Abstract
This systematic review aims to investigate the possibilities of ultrasound imaging in the field of periodontal tissues exploration to visualize periodontal anatomical structures and to assess reliability in clinical evaluation using the PRISMA guidelines. An electronic search through the MEDLINE database was realized to identify studies that have explored ultrasonography in the field of periodontal imaging published from 2000 to March 2022. The search resulted in 245 records; after exclusions, a total of 15 papers were included in the present review. Various publications have shown the possibility of using intraoral ultrasound for a precise exploration of intraoral tissues and to perform measurements of periodontal structures. Studies argue that ultrasounds open the prospect of a complete paradigm shift on the diagnosis and follow-up of periodontal disease. However, there is currently no clinical device dedicated to periodontal ultrasound. This field is still under-studied, and studies are needed to explore the large field of applications from periodontal assessment to treatment reassessment, including surgery. Researchers should focus their efforts to develop special intraoral ultrasound device and explore the possibilities of clinical periodontal applications.
1. Introduction
Periodontitis is an inflammatory disease of the periodontium (tooth supporting tissues) of bacterial origin. It can be treated and controlled successfully, to avoid the progressive destruction of the tissues (the gum, the periodontal ligament and the alveolar bone) [1]. A patient with periodontitis treated and stabilized with initial treatment nevertheless presents a risk of recurrence. Therefore, a continuous and individual assessment of the patient’s risks is necessary in order to monitor the non-evolution of the pathology [2]. Clinically, gingival health is defined by the absence of bleeding on probing, erythema and edema, patient symptoms, attachment and bone loss [3]. The bleeding on probing test performed by a periodontal probe into the bottom of the sulcus is one element of monitoring the health or inflammation of the gingival tissues that is best documented in the literature [4]. This test is important because it has been shown that bleeding is an earlier sign of gingivitis than are the visual signs of inflammation [5]. Bleeding on probing is an indicator of pathological phenomenon with presence of pocketing. On the other hand, it has been demonstrated that bleeding on probing provoked with pressures greater than 0.25 Newtons (N) results in false-positive readings in lack of pathology [6]. Despite this, periodontal probing has its limitations. This test is time-consuming, and operator-dependent errors are possible, such as incorrect angulation of the probe, excessive probing pressure on the gingival tissues, and incorrect reading of the measurement on the probe. An error in calculating the loss of attachment may occur. Reading errors can also result from interference from calculus, the presence of an overhanging restoration, or the contour of the crown. Other factors, such as the size of the probe tip, angle of probe insertion, accuracy of probe calibration, and degree of inflammation in the periodontal tissues affect sensitivity and the reproducibility of the measurements [7].
Radiographic evaluation is an essential element of periodontal diagnosis. A radiograph of periodontal health includes a normal lamina dura, presence of bone in furcation areas, and 2 mm distance from the most coronal portion of the alveolar bone crest to the cementoenamel junction of the tooth [8]. Alveolar bone loss has the result of bone resorption in response of inflammatory process during periodontitis development. That is why it is very difficult to evaluate clinical periodontal health only using routine radiographs [3].
In view of these limitations, it is important to consider a new type of exploration with less time-consuming and easier to use in clinical practice. Since the middle of 1980s, several publications have presented ultrasound imaging applied to the exploration of the oral cavity in animals [9]. Ultrasonographic of the periodontal tissues could constitute an alternative for the visualization of the periodontal structures and for the detection of periodontal disease with the detection of periodontal pocket and the presence of deep tissue inflammation.
Ultrasonography is an ultrasonic medical imaging technique used in a large field of medicine. The ultrasounds used in medical ultrasonography are mechanical waves which propagate in a medium. A mechanical wave is a local deformation which is propagated step by step in solid, liquid or gas medium [10,11]. The frequency of the wave corresponds to the number of periods per second in Hertz (Hz). The field of ultrasound is between 20 kHz and 200 MHz [12]. In medical imaging, ultrasound scanners from 1 to 15 MHz are used. High frequency ultrasound is defined above 20 MHz [13]. This non-ionizing technique allows soft tissues exploration of the human body through ultrasonic waves, which makes it possible to have resolutions between 0.4 and 2 mm. High frequency techniques were used for medical applications such as obstetric, cardiology, abdominal and muscular explorations [14,15,16]. Optimization in B-mode ultrasound has permitted to achieve the best possible image quality in medical applications and develop a wild field of applications [17]. To obtain resolutions better than 0.1 mm, it is necessary to use ultrasound frequencies above 20 MHz. This field of ultra-high resolution ultrasound imaging has permitted the exploration of the skin [18] and the eye [19] for small animal imaging or angiology by submillimeter resolution of small structures [16,20].
Ultrasound imaging has the potential to complement routine radiographic imaging in periodontology and provides instantaneous images of anatomical structures during the same examination. The practitioner can directly modify the incidence of the probe according to the tissue anatomy. It can be used without risk in all patients because it is not ionizing. Overcoming the phenomenon of superposition is possible with this type of imaging, unlike with 2D imaging [21]. For application in oral and dental imaging, its qualities depend on its ability to accurately capture these complex structures in a simple and rapid manner. These complementary methods are attractive because they are non-irradiating, non-invasive and comfortable for the patient since they allow direct reading of the images.
In these perspectives, studies showed the validity and reliability of ultrasonography in the measurement not only of gingival thickness but also of other periodontal structures which cannot be assessed through inspection and palpation [22,23]. A 25 MHz high-frequency resolution ultrasound probe, specially designed for intraoral applications, provides additional morphological information that is not accessible by conventional dental X-rays in daily dental practice with a large-scale of application in the diagnosis of pathologies [24].
Therefore, the aim of this systematic review was to investigate the possibilities of ultrasound imaging in the exploration of periodontal tissues to visualize periodontal anatomical structures and to assess reliability in clinical evaluation.
2. Materials and Methods
This systematic review was reported according to the PRISMA guidelines for Systematic Reviews [25].
2.1. Search Strategy
An electronic search was conducted through the MEDLINE (PubMed) database to identify publications that met the inclusion criteria. (9) The search was performed from 2000 up to March 2022, in order to identify the studies that explore the contribution of ultrasound imaging in periodontology, using the following search terms and keywords alone or in combination with the Boolean operator “AND”/“OR” according to the following equation (“alveolar ridge” [Mesh]) OR (“alveolar bone” [Mesh]) OR (“caries” [Mesh]) OR (“cementoenamel junction” [Mesh]) OR (“periodontal attachment” [Mesh]) OR (“periodontal probing” [Mesh]) OR (“periodontal charting” [Mesh]) OR (“dental implant” [Mesh]) OR (“periodontitis” [Mesh]) OR (“gingivitis” [Mesh]) OR (“periodontium” [Mesh]) AND (“sonography” [Mesh]) OR (“diagnostic ultrasound” [Mesh]) OR (“ultrasonography” [Mesh]).
2.2. Study Detection
References of the eligible studies on the topic were manually checked, and two independent operators (F.D. and M.R.) screened the studies according to the inclusion/exclusion criteria. In case of disagreement, a 3rd reviewer (R.M.) was asked.
Inclusion and Exclusion Criteria
We included experimental or clinical studies (longitudinal, cross-sectional or randomized studies), in healthy patients or patients with periodontitis, that explored the link between ultrasound imaging and periodontology or oral tissues or explored an association between ultrasonography and histological or histometric characterization of periodontal tissues. According to the type of ultrasound imaging, we included only studies that presented a mode B ultrasound device. We excluded conferences, abstracts, reviews and editorials. Publications concerning the detection of carious lesions and publications relating the utilization of ultrasound to increase the healing and osseointegration potential of dental implants were not included, and we excluded publications related to other medical disciplines.
Each study that met the inclusion criteria was analyzed from several aspects such as authors, date of publication, study design, ultrasound device description, image classification, results, limitations and discussion.
2.3. Data Collection
The list of titles and abstracts to identify the potentially relevant papers based on the inclusion criteria previously announced were independently screened by two reviewers (F.D. and R.M.). If the abstracts were identified as non-relevant, the full studies were reviewed to decide if they should be included or not according to the inclusion criteria. A scan of the references of the previously selected articles completed the selection to improve the systematic review. When a discrepancy in the selection decision appeared, the two reviewers engaged in discussion until a consensus was found. If needed, a third reviewer (M.R.) resolved the possible conflicts concerning eligibility.
3. Results
3.1. Study Selection
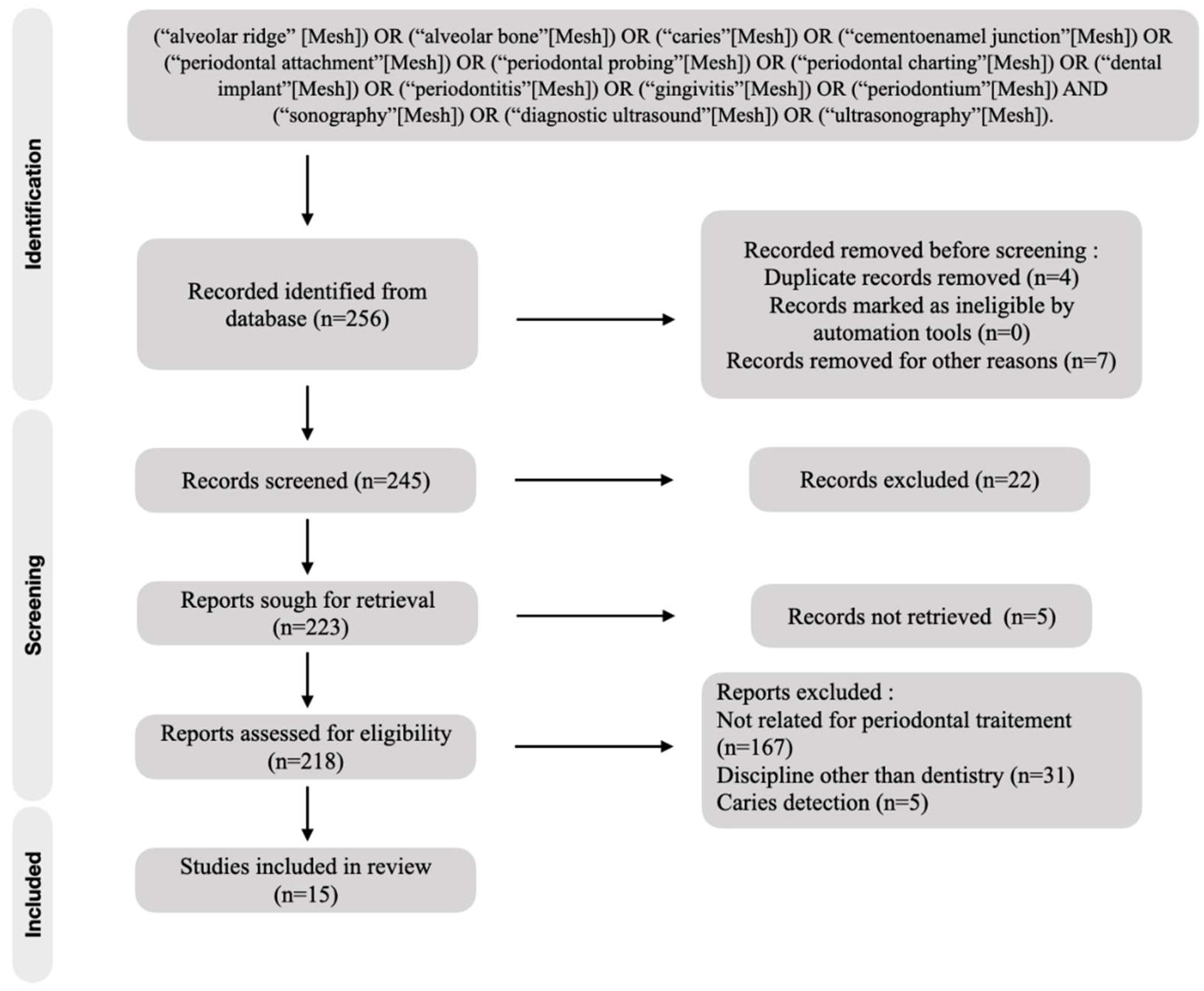
The initial studies retrieved from the databases were first selected, and studies that met the eligibility criteria were reviewed and analyzed. After 220 reading abstracts and 7 full articles, only 15 articles were selected from the 245 studies. The percentage of agreement between the reviewers was 100%. The complementary detection did not result in the selection of new publication for analysis. Finally, a total of 15 articles were included in the analysis (Figure 1).
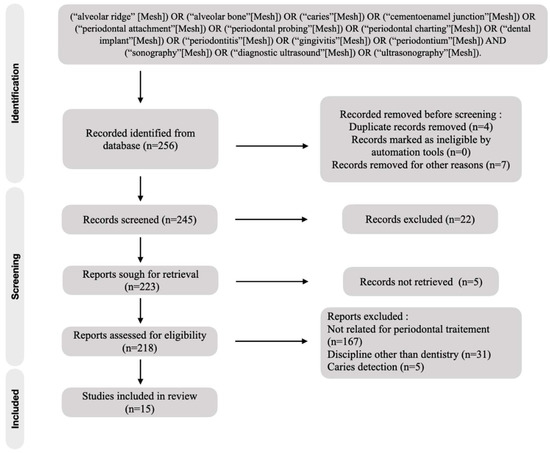
Figure 1.
Flow chart of included studies.
Among the 15 studies included in the analysis (Table 1), we were able to distinguish four common themes emerging such as the evolution of trials, ultrasound device presentation, the description of periodontal tissues using ultrasonography and comparative ultrasonography measurement. Studies included in the analysis were in vitro or ex vivo studies or clinical comparative trials using different tools to validate the use of ultrasound devices. Most of them applied low ultrasound frequencies.

Table 1.
Data analysis.
3.2. Test Evolution
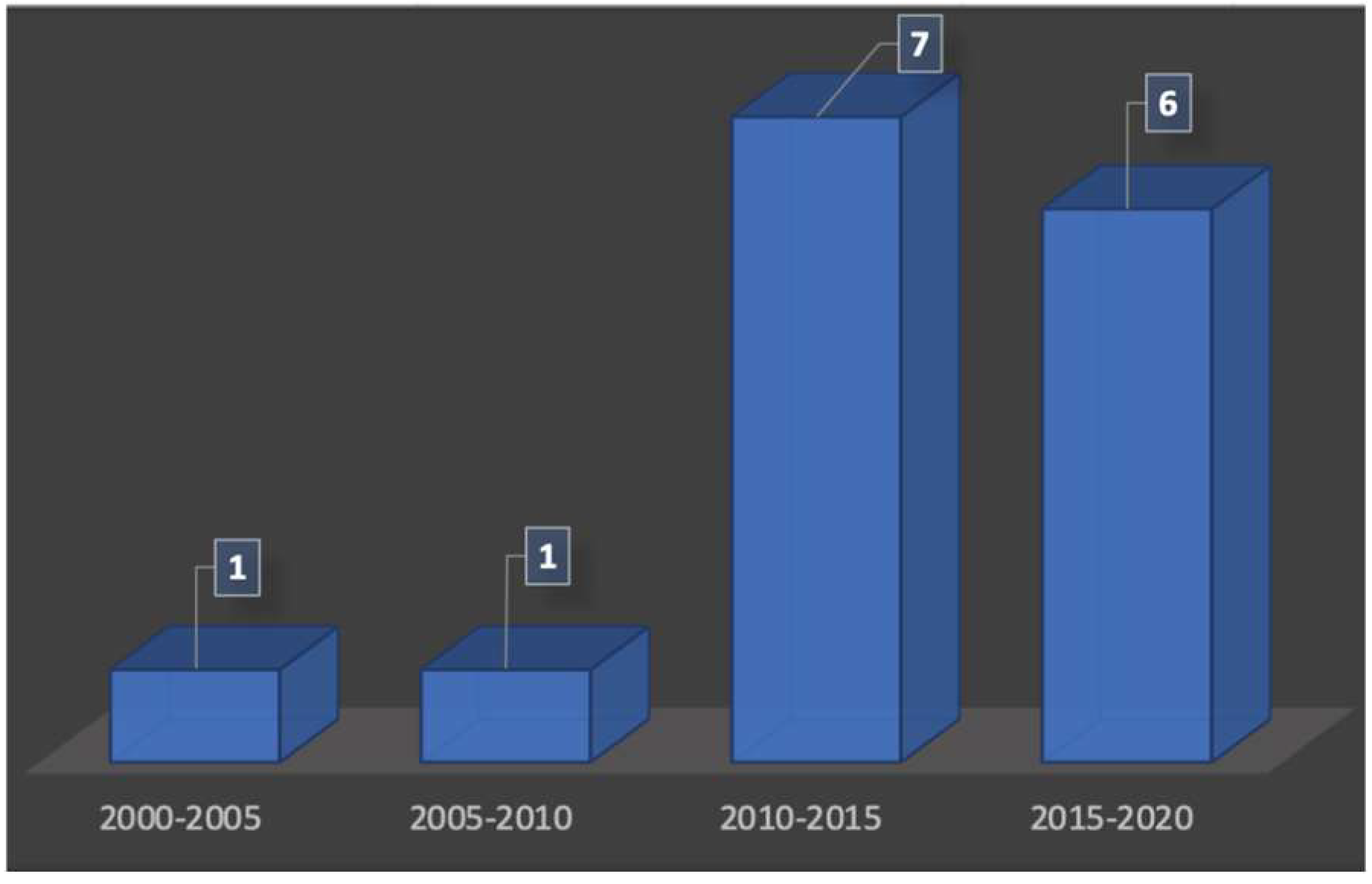
Using ultrasonography for the visualization of the structures of the oral cavity was not an actual possibility, and publications existed for many years already. According to the periods of publications used in this review, we have noticed that there is a renewed interest in ultrasound technologies for periodontal exploration from the 2010s (Figure 2). There were only two publications between the years 2000 and 2010, whereas the number of publications increased after 2010 with 13 articles found.
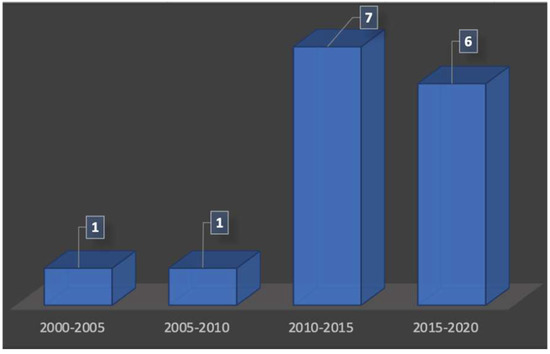
Figure 2.
Number of publications over the time.
According to our analysis, researchers have set up feasibility trials [23,33] for imaging and measuring the periodontal tissue. On the other hand, they have subsequently set up pilot trials [24,37] to evaluate the measurements provided by ultrasound images. In only one publication [29], ultrasonography is used to assess the size of the gingival tissue before and after professional periodontal cleaning.
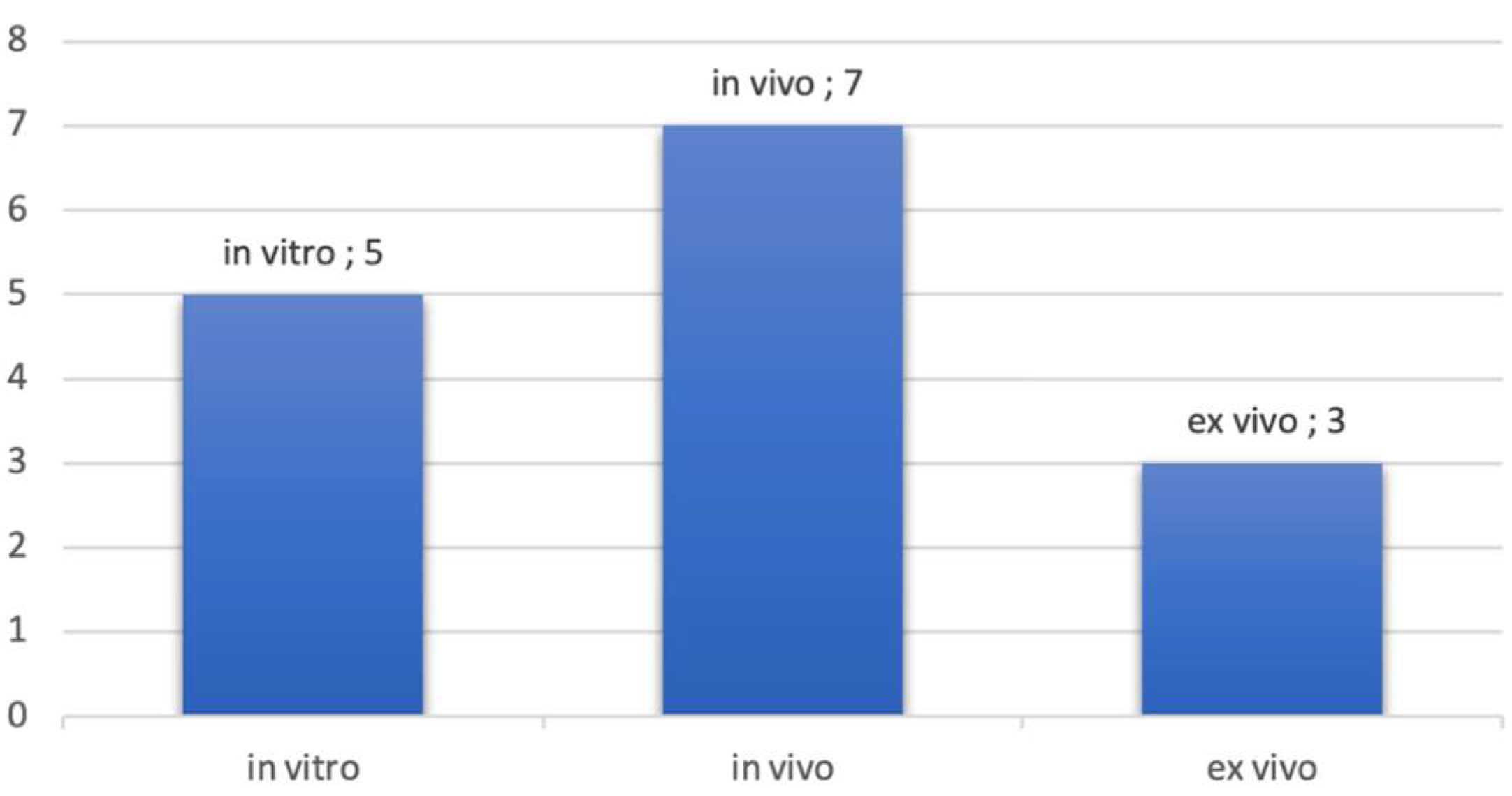
Most of the studies were in vivo studies. In vitro studies used pig jawbones while ex vivo studies were performed on cadavers. Figure 3 presents the proportion of studies according to the nature of the exploration. It should be noted that some studies have been realized in vitro and in vivo topic. This is the case, for example, in the studies of Sun et al., where measurements were realized on pig jawbones and on patients directly [36].
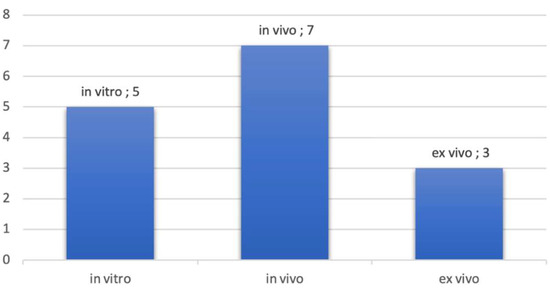
Figure 3.
Number of publications by type of exploration.
3.3. Overview of Ultrasonographic Devices
The literature presents a set of ultrasound probes used for the majority with an intraoral approach. The probe is in contact directly with intra oral tissue for image production providing a sagittal slice. In two publications [28,33], we have found ultrasound probes for extra-oral approach. For the most part of them, ultrasound probes were not used for intra-oral utilization. In some studies, ultrasonography was used for monitoring skin [26,30] or used in small animals’ studies [34].
In the study by Salmon et al., [24] the probe has been designed for intraoral use. It was manufactured on the model of a handpiece to use on all surfaces of the tooth. Tattan et al., [37] as well as Chan et al., [32,33] also have used probes designed for intraoral use. The prototypes for the oral cavity are smaller and used a head probe with a special angle allowing the tightest spaces of the oral cavity access. Components such as the transducer are necessarily miniaturized [24] for ease of use. Coupling agent is often a commercial coupling gel which is similar to water. The coupling gels used were not specific to the oral environment or to use in periodontology.
Regarding the acoustic parameters of the probes, it should be remembered that periodontal imaging aims to image structures close to the probe head. The depth of exploration is less than 10 mm, and the structures to be explored are sub-millimetric in size. In this specific case, emission of frequencies needs to be between 15 MHz [33] up to 40 MHz [23,28] for best results. Beyond 20 MHz, we enter in the field of high frequency ultrasound to obtain high resolution images, with a resolution of less than 100 microns [24,34,35]. The images obtained can be modified to allow better readability as with X-ray images. For example, in the study by Chifor et al. [30] the authors have implemented an image processing technique that automatically delimits the sulcus successfully.
3.4. Periodontal Tissues Description with Ultrasonographic Imagery
Within the periodontal tissue, bone structures are more echogenic because they reflect more ultrasound waves. Impedance rupture between hard and soft tissues allows them to be clearly distinguished. Therefore, hard tissues appeared whiter than soft tissues on the gray scale. First images need to be processed by computer. The authors added different colors according to the tissues in order to allow a good tissue differentiation. Periodontal tissues images from more recent publications, such as that of Tattan et al. [37] show better resolution with an adapted gray scale. Analysis of selected articles showed that ultrasound provided images of all periodontal structures, in pigs and humans, as listed in Figure 4.

Figure 4.
Types of periodontal tissues imaged by US imaging.
3.5. Types of Measurements and Comparison
Detection and classification of periodontal disease is based on measurements of periodontal tissue. The studies almost systematically present a comparison between the ultrasound measurements of the periodontal tissue and the measurements obtained by procedures used in clinical routine (clinical probing), qualified as the gold standard. Publications show measurements of the sulcular depth [23], the thickness of the free gingiva [23], the thickness of the attached gingiva [26,28], the biological width [23,26,28], the level of the alveolar crest in relation to the cemento-enamel junction [21,27,28,32,36], the thickness of the cortical bone [26,32], the height of the interdental papilla [37] and the gingival thickness on the edentulous ridge [37].
Studies on porcine jaws show direct transgingival measurements using an endodontic file [21,34,36], direct histological measurements [21] and direct clinical measurements using periodontal probe [21,23,28,32,34,37]. Conventional imaging methods have also been used: cone been computerized tomography (CBCT) [27,31,32,37], retro-alveolar radiography [28] and optical microscopy [27,30,35].
Several authors have used statistical tools to establish the correlation between ultrasound measurements and other means of measurement [27,28,30,32,36,37]. In the work of Zimbran et al. [23] the measurement of the sulcular space using the periodontal probe (gold standard) was not statistically different (p < 0.05) compared to the ultrasound measurement. The greatest measurement variation between the two means of measurement did not exceed 0.5 mm. Tsiolis et al. [21] have calculated the repeatability coefficient for the ultrasound measurements in comparison with in vitro measurement. Ultrasound measurements were better. Chifor et al. [29] highlighted the reproducible nature of ultrasound measurements. The intra-observer ICC calculated for ultrasound measurements was 98.8 with p < 0.001 for the measurement of the distance between the cemento-enamel junction and the alveolar ridge. After publications analysis, ultrasonography demonstrates reproducibility and precision. Ultrasonography appeared reliable in comparison with to other means of measurement (clinical and radiographic) in periodontal tissue application.
4. Discussion
Ultrasound for periodontology has benefited from renewed interest since the 2010s. However, there are only few teams working on this topic. The teams that presented several publications were made up of researchers from different research centers with different levels of expertise. The team of Chan et al. [32,33] was made up of professionals skilled in medical imaging and dental surgery, like the study by Tattan et al. [37] which involved both South Korean and American practitioners. Moreover, this review exposed in vitro studies that allowed direct measurements only possible in animals, such as transgingival measurements and histological samples. Tattan et al. [37], in the context of measuring gingival thickness on an edentulous ridge, were able to verify their results by direct measurements on humans. Only one publication [29] presented measurements on a pathological periodontium before and after a treatment session using ultrasonography. Patients recruitment for future studies should include patients requiring periodontal surgery to benefit from direct measurement of periodontal tissues. The results of the ultrasonography measurements are encouraging. Due to the diversity of the in vitro and in vivo protocols and the devices used, it was not possible to perform a meta-analysis.
Ultrasound imagery produces artifacts that are unique such as the “drop shadow”. This is due to the fact that a structure always appears less echogenic (blacker) than it really is when it is preceded (on the path of the ultrasound) by a hyperechoic structure. The study by Salmon et al. [24] reported the presence of artifacts due to non-specific coupling gel and distortions on ultrasound images. Ultrasound imagery offers a systematic buccal-palatal section of the tooth and the periodontal tissues. This reconstruction view is incomplete, as it does not allow the entire tooth to be imaged. To be precise, the ultrasound probe must be systematically passed over the vestibular and palatal surfaces to image the globality of the tissues around tooth. Most ultrasonography devices are borrowed from other medical disciplines, and for those that are not, they are prototypes. In addition to the evaluation of the ultrasound images, their handling and ergonomics were evaluated. Most studies show that the tool must be handy and small enough for use in the mouth. The miniaturization of the ultrasound system must also allow a high definition of the image which is a real challenge in terms of technological development. The articles on the PubMed database are mainly addressed to the medical community. However, the description of the acquisition protocol of the ultrasound imaging is not detailed or sometimes not presented. In the context of this research, none of the publications clearly mentioned the participation of a commercial partner or the cost/effectiveness ratio of ultrasound imaging compared to existing means. Technological locks persist and require the continuation of research funding. According to dental access, to produce new probe heads suitable for perfecting image processing software and continuing the process of miniaturization of the transducer, it is necessary to improve the utilization of the imagery approach. Dentists are not trained in reading ultrasound images, and the results obtained with an ultrasonography device must be clear and easy to read and to analyze.
The patients recruited for the studies were mostly healthy patients. Only Chifor et al. [29] enrolled patients with gingivitis. We can legitimately assume that the scanning techniques will have to adapt in the event of a bone defect or in the event of the presence of deep pockets (more than 6 mm). In addition, clinical measures such as periodontal probing are estimated to within half a millimeter, while the ultrasound technic proposes a measure less than tenths of a millimeter. There is a difference in scale between the interpretability of the measurements that can be obtained in the clinic and the precision of the ultrasonography. However, this literature review showed the accuracy of ultrasound for measurements at the level of anatomical structures. [35] Ultrasonic imaging can be valuable for accurate and real-time periodontal diagnosis without concerns about ionizing radiation [35,37]. The possibility to use ultrasound for minimally invasive surgery is founded [32]. In the future, the new prototypes should aim for a scanning time of 5 min for the entire oral cavity, compared to less than one minute per tooth today [37] with an intraoral approach. To do this, dental surgeons must appropriate this technology to be more efficient in the detection of the periodontal diseases.
5. Limitations
This work should not give rise to hasty conclusions due to the low number of publications and the intrinsic limits of the studies. Indeed, the use of only one electronic database is a particular limitation of this study. On the other hand, there are many biases in these studies and those related to the operators’ manipulations. In addition, many of the studies presented are animal studies that do not fully correspond to clinical conditions. These are feasibility studies and pilot tests with a medium level of proof (grade B). In addition, the heterogeneity of frequencies and acquisition protocols used in the current literature may hinder direct comparison between studies and necessitate more studies with homogeneous ultrasound measurements. All of the studies presented here do not allow to draw conclusions on the routine clinical use of ultrasounds in periodontology and require the setting up of randomized clinical studies.
To improve our analysis of the literature, certain sources of complementary information from other fields of science are necessary. For example, there are specific databases for biotechnology engineers and acousticians. We can find technical information that would consolidate the state of the art. The articles exclusively dedicated to implantology also present US technologies allowing indirect exploration of periodontal tissues.
6. Perspectives
High-resolution images have an equal or greater degree of precision than conventional imagery. It is a non-ionizing imaging that overcomes the discomfort of periodontal probing for diagnosis. Ultrasound pocket depth measurements appear to be as reliable and reproducible as those obtained by manual probing, even with an electronic probe.
The ultrasound system combined with an artificial intelligence (AI) data processing system opens the way to new perspectives regarding the distinction of the sulcular space and the measurement between the cemento-enamel junction and the sulcus. On this type of standardized measurements, the process could be automatized, and the data integrated into periodontal charting. We would then have a reliable, less time-consuming device involved in patient follow-up. The AI will allow a better handling of the device.
The use of ultrasound can be beneficial at all times of periodontal surgery. The ultrasonography can be allowed to see the quality of the graft directly before surgery. They would allow the incision path to be refined to avoid vasculo-nervous structures. Postoperatively, the healing or not of the graft could be monitored by inspecting the interactions with the recipient bed. Finally, inter-site contamination that may be caused by manual probing could be avoided.
7. Conclusions
Interest in intraoral ultrasound technologies has grown over the past decade. The various publications highlighted a reliable means of imaging allowing a precise exploration of the periodontal tissues offering the possibility of carrying out measurements of the periodontal structures themselves or between them. However, there is currently no clinical device dedicated to periodontal ultrasound. For the moment, the use of ultrasound in periodontology remains confined to the field of research. Ultrasound opens the prospect of a complete paradigm shift on the diagnosis and follow-up of periodontal disease, by reducing the examination time of periodontal pockets, being more reproducible and more efficient. In addition, the implementation of software including an artificial intelligence system allowing the direct measurement of the periodontal pocket and the early detection of the inflammation of the deep periodontium could permit easy diagnosis and early treatment of periodontal disease. Moreover, this new approach could allow an evaluation of the initial treatment and permit more reliable periodontal maintenance. In addition, the direct measurement of periodontal pockets without ionizing radiation is a major advance on the complementary examination allowing to reduce the number of periapical X-rays. Periodontal ultrasound is not intended to replace the conventional means of evaluation of periodontal tissues. However, the associated opportunities are immense with a lot of applications from periodontal assessment to treatment reassessment, including surgery.
Author Contributions
Conceptualization, M.R. and F.D.; Methodology, F.D., M.R. and D.C.; Resources, M.R. and R.M.; Data curation, M.R.; Writing—original draft preparation, M.R. and F.D.; Writing—review and editing, M.R. and D.C.; Visualization, A.D.; Supervision, A.D., H.B., G.S. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wolf, D.L.; Lamster, I.B. Contemporary Concepts in the Diagnosis of Periodontal Disease. Dent. Clin. N. Am. 2011, 55, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Periodontol. 2018, 89, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Joss, A.; Tonetti, M.S. Monitoring disease during supportive periodontal treatment by bleeding on probing. Periodontology 2000, 12, 44–48. [Google Scholar] [CrossRef]
- Greenstein, G. The Role of Bleeding upon Probing in the Diagnosis of Periodontal Disease: A Literature Review. J. Periodontol. 1984, 55, 684–688. [Google Scholar] [CrossRef]
- Karayiannis, A.; Lang, N.P.; Joss, A.; Nyman, S. Bleeding on probing as it relates to probing pressure and gingival health in patients with a reduced but healthy periodontium. J. Clin. Periodontol. 1992, 19, 471–475. [Google Scholar] [CrossRef]
- Kour, A.; Kumar, A.; Puri, K.; Khatri, M.; Bansal, M.; Gupta, G. Comparative evaluation of probing depth and clinical attachment level using a manual probe and Florida probe. J. Indian Soc. Periodontol. 2016, 20, 299. [Google Scholar]
- Wikner, S.; Söder, P.Ö.; Frithiof, L.; Wouters, F. The approximal bone height and intrabony defects in young adults, related to the salivary buffering capacity and counts of Streptococcus mutans and lactobacilli. Arch. Oral Biol. 1990, 35, 213–215. [Google Scholar] [CrossRef]
- Fukukita, H.; Yano, T.; Fukumoto, A.; Sawada, K.; Fujimasa, T.; Sunada, I. Development and application of an ultrasonic imaging system for dental diagnosis. J. Clin. Ultrasound 1985, 13, 597–600. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Cootney, R.W.; Coatney, R.W. Ultrasound Imaging: Principles and Applications in Rodent Research. ILAR J. 2001, 42, 233–247. [Google Scholar] [CrossRef]
- Banquart, A.; Callé, S.; Levassort, F.; Fritsch, L.; Ossant, F.; Toffessi Siewe, S.; Chevalliot, S.; Capri, A.; Grégoire, J.-M. Piezoelectric P(VDF-TrFE) film inkjet printed on silicon for high-frequency ultrasound applications. J. Appl. Phys. 2021, 129, 195107. [Google Scholar] [CrossRef]
- Siewe, S.T.; Calle, S.; Banquart, A.; Ossant, F.; Gregoire, J.M.; Levassort, F. Properties comparison of three HF (50 MHz) single-element transducer radiation patterns with different focusing principles. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Vegas, NV, USA, 7–11 September 2020; pp. 1–4. [Google Scholar]
- Kasban, H.; El-Bendary, M.A.M.; Salama, D.H. A Comparative Study of Medical Imaging Techniques. Int. J. Inf. Sci. Intell. Syst. 2015, 4, 37–58. [Google Scholar]
- Azhari, H. Ultrasound: Medical Imaging and Beyond (An Invited Review). Curr. Pharm. Biotechnol. 2012, 13, 2104–2116. [Google Scholar] [CrossRef] [PubMed]
- Izzetti, R.; Vitali, S.; Aringhieri, G.; Nisi, M.; Oranges, T.; Dini, V.; Ferro, F.; Baldini, C.; Romanelli, M.; Caramella, D.; et al. Ultra-High Frequency Ultrasound, A Promising Diagnostic Technique: Review of the Literature and Single-Center Experience. Can. Assoc. Radiol. J. 2021, 72, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Zander, D.; Hüske, S.; Hoffmann, B.; Cui, X.W.; Dong, Y.; Lim, A.; Jenssen, C.; Löwe, A.; Koch, J.B.; Dietrich, C.F. Ultrasound Image Optimization (Knobology): B-Mode. Ultrasound Int. Open 2020, 6, 14–24. [Google Scholar] [CrossRef]
- Krzysztof Mlosek, R.; Malinowska, S. Ultrasonograficzny obraz skóry, aparatura i podstawy obrazowania Ultrasound image of the skin, apparatus and imaging basics. J. Ultrason. 2013, 13, 212–221. [Google Scholar] [CrossRef]
- Silverman, R.H. High-resolution ultrasound imaging of the eye—A review. Clin. Exp. Ophthalmol. 2009, 37, 54–67. [Google Scholar] [CrossRef]
- Kagadis, G.C.; Loudos, G.; Katsanos, K.; Langer, S.G.; Nikiforidis, G.C. In vivo small animal imaging: Current status and future prospects. Med. Phys. 2010, 37, 6421–6442. [Google Scholar] [CrossRef]
- Tsiolis, F.I.; Needleman, I.G.; Griffiths, G.S. Periodontal ultrasonography. J. Clin. Periodontol. 2003, 30, 849–854. [Google Scholar] [CrossRef]
- Eger, T.; Müller, H.P.; Heinecke, A. Ultrasonic determination of gingival thickness. J. Clin. Periodontol. 1996, 23, 839–845. [Google Scholar] [CrossRef]
- Zimbran, A.; Dudea, S.; Dudea, D. Evaluation of periodontal tissues using 40MHz ultrasonography. Preliminary report. Med. Ultrason. 2013, 15, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Salmon, B.; le Denmat, D. Intraoral ultrasonography: Development of a specific high-frequency probe and clinical pilot study. Clin. Oral Investig. 2011, 16, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Chifor, R.; Badea, M.E.; Hedeşiu, M.; Şerbănescu, A.; Badea, A.F. Experimental model for measuring and characterisation of the dento-alveolar system using high frequencies ultrasound techniques. Med. Ultrason. 2010, 12, 127–132. [Google Scholar]
- Chifor, R.; HedeÅŸiu, M.; Bolfa, P.; Catoi, C.; Crisan, M.; Serbanescu, A.; Badea, A.; Moga, I.; Badea, E. The evaluation of 20 MHz ultrasonography, computed tomography scans as compared to direct microscopy for periodontal system assessment. Med. Ultrason. 2011, 13, 120–126. [Google Scholar]
- Chifor, R.; Eugenia Badea, M.; Hedeşiu, M.; Chifor, I. Identification of the anatomical elements used in periodontal diagnosis on 40 MHz periodontal ultrasonography. Rom. J. Morphol. Embryol. 2015, 56, 149–153. [Google Scholar]
- Chifor, R.; Badea, M.E.; Vesa, Ş.C.; Chifor, I. The utility of 40 MHz periodontal ultrasonography in the assessment of gingival inflammation evolution following professional teeth cleaning. Med. Ultrason. 2015, 17, 34–38. [Google Scholar] [CrossRef]
- Chifor, R.; Badea, M.E.; Mitrea, D.A.; Badea, I.C.; Crisan, M.; Chifor, I.; Avram, R. Computer-assisted identification of the gingival sulcus and periodontal epithelial junction on high-frequency ultrasound images. Med. Ultrason. 2015, 17, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.T.; Le, L.H.; Kaipatur, N.R.; Zheng, R.; Lou, E.H.; Major, P.W. High-Resolution Ultrasonic Imaging of Dento-Periodontal Tissues Using a Multi-Element Phased Array System. Ann. Biomed. Eng. 2016, 44, 2874–2886. [Google Scholar] [CrossRef]
- Chan, H.L.; Sinjab, K.; Chung, M.P.; Chiang, Y.C.; Wang, H.L.; Giannobile, W.V.; Kripfgans, O.D. Non-invasive evaluation of facial crestal bone with ultrasonography. PLoS ONE 2017, 12, e0171237. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Wang, H.L.; Fowlkes, J.B.; Giannobile, W.V.; Kripfgans, O.D. Non-ionizing real-time ultrasonography in implant and oral surgery: A feasibility study. Clin. Oral Implant Res. 2017, 28, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chen, F.; Hariri, A.; Chen, C.J.; Wilder-Smith, P.; Takesh, T.; Jokerst, J.V. Photoacoustic Imaging for Noninvasive Periodontal Probing Depth Measurements. J. Dent. Res. 2018, 97, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Barootchi, S.; Chan, H.L.; Namazi, S.S.; Wang, H.L.; Kripfgans, O.D. Ultrasonographic characterization of lingual structures pertinent to oral, periodontal, and implant surgery. Clin. Oral Implants Res. 2020, 31, 352–359. [Google Scholar] [CrossRef]
- Sun, M.; Yao, W.; Deng, Y.; Cao, J.; Meng, H. Measurements of buccal gingival and alveolar crest thicknesses of premolars using a noninvasive method. Med. Ultrason. 2020, 22, 409–414. [Google Scholar] [CrossRef]
- Tattan, M.; Sinjab, K.; Lee, E.; Arnett, M.; Oh, T.J.; Wang, H.L.; Chan, H.-L.; Kripfgans, O.D. Ultrasonography for chairside evaluation of periodontal structures: A pilot study. J. Periodontol. 2020, 91, 890–899. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).