Preliminary Investigation into the Prevalence of G6PD Deficiency in a Pediatric African American Population Using a Near-Patient Diagnostic Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. G6PD Enzymatic Activity Measurement
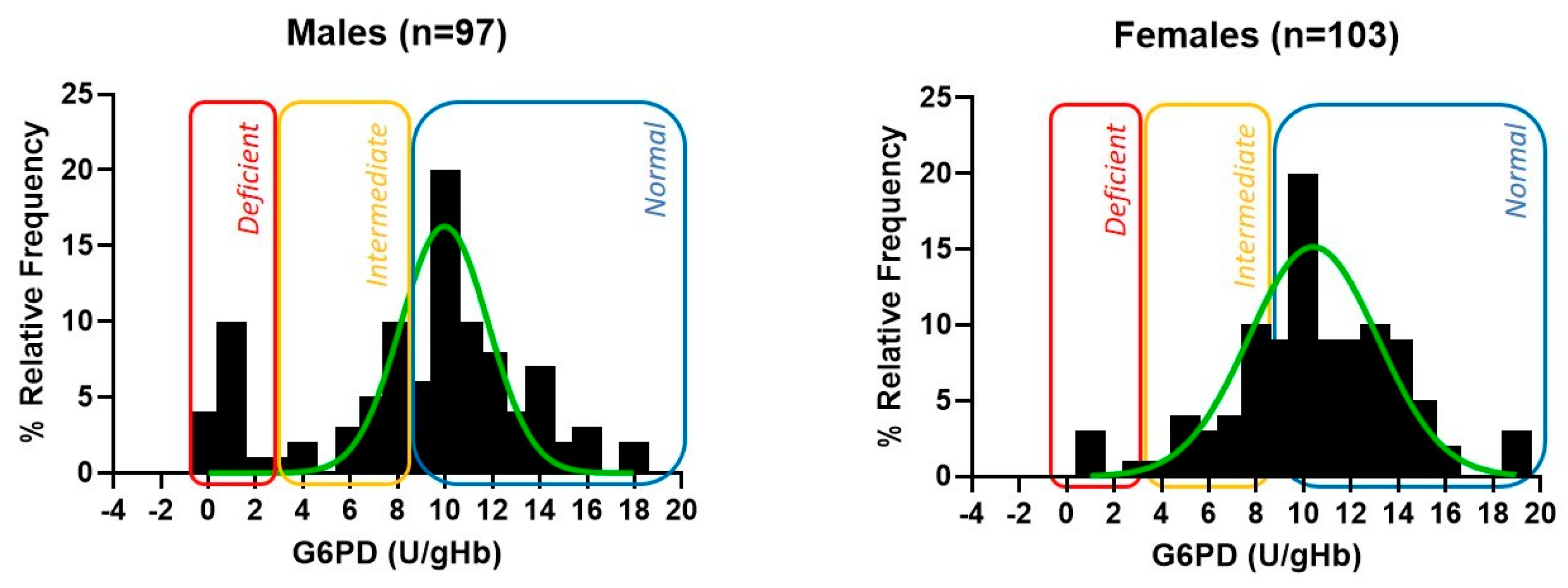
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahr, T.M.; Agarwal, A.M.; Meznarich, J.A.; Prince, W.L.; Wait, T.W.; Prchal, J.T.; Christensen, R.D. Thirty-five males with severe (Class 1) G6PD deficiency (c.637G>T) in a North American family of European ancestry. Blood Cells Mol. Dis. 2021, 92, 102625. [Google Scholar] [CrossRef] [PubMed]
- Glader, B. Diagnosis and Management of Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency. UpToDate. 2022. Available online: https://www.uptodate.com/contents/diagnosis-and-management-of-glucose-6-phosphate-dehydrogenase-g6pd-deficiency (accessed on 24 October 2023).
- Youngster, I.; Arcavi, L.; Schechmaster, R.; Akayzen, Y.; Popliski, H.; Shimonov, J.; Beig, S.; Berkovitch, M. Medications and glucose-6-phosphate dehydrogenase deficiency: An evidence-based review. Drug Saf. 2010, 33, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.V.; Redaelli, F.; Gualdi, V.; Rizzi, V.; Mameli, C.; Dilillo, D.; Fabiano, V. Hemolytic crisis in a G6PD-deficient infant after ingestion of pumpkin. Ital. J. Pediatr. 2014, 40, 71. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.H.; Chiu, Y.W. Clinical characteristics of G6PD deficiency in infants with marked hyperbilirubinemia. J. Pediatr. Hematol. Oncol. 2010, 32, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Kheir, A.; Gaber, I.; Gafer, S.; Ahmed, W. Life-threatening haemolysis induced by henna in a Sudanese child with glucose-6-phosphate dehydrogenase deficiency. East. Mediterr. Heal. J. 2017, 23, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Drugs and Lactation Database (LactMed). Bethesda (MD): National Institute of Child Health and Human Development. Fava Beans. 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532498/ (accessed on 24 October 2023).
- Slusher, T.M.; Vreman, H.J.; McLaren, D.W.; Lewison, L.J.; Brown, A.K.; Stevenson, D.K. Glucose-6-phosphate dehydrogenase deficiency and carboxyhemoglobin concentrations associated with bilirubin-related morbidity and death in Nigerian infants. J. Pediatr. 1995, 126, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Guide to G6PD Deficiency Rapid Diagnostic Testing to Support P. vivax Radical Cure—World Health Organization. 30 June 2018. Available online: https://www.who.int/publications/i/item/9789241514286 (accessed on 24 October 2023).
- Hammami, M.B.; Qasim, A.; Thakur, R.; Vegivinti, C.T.R.; Patton, C.D.; Vikash, S.; Kumar, A. Rasburicase-induced hemolytic anemia and methemoglobinemia: A systematic review of current reports. Ann. Hematol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Subcomittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Maisels, M.J.; Bhutani, V.K.; Bogen, D.; Newman, T.B.; Stark, A.R.; Watchko, J.F. Hyperbilirubinemia in the newborn infant greater than or equal to 35 weeks’ gestation: An update with clarifications. Pediatrics 2009, 124, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Senate Bill S4316. New York State Senate 2021–2022. (ACTIVE)—Summary, List of Screenings for Newborns. Available online: https://www.nysenate.gov/legislation/bills/2021/S4316#:~:text=S4316 (accessed on 24 October 2023).
- New NY Law Mandates Newborns Be Tested for Enzyme Deficiency. Babylon Village Patch. 22 June 2022. Available online: https://patch.com/new-york/babylonvillage/new-law-mandates-newborns-be-tested-enzyme-deficiency (accessed on 24 October 2023).
- Chinevere, T.D.; Murray, C.K.; Grant, E.; Johnson, G.A.; Duelm, F.; Hospenthal, D.R. Prevalence of glucose-6-phosphate dehydrogenase deficiency in U.S. Army personnel. Mil. Med. 2006, 171, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Herschel, M.; Hammerman, C.; Hoyer, J.D.; Stevenson, D.K. Hyperbilirubinemia Among African American, Glucose-6-Phosphate Dehydrogenase-Deficient Neonates. Pediatrics 2004, 114, e213–e219. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Newman, T.B.; Slaughter, J.L.; Maisels, M.J.; Watchko, J.F.; Downs, S.M.; Grout, R.W.; Bundy, D.G.; Stark, A.R.; Bogen, D.L.; et al. Clinical Practice Guideline Revision: Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2022, 150, 2022058859. [Google Scholar] [CrossRef] [PubMed]
- Sista, R.S.; Ng, R.; Nuffer, M.; Basmajian, M.; Coyne, J.; Elderbroom, J.; Hull, D.; Kay, K.; Krishnamurthy, M.; Roberts, C.; et al. Digital Microfluidic Platform to Maximize Diagnostic Tests with Low Sample Volumes from Newborns and Pediatric Patients. Diagnostics 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Montiel, C.; Kunda, M.; Stevenson, D.K.; Bhutani, V.K. A Novel Point-of-Care Device for Measuring Glucose-6-Phosphate Dehydrogenase Enzyme Deficiency. Semin. Perinatol. 2021, 45, 151356. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, V.K.; Kaplan, M.; Glader, B.; Cotten, M.; Kleinert, J.; Pamula, V. Point-of-care quantitative measure of glucose-6-phosphate dehydrogenase enzyme deficiency. Pediatrics 2015, 136, e1268–e1275. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration 510(k) Premarket Notification. K201049: FINDER G6PD. 20 February 2023. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K201049 (accessed on 24 October 2023).
- Kaplan, M.; Herschel, M.; Hammerman, C.; Hoyer, J.D.; Heller, G.Z.; Stevenson, D.K. Neonatal hyperbilirubinemia in African American males: The importance of glucose-6-phosphate dehydrogenase deficiency. J. Pediatr. 2006, 149, 83–88. [Google Scholar] [CrossRef] [PubMed]
| Patient Demographics | |
|---|---|
| All (n = 200) | |
| Median Age | 11 y |
| Low Age | 10 d |
| High Age | 20 y |
| Female (n = 103) | |
| Median Age | 12 y |
| Low Age | 17 d |
| High Age | 20 y |
| Male (n = 97) | |
| Median Age | 11 y |
| Low Age | 10 d |
| High Age | 19 y |
| G6PD Activity | |||
|---|---|---|---|
| All Samples (n = 200) | |||
| All | Female | Male | |
| Median G6PD value (U/gHb) | 10.5 | 10.7 | 10.3 |
| Mean G6PD value (U/gHb) | 10.2 | 10.9 | 9.4 |
| SD of G6PD values (U/gHb) | 4.0 | 3.5 | 4.4 |
| Deficient sample (n) | 18 | 3 | 15 |
| Deficiency frequency | 9.00% | 2.91% | 15.46% |
Samples with Normal and Intermediate Results | |||
| Median G6PD value (U/gHb) | 10.8 | 10.8 | 10.8 |
| Mean G6PD value (U/gHb) | 11.0 | 11.2 | 10.9 |
| SD of G6PD values (U/gHb) | 3.0 | 3.2 | 2.9 |
Samples with Deficient Results | |||
| Median G6PD value (U/gHb) | 1.4 | 1.3 | 1.5 |
| Mean G6PD value (U/gHb) | 1.4 | 1.5 | 1.4 |
| SD of G6PD values (U/gHb) | 0.5 | 0.4 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung-Pineda, V.; Weinzierl, E.P.; Rogers, B.B. Preliminary Investigation into the Prevalence of G6PD Deficiency in a Pediatric African American Population Using a Near-Patient Diagnostic Platform. Diagnostics 2023, 13, 3647. https://doi.org/10.3390/diagnostics13243647
Leung-Pineda V, Weinzierl EP, Rogers BB. Preliminary Investigation into the Prevalence of G6PD Deficiency in a Pediatric African American Population Using a Near-Patient Diagnostic Platform. Diagnostics. 2023; 13(24):3647. https://doi.org/10.3390/diagnostics13243647
Chicago/Turabian StyleLeung-Pineda, Van, Elizabeth P. Weinzierl, and Beverly B. Rogers. 2023. "Preliminary Investigation into the Prevalence of G6PD Deficiency in a Pediatric African American Population Using a Near-Patient Diagnostic Platform" Diagnostics 13, no. 24: 3647. https://doi.org/10.3390/diagnostics13243647
APA StyleLeung-Pineda, V., Weinzierl, E. P., & Rogers, B. B. (2023). Preliminary Investigation into the Prevalence of G6PD Deficiency in a Pediatric African American Population Using a Near-Patient Diagnostic Platform. Diagnostics, 13(24), 3647. https://doi.org/10.3390/diagnostics13243647





