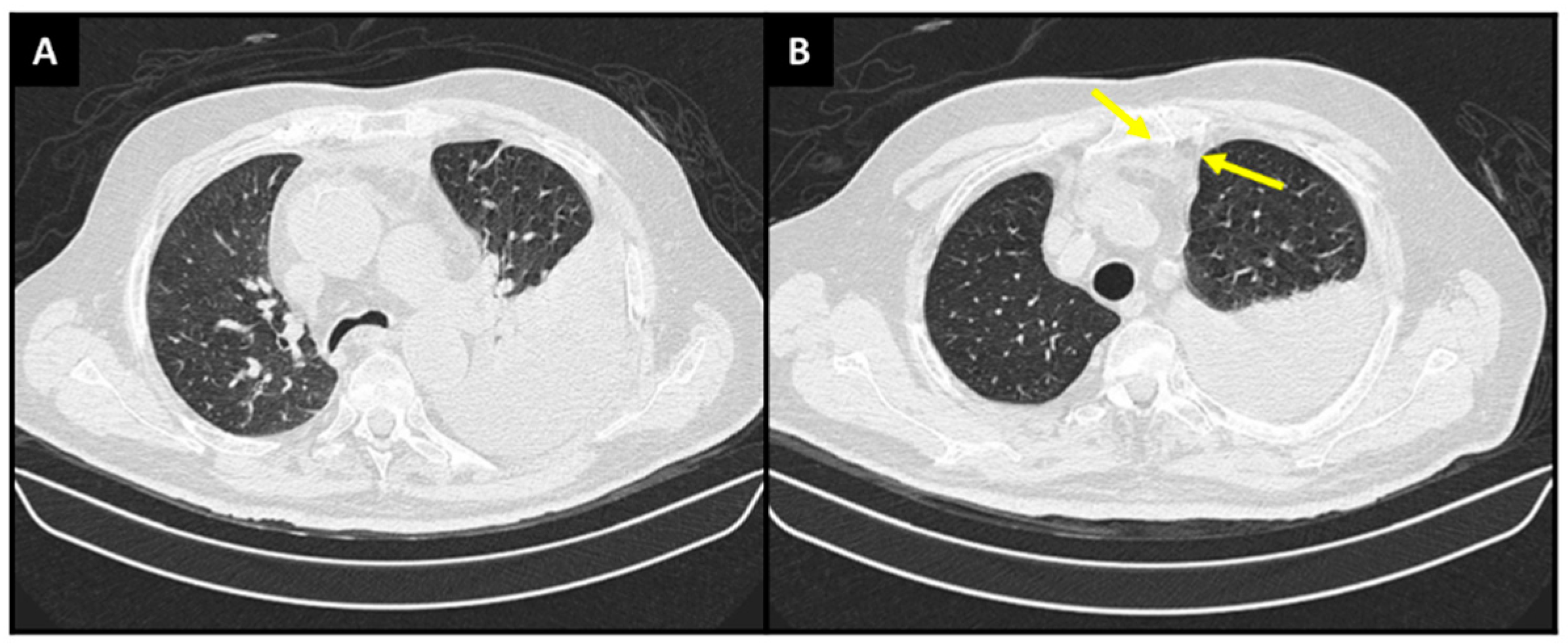
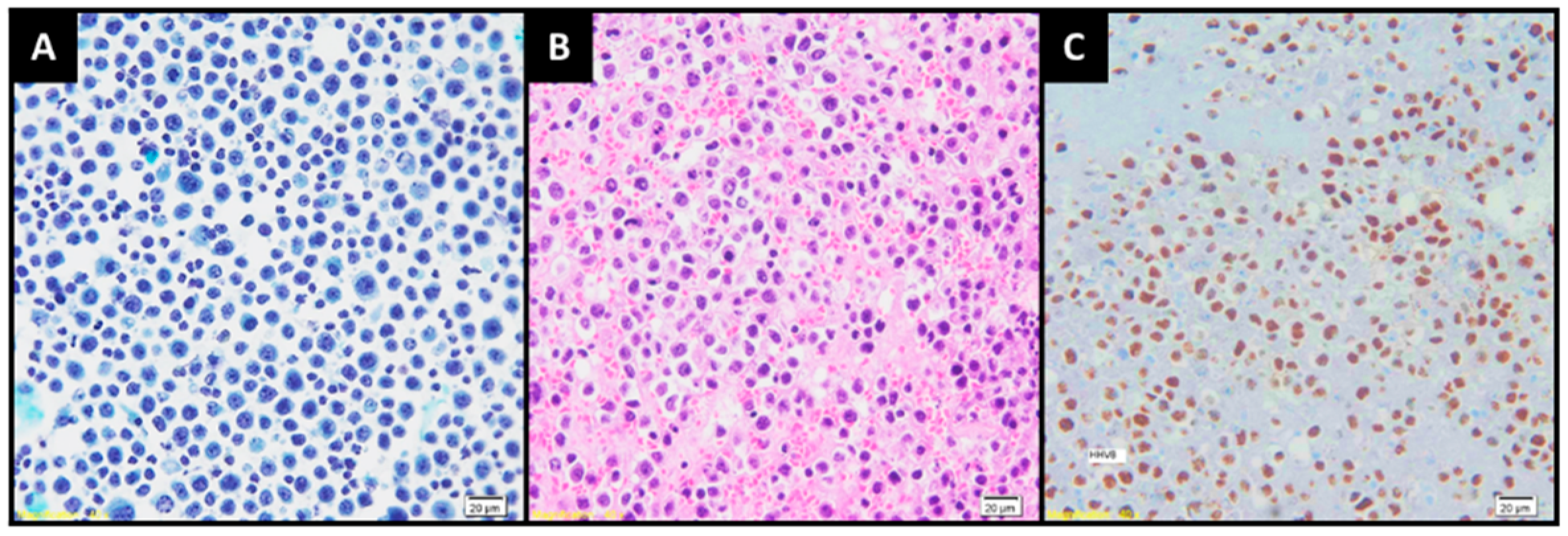
:
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreiro, L.; Toubes, M.E.; San José, M.E.; Suárez-Antelo, J.; Golpe, A.; Valdés, L. Advances in Pleural Effusion Diagnostics. Expert Rev. Respir. Med. 2020, 14, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Feller-Kopman, D.; Light, R. Pleural Disease. N. Engl. J. Med. 2018, 378, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, S.; Gonzalez, A.V. Evaluation of the Patient with Pleural Effusion. CMAJ 2018, 190, E291–E295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, E.E.; Anderson, P.B. Diagnosis of Pleural Effusion: A Systematic Approach. Am. J. Crit. Care 2011, 20, 119–128. [Google Scholar] [CrossRef]
- Walker, S.; Mercer, R.; Maskell, N.; Rahman, N.M. Malignant Pleural Effusion Management: Keeping the Flood Gates Shut. Lancet Respir. Med. 2020, 8, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Hayakawa, F.; Kiyoi, H. Biology and Management of Primary Effusion Lymphoma. Blood 2018, 132, 1879–1888. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-B.; Rahemtullah, A.; Hochberg, E. Primary Effusion Lymphoma. Oncologist 2007, 12, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Arora, N.; Gupta, A.; Sadeghi, N. Primary Effusion Lymphoma. Curr. Opin. Pulm. Med. 2017, 23, 365–370. [Google Scholar] [CrossRef]
- Aguilar, C.; Laberiano, C.; Beltran, B.; Diaz, C.; Taype-Rondan, A.; Castillo, J.J. Clinicopathologic Characteristics and Survival of Patients with Primary Effusion Lymphoma. Leuk. Lymphoma 2020, 61, 2093–2102. [Google Scholar] [CrossRef]
- Grimm, K.E.; O’Malley, D.P. Aggressive B Cell Lymphomas in the 2017 Revised WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. Ann. Diagn. Pathol. 2019, 38, 6–10. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, A.; Vaccher, E.; Gloghini, A. Hematologic Cancers in Individuals Infected by HIV. Blood 2022, 139, 995–1012. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chen, B.-J.; Chuang, S.-S. Primary Effusion Lymphoma: A Timely Review on the Association with HIV, HHV8, and EBV. Diagnostics 2022, 12, 713. [Google Scholar] [CrossRef]
- Calvani, J.; Gérard, L.; Fadlallah, J.; Poullot, E.; Galicier, L.; Robe, C.; Garzaro, M.; Bertinchamp, R.; Boutboul, D.; Cuccuini, W.; et al. A Comprehensive Clinicopathologic and Molecular Study of 19 Primary Effusion Lymphomas in HIV-Infected Patients. Am. J. Surg. Pathol. 2022, 46, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, M.; Müller, M.; Wyen, C.; Bogner, J.; Thomssen, H.; Wasmuth, J.; Wolf, T.; Hoffmann, C.; Schommers, P. Characteristics and Outcome of Human Immunodeficiency Virus (HIV)-associated Primary Effusion Lymphoma as Observed in the German HIV-related Lymphoma Cohort Study. Br. J. Haematol. 2021, 194, 642–646. [Google Scholar] [CrossRef]
- Kropf, J.; Gerges, M.; Perez Perez, A.; Ellis, A.; Mathew, M.; Ayesu, K.; Ge, L.; Carlan, S.J. T Cell Primary Effusion Lymphoma in an HIV-Negative Man with Liver Cirrhosis. Am. J. Case Rep. 2020, 21, e919032. [Google Scholar] [CrossRef] [PubMed]
- Paner, G.P.; Jensen, J.; Foreman, K.E.; Reyes, C.V. HIV and HHV-8 Negative Primary Effusion Lymphoma in a Patient with Hepatitis C Virus-Related Liver Cirrhosis. Leuk. Lymphoma 2003, 44, 1811–1814. [Google Scholar] [CrossRef]
- Sasaki, Y.; Isegawa, T.; Shimabukuro, A.; Yonaha, T.; Yonaha, H. Primary Effusion Lymphoma in an Elderly HIV-Negative Patient with Hemodialysis: Importance of Evaluation for Pleural Effusion in Patients Receiving Hemodialysis. Case Rep. Nephrol. Urol. 2014, 4, 95–102. [Google Scholar] [CrossRef]
- Zanelli, M.; Sanguedolce, F.; Zizzo, M.; Palicelli, A.; Bassi, M.C.; Santandrea, G.; Martino, G.; Soriano, A.; Caprera, C.; Corsi, M.; et al. Primary Effusion Lymphoma Occurring in the Setting of Transplanted Patients: A Systematic Review of a Rare, Life-Threatening Post-Transplantation Occurrence. BMC Cancer 2021, 21, 468. [Google Scholar] [CrossRef]
- Malik, P.; Khader, S.N.; Asiry, S. A Rare Case of Primary Effusion Lymphoma in HIV Negative Patient: Diagnostic Challenges and Literature Review. Diagn. Cytopathol. 2021, 49, 785–789. [Google Scholar] [CrossRef]
- Hu, Z.; Pan, Z.; Chen, W.; Shi, Y.; Wang, W.; Yuan, J.; Wang, E.; Zhang, S.; Kurt, H.; Mai, B.; et al. Primary Effusion Lymphoma: A Clinicopathological Study of 70 Cases. Cancers 2021, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Cook, J.R.; Elsheikh, T.M. Primary Effusion Lymphoma in Human Immune Deficiency (HIV)-negative Non-organ Transplant Immunocompetent Patients. Diagn. Cytopathol. 2020, 48, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, I.; Rossi, G.; Rullo, E.; Ascoli, V. Classic kshv/hhv-8-positive primary effusion lymphoma (pel): A systematic review and meta-analysis of case reports. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022020. [Google Scholar] [CrossRef]
- El-Fattah, M.A. Clinical Characteristics and Survival Outcome of Primary Effusion Lymphoma: A Review of 105 Patients. Hematol. Oncol. 2017, 35, 878–883. [Google Scholar] [CrossRef]
- Baidoun, F.; Moustafa, M.A.; Tun, H.W.; Hill, B.T. Clinical Characteristics and Survival Outcomes of Primary Effusion Lymphoma: A National Cancer Database Study. Clin. Lymphoma Myeloma Leuk. 2022, 22, e485–e494. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Xiao, P. Primary Effusion Lymphoma. Arch. Pathol. Lab. Med. 2013, 137, 1152–1154. [Google Scholar] [CrossRef] [Green Version]
- Rossi, G.; Cozzi, I.; della Starza, I.; de Novi, L.A.; de Propris, M.S.; Gaeta, A.; Petrucci, L.; Pulsoni, A.; Pulvirenti, F.; Ascoli, V. Human Herpesvirus-8–Positive Primary Effusion Lymphoma in HIV-negative Patients: Single Institution Case Series with a Multidisciplinary Characterization. Cancer Cytopathol. 2021, 129, 62–74. [Google Scholar] [CrossRef]
- Cesarman, E.; Chadburn, A.; Rubinstein, P.G. KSHV/HHV8-Mediated Hematologic Diseases. Blood 2022, 139, 1013–1025. [Google Scholar] [CrossRef]
- Gisriel, S.D.; Yuan, J.; Braunberger, R.C.; Maracaja, D.L.V.; Chen, X.; Wu, X.; McCracken, J.; Chen, M.; Xie, Y.; Brown, L.E.; et al. Human Herpesvirus 8-Negative Effusion-Based Large B-Cell Lymphoma: A Distinct Entity with Unique Clinicopathologic Characteristics. Mod. Pathol. 2022, 35, 1411–1422. [Google Scholar] [CrossRef]
- Gathers, D.; Galloway, E.; Kelemen, K.; Rosenthal, A.; Gibson, S.; Munoz, J. Primary Effusion Lymphoma: A Clinicopathologic Perspective. Cancers 2022, 14, 722. [Google Scholar] [CrossRef]
- Sukswai, N.; Lyapichev, K.; Khoury, J.D.; Medeiros, L.J. Diffuse Large B-Cell Lymphoma Variants: An Update. Pathology 2020, 52, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessamy, K.; Ojevwe, F.O.; Doobay, R.; Naous, R.; Yu, J.; Lemke, S.M. Primary Effusion Lymphoma: Is Dose-Adjusted-EPOCH Worthwhile Therapy? Case Rep. Oncol. 2016, 9, 273–279. [Google Scholar] [CrossRef] [PubMed]
- El-Ayass, W.; Yu, E.M.; Karcher, D.S.; Aragon-Ching, J.B. Complete response to EPOCH in a patient with HIV and extracavitary primary effusion lymphoma involving the colon: A case report and review of literature. Clin. Lymphoma Myeloma Leuk. 2012, 12, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Goto, H.; Yotsumoto, M. Current status of treatment for primary effusion lymphoma. Intractable Rare Dis. Res. 2014, 3, 65–74. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, L.J.; Mohrbacher, S.; Neves, P.D.M.d.M.; Zacchi, F.F.S.; Medeiros, I.U.D.; Sato, V.A.H.; Oliveira, É.S.; Pereira, L.V.B.; Cuvello-Neto, A.L.; Baiocchi, O.; et al. Primary Effusion Lymphoma: A Rare and Challenging Diagnosis for Recurrent Pleural Effusion. Diagnostics 2023, 13, 370. https://doi.org/10.3390/diagnostics13030370
Pereira LJ, Mohrbacher S, Neves PDMdM, Zacchi FFS, Medeiros IUD, Sato VAH, Oliveira ÉS, Pereira LVB, Cuvello-Neto AL, Baiocchi O, et al. Primary Effusion Lymphoma: A Rare and Challenging Diagnosis for Recurrent Pleural Effusion. Diagnostics. 2023; 13(3):370. https://doi.org/10.3390/diagnostics13030370
Chicago/Turabian StylePereira, Letícia Jacome, Sara Mohrbacher, Precil Diego Miranda de Menezes Neves, Flavia Fernandes Silva Zacchi, Ivan Ucella Dantas Medeiros, Victor Augusto Hamamoto Sato, Érico Souza Oliveira, Leonardo Victor Barbosa Pereira, Américo Lourenço Cuvello-Neto, Otávio Baiocchi, and et al. 2023. "Primary Effusion Lymphoma: A Rare and Challenging Diagnosis for Recurrent Pleural Effusion" Diagnostics 13, no. 3: 370. https://doi.org/10.3390/diagnostics13030370
APA StylePereira, L. J., Mohrbacher, S., Neves, P. D. M. d. M., Zacchi, F. F. S., Medeiros, I. U. D., Sato, V. A. H., Oliveira, É. S., Pereira, L. V. B., Cuvello-Neto, A. L., Baiocchi, O., & Chocair, P. R. (2023). Primary Effusion Lymphoma: A Rare and Challenging Diagnosis for Recurrent Pleural Effusion. Diagnostics, 13(3), 370. https://doi.org/10.3390/diagnostics13030370





