Utility of Fetal Cardiac Resonance Imaging in Prenatal Clinical Practice: Current State of the Art
Abstract
1. Introduction
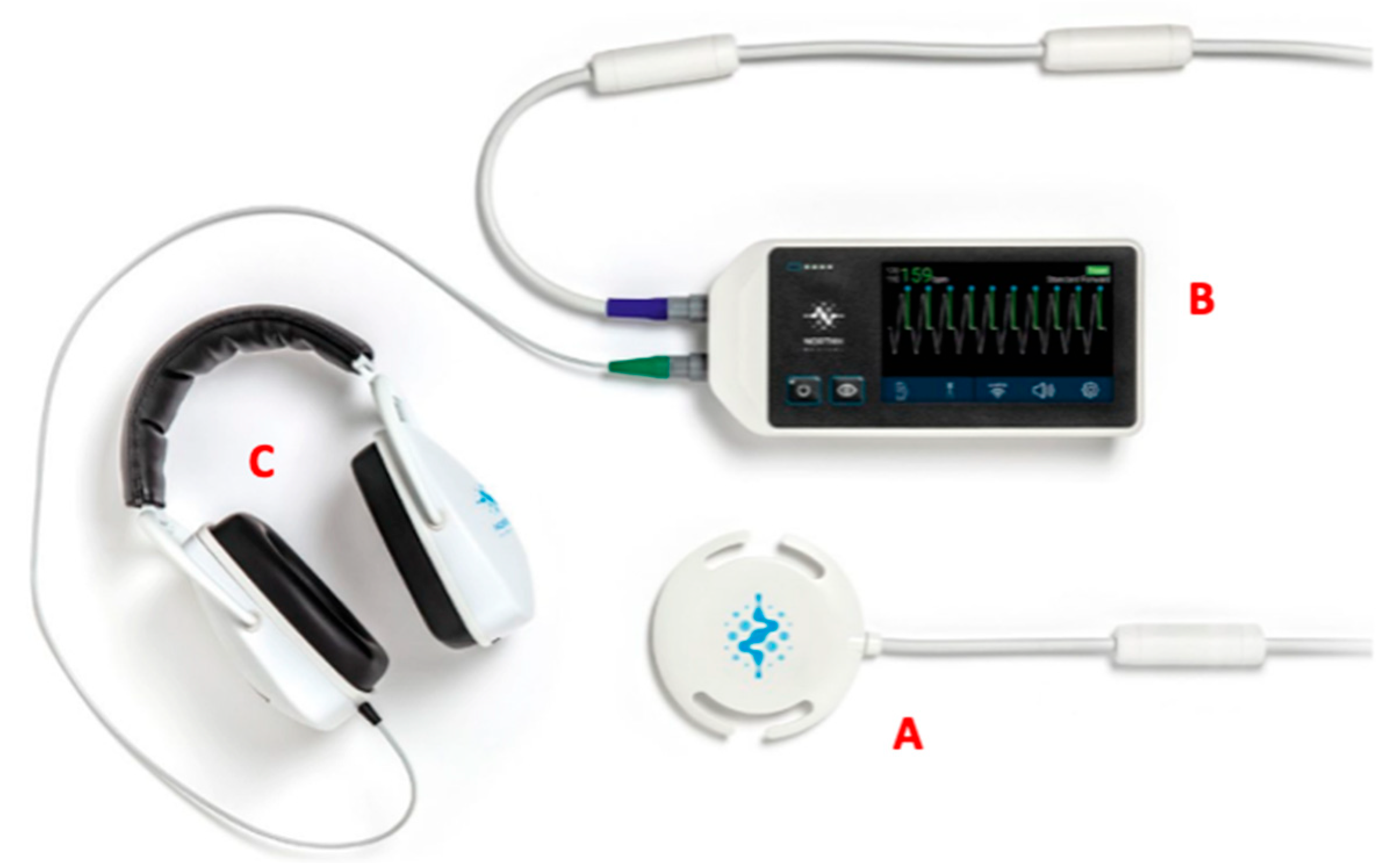
2. Hardware Consideration
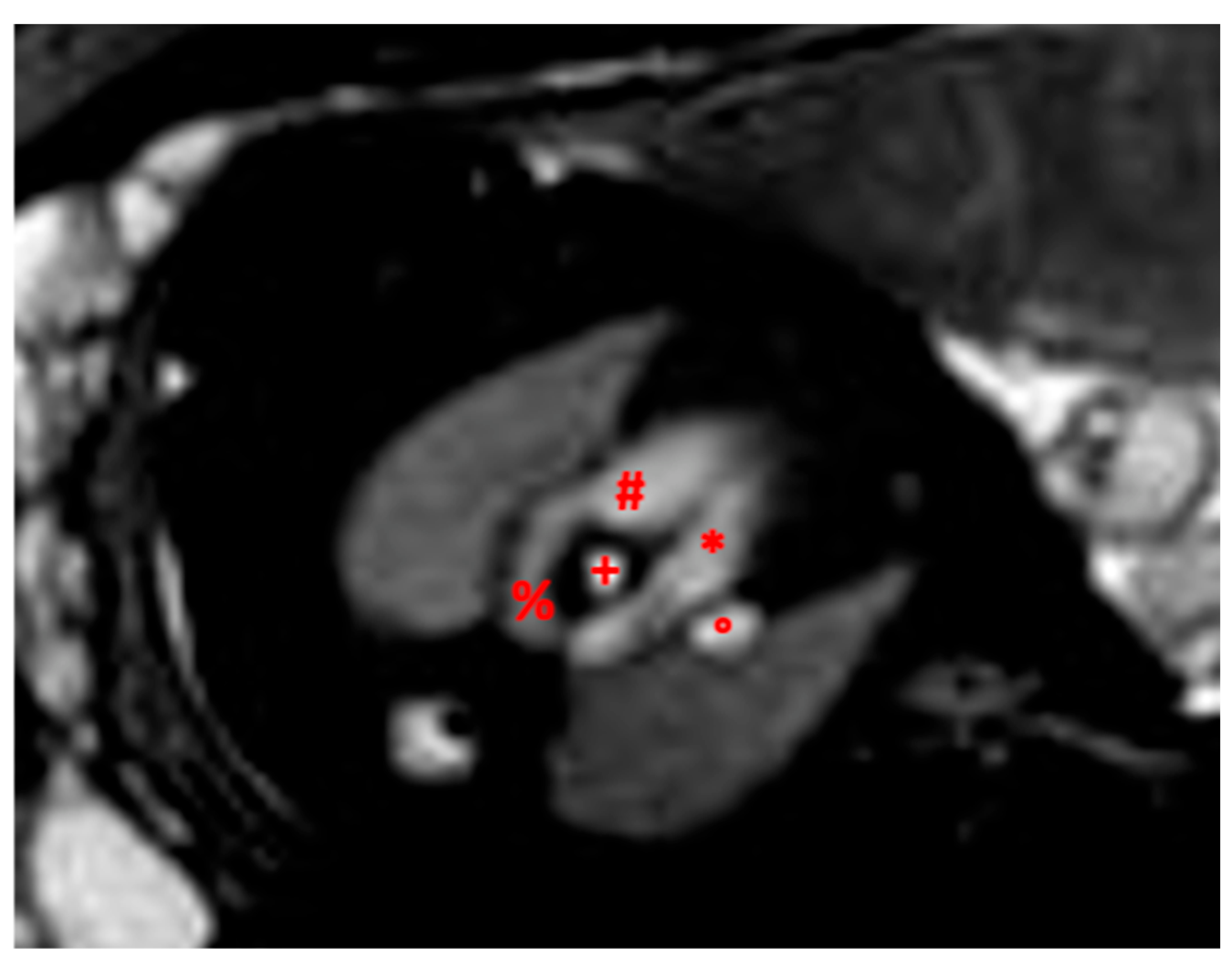
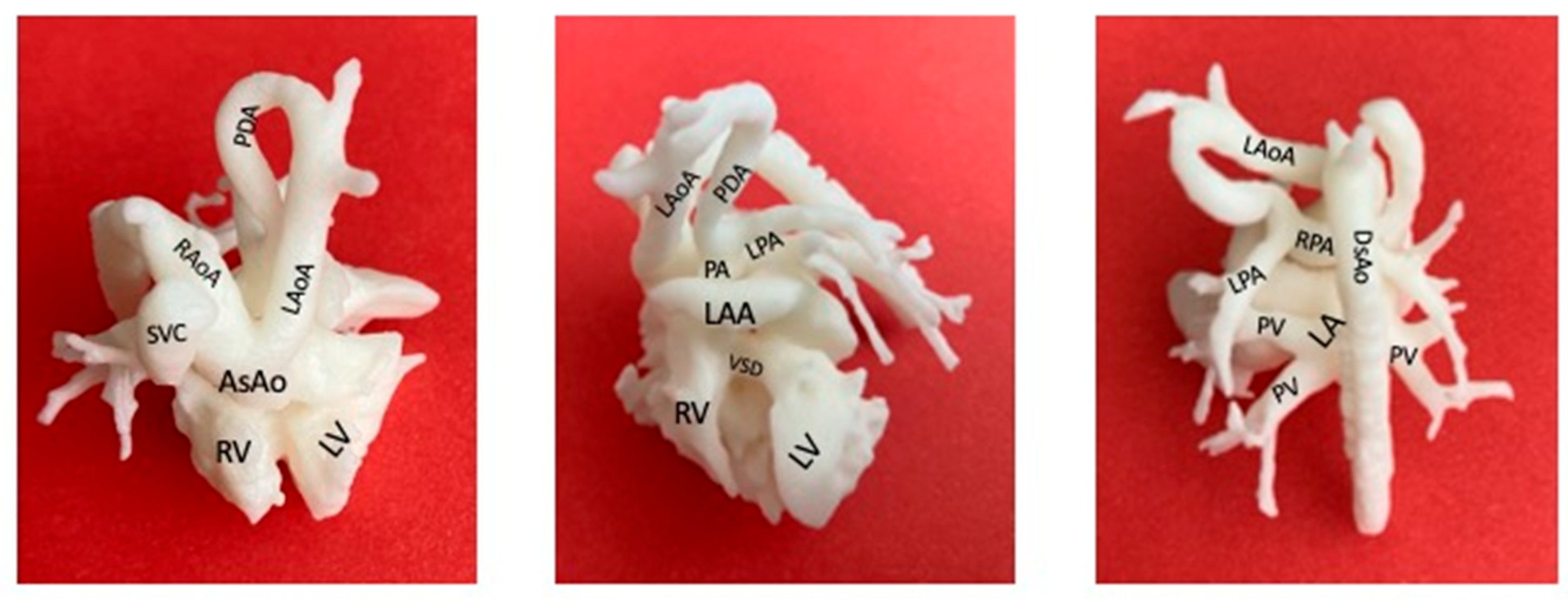
3. Application of Fetal CMR Imaging in Understanding CHD
4. Challenges of Fetal CMR
5. Limitations of Fetal CMR
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balasubramanya, R.; Valle, C. Uterine Imaging; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551551/ (accessed on 19 August 2023).
- McBrien, A.; Hornberger, L.K. Early fetal echocardiography. Birth Defects Res. 2018, 111, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Fesslova, V.; Farina, A.; Kagan, K.O.; Candiani, M.; Morelli, M.; Crispi, F.; Cavoretto, P.I. How to Do a Fetal Cardiac Scan. Arch. Gynecol. Obstet. 2023, 307, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Russo, M.G.; Paladini, D.; Felicetti, M.; Castaldi, B.; Tartaglione, A.; di Pietto, L.; Ricci, C.; Morelli, C.; Pacileo, G.; et al. Two-dimensional strain to assess regional left and right ventricular longitudinal function in 100 normal foetuses. Eur. J. Echocardiogr. 2008, 9, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Russo, M.G.; Paladini, D.; Pacileo, G.; Felicetti, M.; Ricci, C.; Cardaropoli, D.; Palma, M.; Caso, P.; Calabro, R. Quantification of regional left and right ventricular longitudinal function in 75 normal fetuses using ultrasound-based strain rate and strain imaging. Ultrasound Med. Biol. 2005, 31, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.; Pacileo, G.; Russo, M.G. (Eds.) New Insight in Pediatric Cardiology: From Basic to Therapeutics; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012. [Google Scholar] [CrossRef]
- Marella, N.T.; Gil, A.M.; Fan, W.; Aristizabal, C.A.; Asrani, P.; Harrington, J.K.; Channing, A.; Setton, M.; Shah, A.M.; Levasseur, S.; et al. 3D-Printed Cardiac Models for Fetal Counseling: A Pilot Study and Novel Approach to Improve Communication. Pediatr. Cardiol. 2023, 44, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.L.B.; da Silva, M.G.; Faria, S.C.C.; Salemi, V.M.C.; Mady, C.; Baltabaeva, A.; Sutherland, G.R. Quantification of Regional Left and Right Ventricular Deformation Indices in Healthy Neonates by Using Strain Rate and Strain Imaging. J. Am. Soc. Echocardiogr. 2009, 22, 369–375. [Google Scholar] [CrossRef]
- Goncalves, L.F.; Lindblade, C.L.; Cornejo, P.; Patel, M.C.; McLaughlin, E.S.; Bardo, D.M.E. Contribution of fetal magnetic resonance imaging in fetuses with congenital heart disease. Pediatr. Radiol. 2022, 52, 513–526. [Google Scholar] [CrossRef]
- Lloyd, D.F.A.; van Amerom, J.F.P.; Pushparajah, K.; Simpson, J.M.; Zidere, V.; Miller, O.; Sharland, G.; Allsop, J.; Fox, M.; Lohezic, M.; et al. An exploration of the potential utility of fetal cardiovascular MRI as an adjunct to fetal echocardiography. Prenat. Diagn. 2016, 36, 916–925. [Google Scholar] [CrossRef]
- Mamalis, M.; Bedei, I.; Schoennagel, B.; Kording, F.; Reitz, J.G.; Wolter, A.; Schenk, J.; Axt-Fliedner, R. The Evolution and Developing Importance of Fetal Magnetic Resonance Imaging in the Diagnosis of Congenital Cardiac Anomalies: A Systematic Review. J. Clin. Med. 2022, 11, 7027. [Google Scholar] [CrossRef]
- Loomba, R.S.; Chandrasekar, S.; Shah, P.H.; Sanan, P. The developing role of fetal magnetic resonance imaging in the diagnosis of congenital cardiac anomalies: A systematic review. Ann. Pediatr. Cardiol. 2011, 4, 172–176. [Google Scholar] [CrossRef]
- Rajiah, P.; Kay, F.; Bolen, M.; Patel, A.R.; Landeras, L. Cardiac Magnetic Resonance in Patients with Cardiac Implantable Electronic Devices. J. Thorac. Imaging 2020, 35, W1–W17. [Google Scholar] [CrossRef]
- De Wilde, J.; Rivers, A.; Price, D. A review of the current use of magnetic resonance imaging in pregnancy and safety implications for the fetus. Prog. Biophys. Mol. Biol. 2005, 87, 335–353. [Google Scholar] [CrossRef]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 2016, 316, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Machado-Rivas, F.; Jaimes, C.; Kirsch, J.E.; Gee, M.S. Image-quality optimization and artifact reduction in fetal magnetic resonance imaging. Pediatr. Radiol. 2020, 50, 1830–1838. [Google Scholar] [CrossRef]
- Dong, S.-Z.; Zhu, M.; Li, F. Preliminary experience with cardiovascular magnetic resonance in evaluation of fetal cardiovascular anomalies. J. Cardiovasc. Magn. Reson. 2013, 15, 40. [Google Scholar] [CrossRef]
- Sun, L.; Macgowan, C.K.; Portnoy, S.; Sled, J.G.; Yoo, S.; Grosse-Wortmann, L.; Jaeggi, E.; Kingdom, J.; Seed, M. New advances in fetal cardiovascular magnetic resonance imaging for quantifying the distribution of blood flow and oxygen transport: Potential applications in fetal cardiovascular disease diagnosis and therapy. Echocardiography 2017, 34, 1799–1803. [Google Scholar] [CrossRef]
- Weisstanner, C.; Gruber, G.M.; Brugger, P.C.; Mitter, C.; Diogo, M.C.; Kasprian, G.; Prayer, D. Fetal MRI at 3T—Ready for routine use? Br. J. Radiol. 2017, 90, 20160362. [Google Scholar] [CrossRef] [PubMed]
- Marini, D.; van Amerom, J.; Saini, B.S.; Sun, L.; Seed, M. MR imaging of the fetal heart. J. Magn. Reson. Imaging 2020, 51, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.; Tuura, R.O.; Callaghan, F.M.; Burkhardt, B.E.U.; Kellenberger, C.J.; Buechel, E.R.V. Feasibility of non-gated dynamic fetal cardiac MRI for identification of fetal cardiovascular anatomy. Fetal Diagn. Ther. 2023, 50, 8–16. [Google Scholar] [CrossRef]
- Udine, M.; Loke, Y.-H.; Goudar, S.; Donofrio, M.T.; Truong, U.; Krishnan, A. The current state and potential innovation of fetal cardiac MRI. Front. Pediatr. 2023, 11, 1219091. [Google Scholar] [CrossRef]
- Manganaro, L.; Savelli, S.; Di Maurizio, M.; Francioso, A.; Fierro, F.; Tomei, A.; Coratella, F.; Ballesio, L.; Ventriglia, F. Fetal MRI of the cardiovascular system: Role of steady-state free precession sequences for the evaluation of normal and pathological appearances. Radiol. Med. 2009, 114, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.; de Sousa, M.T.; Lenz, A.; Herrmann, J.; Zhang, S.; Kording, F.; Hergert, B.; Adam, G.; Bannas, P.; Schoennagel, B.P. Fetal 4D flow MRI of the great thoracic vessels at 3 Tesla using Doppler-ultrasound gating: A feasibility study. Eur. Radiol. 2023, 33, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Schrauben, E.M.; Saini, B.S.; Darby, J.R.T.; Soo, J.Y.; Lock, M.C.; Stirrat, E.; Stortz, G.; Sled, J.G.; Morrison, J.L.; Seed, M.; et al. Fetal hemodynamics and cardiac streaming assessed by 4D flow cardiovascular magnetic resonance in fetal sheep. J. Cardiovasc. Magn. Reson. 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.A.; van Amerom, J.F.P.; Uus, A.; Lloyd, D.F.A.; van Poppel, M.P.M.; Price, A.N.; Tournier, J.-D.; Mohanadass, C.A.; Jackson, L.H.; Malik, S.J.; et al. Fetal whole heart blood flow imaging using 4D cine MRI. Nat. Commun. 2020, 11, 4992. [Google Scholar] [CrossRef] [PubMed]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Li, J.; Kuo, A.; Moody, A.; Nathanielsz, P. Cardiac magnetic resonance imaging: Insights into developmental programming and its consequences for aging. J. Dev. Orig. Health Dis. 2021, 12, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Arya, B. Fetal Cardiac Imaging for Congenital Heart Disease—Is Cardiac Magnetic Resonance Imaging the Future? JAMA Netw. Open 2021, 4, e214617. [Google Scholar] [CrossRef] [PubMed]
- Tsuritani, M.; Morita, Y.; Miyoshi, T.; Kurosaki, K.; Yoshimatsu, J. Fetal Cardiac Functional Assessment by Fetal Heart Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2019, 43, 104–108. [Google Scholar] [CrossRef]
- Portnoy, S.; Seed, M.; Sled, J.G.; Macgowan, C.K. Non-invasive evaluation of blood oxygen saturation and hematocrit from T1 and T2 relaxation times: In-vitro validation in fetal blood. Magn. Reson. Med. 2017, 78, 2352–2359. [Google Scholar] [CrossRef]
- Van Amerom, J.F.; Lloyd, D.F.; Deprez, M.; Price, A.N.; Malik, S.J.; Pushparajah, K.; van Poppel, M.P.; Rutherford, M.A.; Razavi, R.; Hajnal, J.V. Fetal whole-heart 4D imaging using motion-corrected multi-planar real-time MRI. Magn. Reson. Med. 2019, 82, 1055–1072. [Google Scholar] [CrossRef]
- Lloyd, D.F.; van Poppel, M.P.; Pushparajah, K.; Vigneswaran, T.V.; Zidere, V.; Steinweg, J.; van Amerom, J.F.; Roberts, T.A.; Schulz, A.; Charakida, M.; et al. Analysis of 3-Dimensional Arch Anatomy, Vascular Flow, and Postnatal Outcome in Cases of Suspected Coarctation of the Aorta Using Fetal Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2021, 14, e012411. [Google Scholar] [CrossRef]
- Vigneswaran, T.V.; Bellsham-Revell, H.R.; Chubb, H.; Simpson, J.M. Early Postnatal Echocardiography in Neonates with a Prenatal Suspicion of Coarctation of the Aorta. Pediatr. Cardiol. 2020, 41, 772–780. [Google Scholar] [CrossRef]
- Collett, R.W.; Edwards, J.E. Persistent Truncus Arteriosus: A Classification According to Anatomic Types. Surg. Clin. North Am. 1949, 29, 1245–1270. [Google Scholar] [CrossRef]
- Van Praagh, R.; Van Praagh, S. The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications: A study of 57 necropsy cases. Am. J. Cardiol. 1965, 16, 406–425. [Google Scholar] [CrossRef]
- Pasieczna, M.; Duliban, J.; Grzyb, A.; Szymkiewicz-Dangel, J. 4D imaging of fetal right ventricle—Feasibility study and a review of the literature. Int. J. Cardiovasc. Imaging 2022, 38, 319–329. [Google Scholar] [CrossRef]
- Rubert, N.C.; Jategaonkar, G.; Plasencia, J.D.; Lindblade, C.L.; Bardo, D.M.E.; Goncalves, L.F. Four-dimensional fetal cardiac imaging in a cohort of fetuses with suspected congenital heart disease. Pediatr. Radiol. 2023, 53, 198–209. [Google Scholar] [CrossRef]
- Mamalis, M.; Koehler, T.; Bedei, I.; Wolter, A.; Schenk, J.; Widriani, E.; Axt-Fliedner, R. Comparison of the Results of Prenatal and Postnatal Echocardiography and Postnatal Cardiac MRI in Children with a Congenital Heart Defect. J. Clin. Med. 2023, 12, 3508. [Google Scholar] [CrossRef]
- Cho, S.K.S.; Darby, J.R.T.; Saini, B.S.; Lock, M.C.; Holman, S.L.; Lim, J.M.; Perumal, S.R.; Macgowan, C.K.; Morrison, J.L.; Seed, M. Feasibility of ventricular volumetry by cardiovascular MRI to assess cardiac function in the fetal sheep. J. Physiol. 2020, 598, 2557–2573. [Google Scholar] [CrossRef]
- Bertelli, F.; Raimondi, F.; Godard, C.; Bergonzoni, E.; Cattapan, C.; Gastino, E.; Galliotto, F.; Boddaert, N.; El Beheiry, M.; Masson, J.-B.; et al. Fast-track virtual reality software to facilitate 3-dimensional reconstruction in congenital heart disease. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad087. [Google Scholar] [CrossRef]
- A Lloyd, D.F.; Pushparajah, K.; Simpson, J.M.; van Amerom, J.F.P.; van Poppel, M.P.M.; Schulz, A.; Kainz, B.; Deprez, M.; Lohezic, M.; Allsop, J.; et al. Three-dimensional visualisation of the fetal heart using prenatal MRI with motion-corrected slice-volume registration: A prospective, single-centre cohort study. Lancet 2019, 393, 1619–1627. [Google Scholar] [CrossRef]
- Cattapan, C.; Bertelli, F.; Guariento, A.; Andolfatto, M.; Veronese, P.; Vida, V.L. 3D ultrasound-based fetal heart reconstruction: A pilot protocol in prenatal counselling. Rev. Esp. Cardiol. 2021, 74, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.W.; Macgowan, C.K. Dynamic MRI of a Large Fetal Cardiac Mass. Radiology 2019, 290, 288. [Google Scholar] [CrossRef] [PubMed]
- Mühler, M.R.; Rake, A.; Schwabe, M.; Schmidt, S.; Kivelitz, D.; Chaoui, R.; Hamm, B. Value of fetal cerebral MRI in sonographically proven cardiac rhabdomyoma. Pediatr. Radiol. 2007, 37, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Vachon-Marceau, C.; Guerra, V.; Jaeggi, E.; Chau, V.; Ryan, G.; Van Mieghem, T. In-utero treatment of large symptomatic rhabdomyoma with sirolimus. Ultrasound Obstet. Gynecol. 2019, 53, 420–421. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Stires, G.; Hahn, E.; Krueger, D.; Franz, D.N. Prenatal Sirolimus Treatment for Rhabdomyomas in Tuberous Sclerosis. Pediatr. Neurol. 2021, 125, 26–31. [Google Scholar] [CrossRef]
- Pluym, I.D.; Sklansky, M.; Wu, J.Y.; Afshar, Y.; Holliman, K.; Devore, G.R.; Walden, A.; Platt, L.D.; Krakow, D. Fetal cardiac rhabdomyomas treated with maternal sirolimus. Prenat. Diagn. 2020, 40, 358–364. [Google Scholar] [CrossRef]
- Seed, M.; van Amerom, J.F.P.; Yoo, S.-J.; Al Nafisi, B.; Grosse-Wortmann, L.; Jaeggi, E.; Jansz, M.S.; Macgowan, C.K. Feasibility of quantification of the distribution of blood flow in the normal human fetal circulation using CMR: A cross-sectional study. J. Cardiovasc. Magn. Reson. 2012, 14, 79. [Google Scholar] [CrossRef]
- Goolaub, D.S.; Xu, J.; Schrauben, E.M.; Marini, D.; Kingdom, J.C.; Sled, J.G.; Seed, M.; Macgowan, C.K. Volumetric Fetal Flow Imaging with Magnetic Resonance Imaging. IEEE Trans. Med. Imaging 2022, 41, 2941–2952. [Google Scholar] [CrossRef]
- Sun, L.; Marini, D.; Saini, B.; Schrauben, E.; Macgowan, C.K.; Seed, M. Understanding Fetal Hemodynamics Using Cardiovascular Magnetic Resonance Imaging. Fetal Diagn. Ther. 2020, 47, 354–362. [Google Scholar] [CrossRef]
- Freud, L.R.; Tworetzky, W. Fetal interventions for congenital heart disease. Curr. Opin. Pediatr. 2016, 28, 156–162. [Google Scholar] [CrossRef]
- Caro-Domínguez, P.; Secinaro, A.; Valverde, I.; Fouilloux, V. Imaging and surgical management of congenital heart diseases. Pediatr. Radiol. 2023, 53, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, H.M. In utero intervention for severe congenital heart disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 58, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Boudjemline, Y. Valve Interventions in Utero: Understanding the Timing, Indications, and Approaches. Can. J. Cardiol. 2017, 33, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, H.J.; Aghajani, F.; Jawwad, M.; Shah, N.; Abuhamad, A.; Khalil, A. Fetal cardiac intervention in hypoplastic left heart syndrome with intact or restrictive atrial septum, systematic review, and meta-analysis. Prenat. Diagn. 2023. [Google Scholar] [CrossRef] [PubMed]
- Marini, D.; Xu, J.; Sun, L.; Jaeggi, E.; Seed, M. Current and future role of fetal cardiovascular MRI in the setting of fetal cardiac interventions. Prenat. Diagn. 2020, 40, 71–83. [Google Scholar] [CrossRef]
- Victoria, T.; Andronikou, S. The fetal MR appearance of “nutmeg lung”: Findings in 8 cases linked to pulmonary lymphangiectasia. Pediatr. Radiol. 2014, 44, 1237–1242. [Google Scholar] [CrossRef]
- Seed, M.; Bradley, T.; Bourgeois, J.; Jaeggi, E.; Yoo, S.-J. Antenatal MR imaging of pulmonary lymphangiectasia secondary to hypoplastic left heart syndrome. Pediatr. Radiol. 2009, 39, 747–749. [Google Scholar] [CrossRef]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and Treatment of Fetal Cardiac Disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef]
- Yamamura, J.; Frisch, M.; Ecker, H.; Graessner, J.; Hecher, K.; Adam, G.; Wedegärtner, U. Self-gating MR imaging of the fetal heart: Comparison with real cardiac triggering. Eur. Radiol. 2011, 21, 142–149. [Google Scholar] [CrossRef]
- Kühle, H.; Cho, S.K.S.; Barber, N.; Goolaub, D.S.; Darby, J.R.T.; Morrison, J.L.; Haller, C.; Sun, L.; Seed, M. Advanced imaging of fetal cardiac function. Front. Cardiovasc. Med. 2023, 10, 1206138. [Google Scholar] [CrossRef]
- Yamamura, J.; Kopp, I.; Frisch, M.; Fischer, R.; Valett, K.; Hecher, K.; Adam, G.; Wedegärtner, U. Cardiac MRI of the fetal heart using a novel triggering method: Initial results in an animal model. J. Magn. Reson. Imaging 2012, 35, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.W.; Seed, M.; van Amerom, J.F.P.; Al Nafisi, B.; Grosse-Wortmann, L.; Yoo, S.; Macgowan, C.K. Dynamic imaging of the fetal heart using metric optimized gating. Magn. Reson. Med. 2013, 70, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Jansz, M.S.; Seed, M.; van Amerom, J.F.P.; Wong, D.; Grosse-Wortmann, L.; Yoo, S.; Macgowan, C.K. Metric optimized gating for fetal cardiac MRI. Magn. Reson. Med. 2010, 64, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.C.; White, R.D.; Laub, G.; McVeigh, E.R.; Li, D.; Simonetti, O.P. Self-gated cardiac cine MRI. Magn. Reson. Med. 2004, 51, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Haris, K.; Hedström, E.; Bidhult, S.; Testud, F.; Maglaveras, N.; Heiberg, E.; Hansson, S.R.; Arheden, H.; Aletras, A.H. Self-gated fetal cardiac MRI with tiny golden angle iGRASP: A feasibility study. J. Magn. Reson. Imaging 2017, 46, 207–217. [Google Scholar] [CrossRef]
- De Sousa, M.T.; Hecher, K.; Yamamura, J.; Kording, F.; Ruprecht, C.; Fehrs, K.; Behzadi, C.; Adam, G.; Schoennagel, B.P. Dynamic fetal cardiac magnetic resonance imaging in four-chamber view using Doppler ultrasound gating in normal fetal heart and in congenital heart disease: Comparison with fetal echocardiography. Ultrasound Obstet. Gynecol. 2019, 53, 669–675. [Google Scholar] [CrossRef]
- Haris, K.; Hedström, E.; Kording, F.; Bidhult, S.; Steding-Ehrenborg, K.; Ruprecht, C.; Heiberg, E.; Arheden, H.; Aletras, A.H. Free-breathing fetal cardiac MRI with doppler ultrasound gating, compressed sensing, and motion compensation. J. Magn. Reson. Imaging 2020, 51, 260–272. [Google Scholar] [CrossRef]
- Kording, F.; Yamamura, J.; Much, C.; Adam, G.; Schoennagel, B.; Wedegärtner, U.; Ueberle, F. Evaluation of an Mr Compatible Doppler-Ultrasound Device as a New Trigger Method in Cardiac Mri: A Quantitative Comparison to ECG. Biomed. Tech. 2013, 58 (Suppl. 1), 000010151520134267. [Google Scholar] [CrossRef]
- Kording, F.; Yamamura, J.; de Sousa, M.T.; Ruprecht, C.; Hedström, E.; Aletras, A.H.; Grant, P.E.; Powell, A.J.; Fehrs, K.; Adam, G.; et al. Dynamic fetal cardiovascular magnetic resonance imaging using Doppler ultrasound gating. J. Cardiovasc. Magn. Reson. 2018, 20, 17. [Google Scholar] [CrossRef]
- Ramdass, S.; Adam, S.; Lockhat, Z.; Masenge, A.; Suleman, F.E. Foetal magnetic resonance imaging: A necessity or adjunct? A modality comparison of in-utero ultrasound and ultrafast foetal magnetic resonance imaging. S. Afr. J. Radiol. 2021, 25, 2010. [Google Scholar] [CrossRef]
- Wielandner, A.; Mlczoch, E.; Prayer, D.; Berger-Kulemann, V. Potential of magnetic resonance for imaging the fetal heart. Semin. Fetal Neonatal Med. 2013, 18, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Ghanchi, A.; Derridj, N.; Bonnet, D.; Bertille, N.; Salomon, L.J.; Khoshnood, B. Children Born with Congenital Heart Defects and Growth Restriction at Birth: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3056. [Google Scholar] [CrossRef] [PubMed]
- Brodwall, K.; Leirgul, E.; Greve, G.; Vollset, S.E.; Holmstrøm, H.; Tell, G.S.; Øyen, N. Possible Common Aetiology behind Maternal Preeclampsia and Congenital Heart Defects in the Child: A Cardiovascular Diseases in Norway Project Study. Paediatr. Perinat. Epidemiology 2016, 30, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Thilaganathan, B. Preeclampsia and Fetal Congenital Heart Defects. Circulation 2017, 136, 49–51. [Google Scholar] [CrossRef]
- Gatta, G.; Di Grezia, G.; Cuccurullo, V.; Sardu, C.; Iovino, F.; Comune, R.; Ruggiero, A.; Chirico, M.; La Forgia, D.; Fanizzi, A.; et al. MRI in Pregnancy and Precision Medicine: A Review from Literature. J. Pers. Med. 2021, 12, 9. [Google Scholar] [CrossRef]
- Mekkaoui, C.; Porayette, P.; Jackowski, M.P.; Kostis, W.J.; Dai, G.; Sanders, S.; Sosnovik, D.E. Diffusion MRI Tractography of the Developing Human Fetal Heart. PLoS ONE 2013, 8, e72795. [Google Scholar] [CrossRef]
- Castaldi, B.; Angelini, A.; De Filippi, E.; Susin, F.; Cattapan, I.; Fedrigo, M.; Boso, D.; Peruzzo, P.; Comunale, G.; Milanesi, O.; et al. Young Investigator Award session—Basic Science. Eur. Heart J. Cardiovasc. Imaging 2017, 18, iii80–iii81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozza, A.; Reffo, E.; Castaldi, B.; Cattapan, I.; Avesani, M.; Biffanti, R.; Cavaliere, A.; Cerutti, A.; Di Salvo, G. Utility of Fetal Cardiac Resonance Imaging in Prenatal Clinical Practice: Current State of the Art. Diagnostics 2023, 13, 3523. https://doi.org/10.3390/diagnostics13233523
Pozza A, Reffo E, Castaldi B, Cattapan I, Avesani M, Biffanti R, Cavaliere A, Cerutti A, Di Salvo G. Utility of Fetal Cardiac Resonance Imaging in Prenatal Clinical Practice: Current State of the Art. Diagnostics. 2023; 13(23):3523. https://doi.org/10.3390/diagnostics13233523
Chicago/Turabian StylePozza, Alice, Elena Reffo, Biagio Castaldi, Irene Cattapan, Martina Avesani, Roberta Biffanti, Annachiara Cavaliere, Alessia Cerutti, and Giovanni Di Salvo. 2023. "Utility of Fetal Cardiac Resonance Imaging in Prenatal Clinical Practice: Current State of the Art" Diagnostics 13, no. 23: 3523. https://doi.org/10.3390/diagnostics13233523
APA StylePozza, A., Reffo, E., Castaldi, B., Cattapan, I., Avesani, M., Biffanti, R., Cavaliere, A., Cerutti, A., & Di Salvo, G. (2023). Utility of Fetal Cardiac Resonance Imaging in Prenatal Clinical Practice: Current State of the Art. Diagnostics, 13(23), 3523. https://doi.org/10.3390/diagnostics13233523






