Unpredictable Metastasis in the Head and Neck Region: A Diagnostic Immunohistochemical Challenge
Abstract
1. Introduction
2. Case Presentation
2.1. Case 1
2.1.1. Clinical Findings
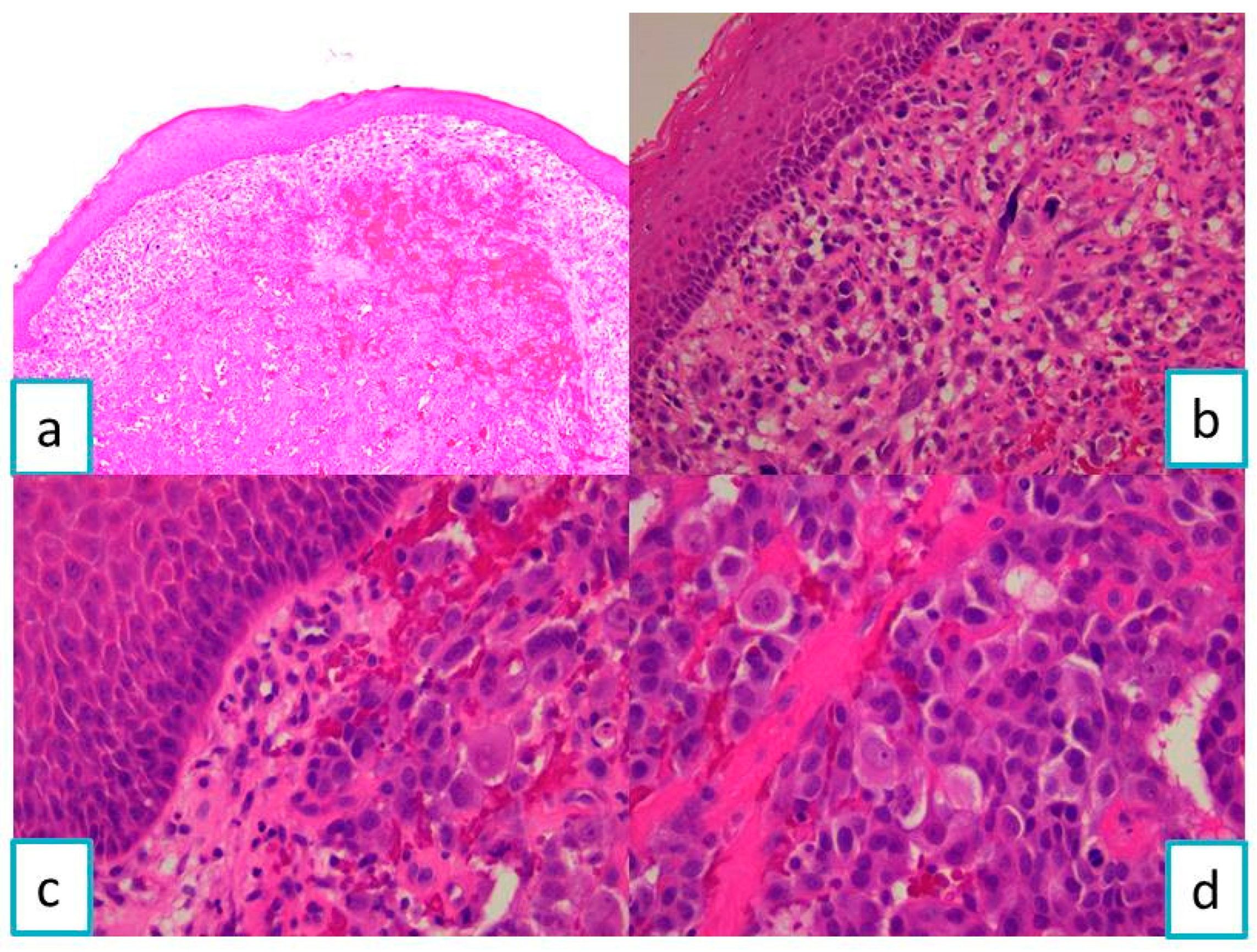
2.1.2. Pathological Findings
2.2. Case 2
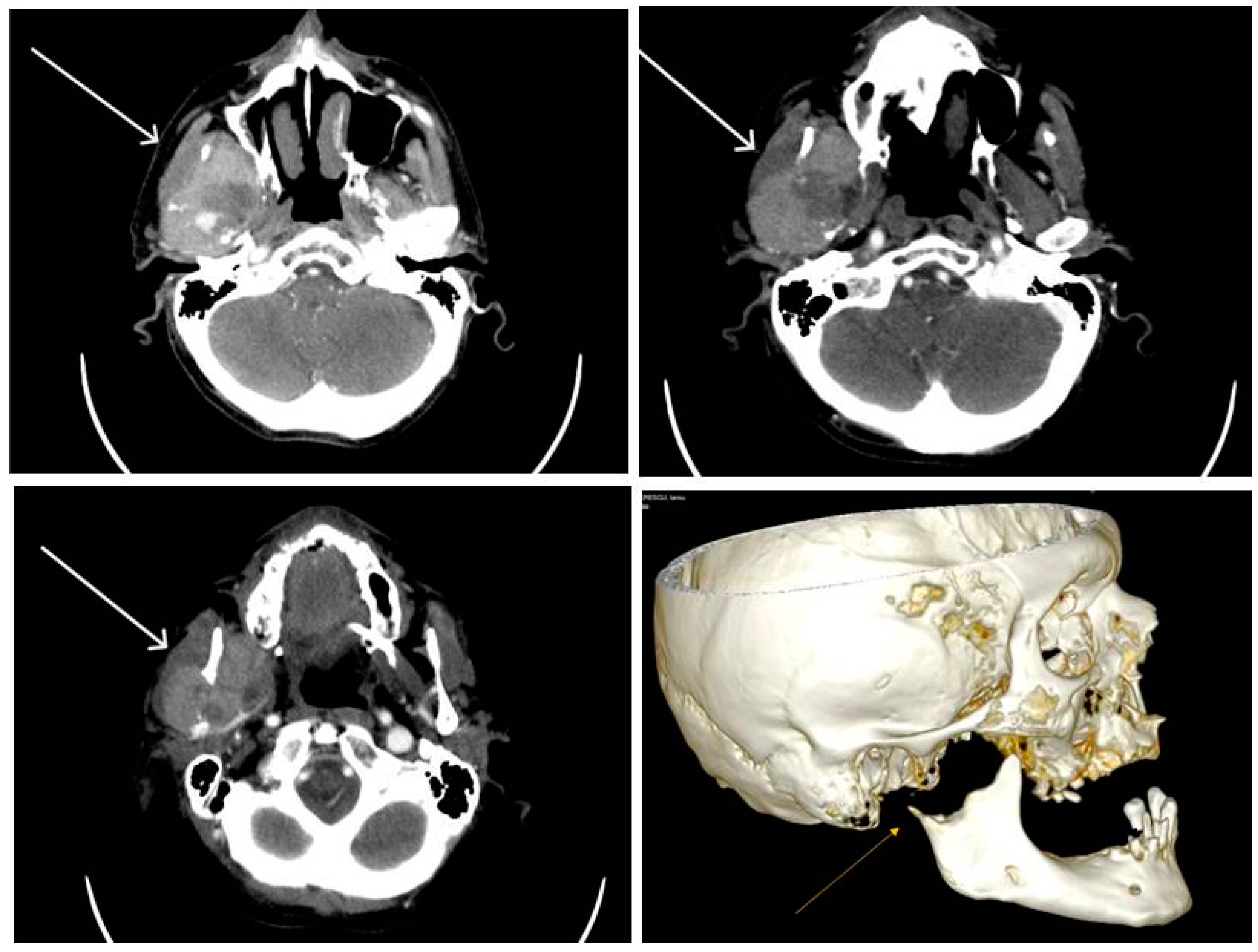
2.2.1. Clinical Findings
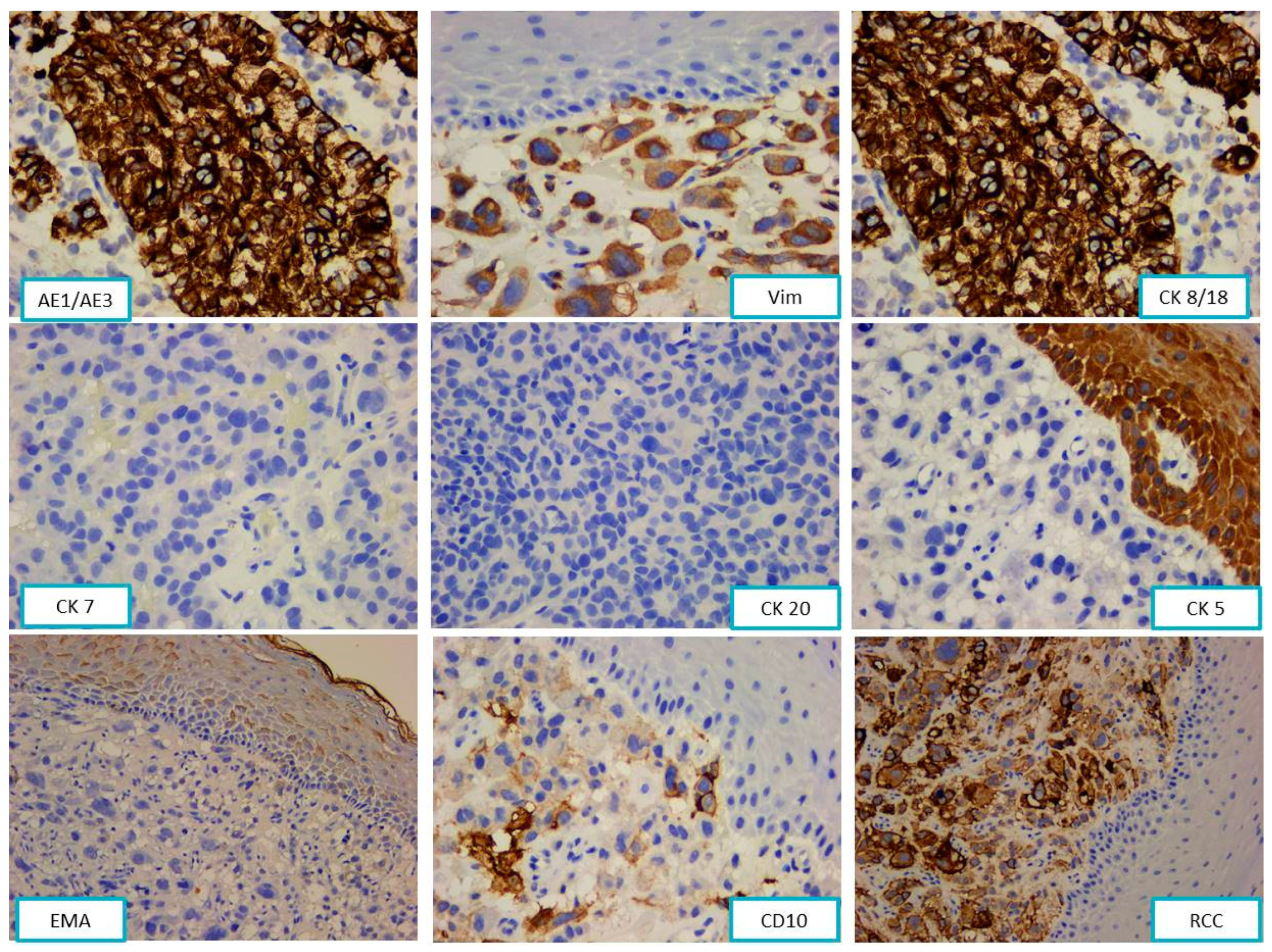
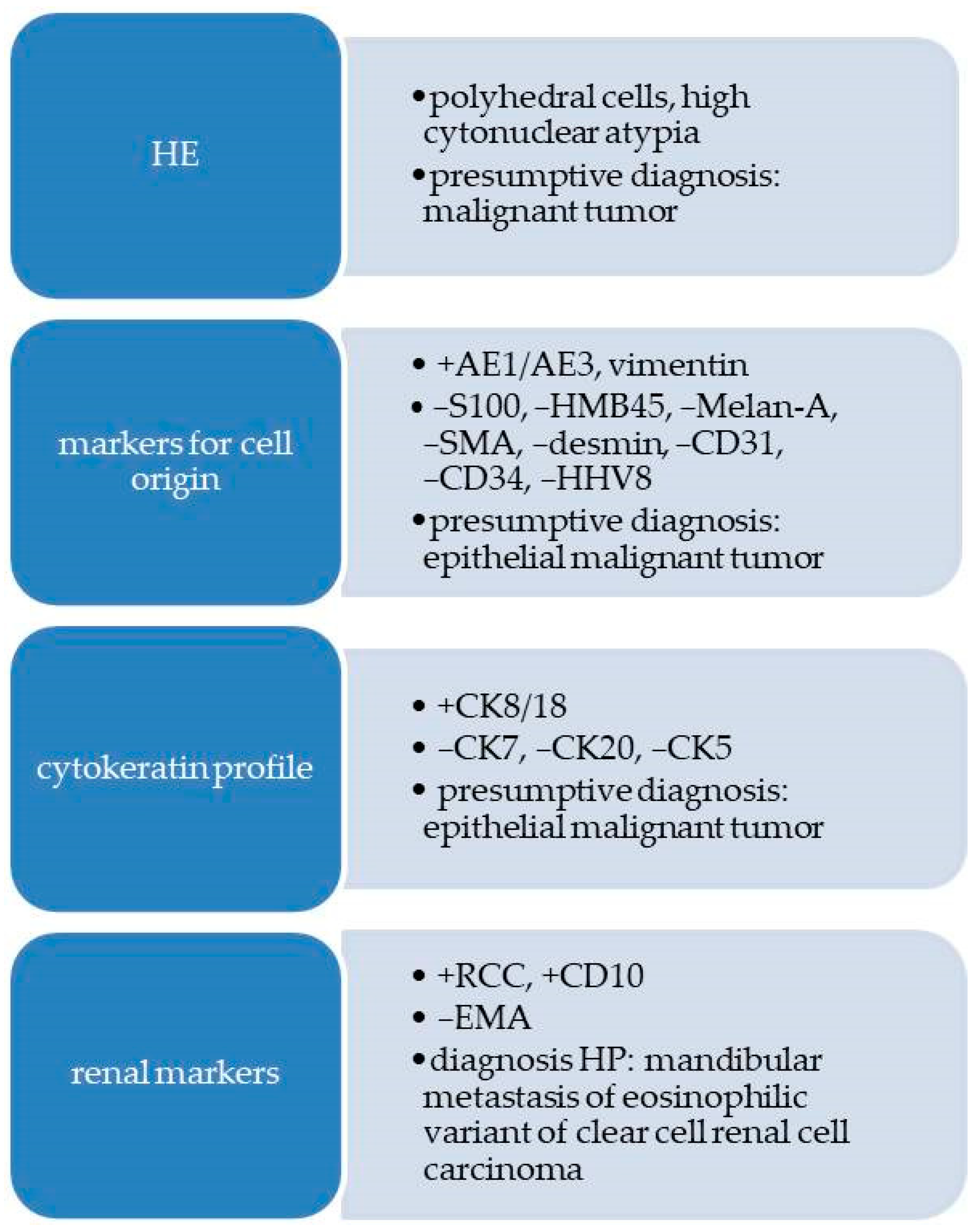
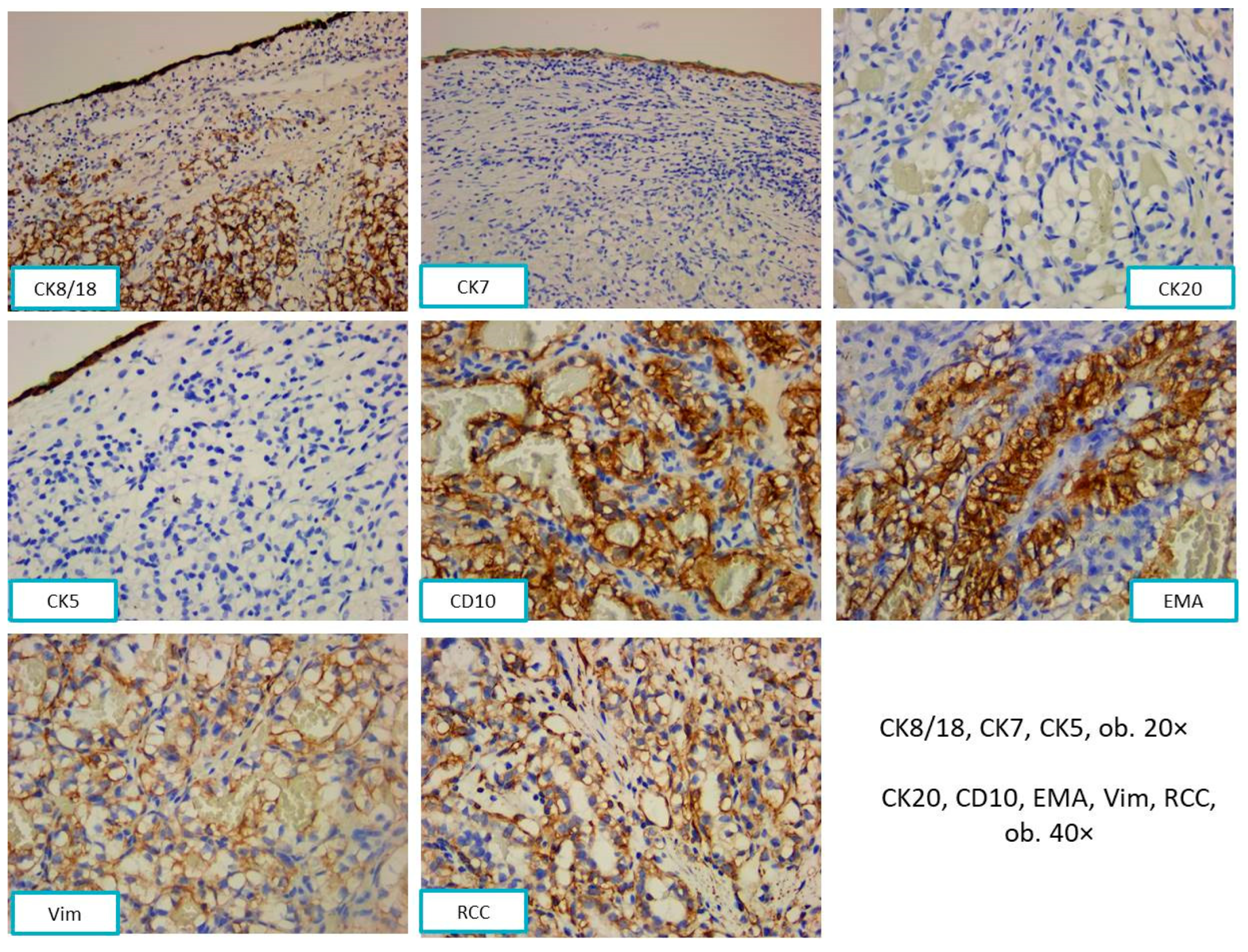
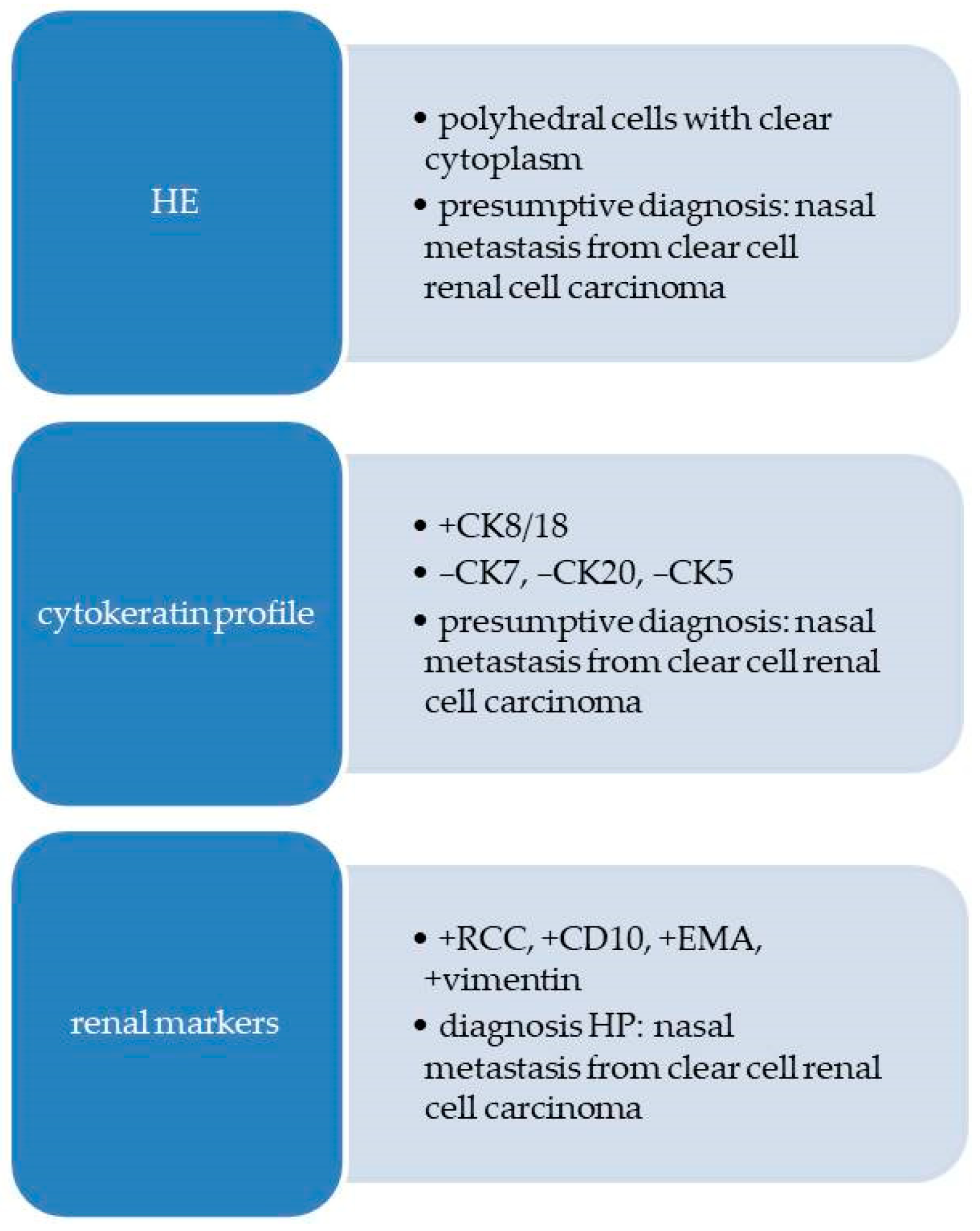
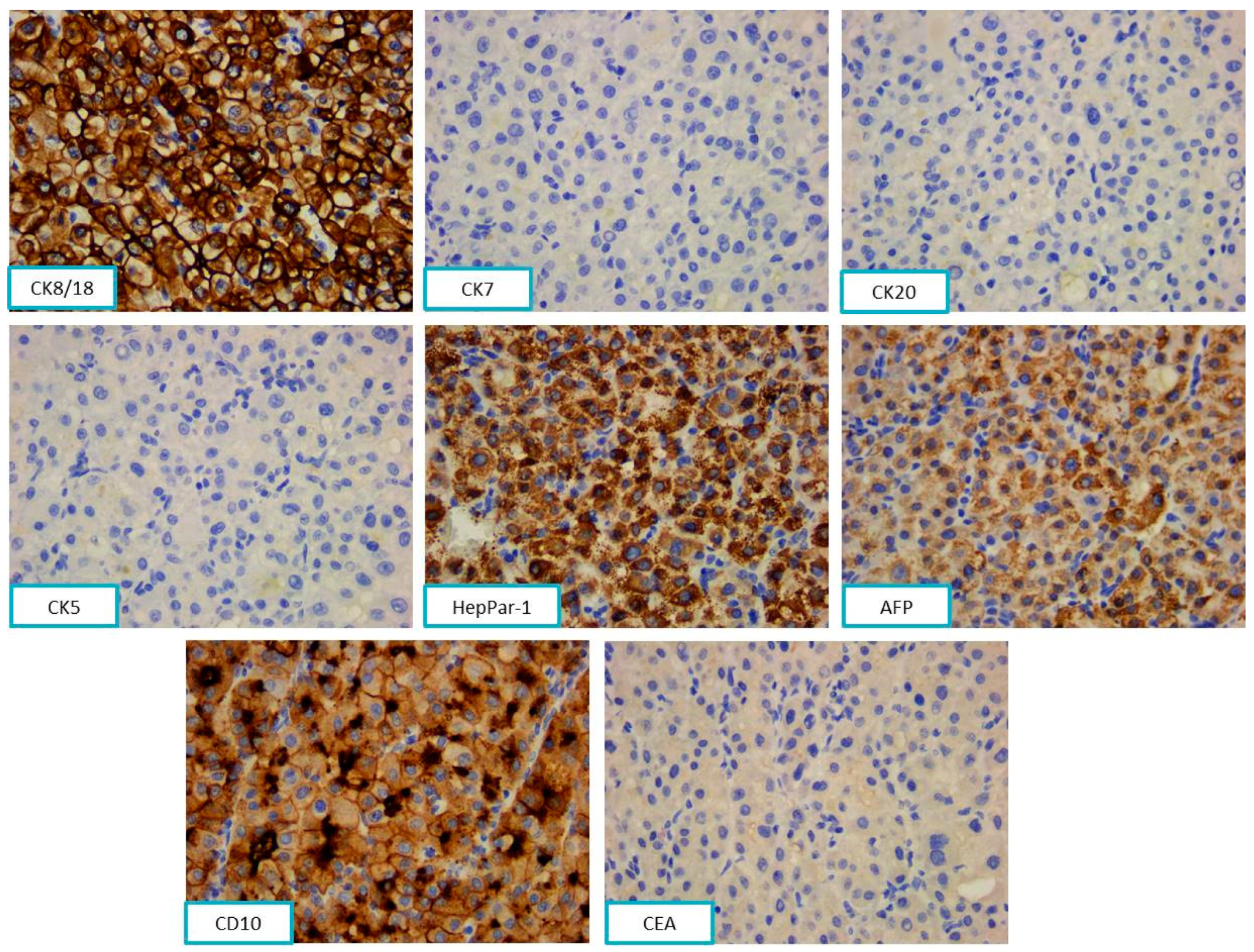
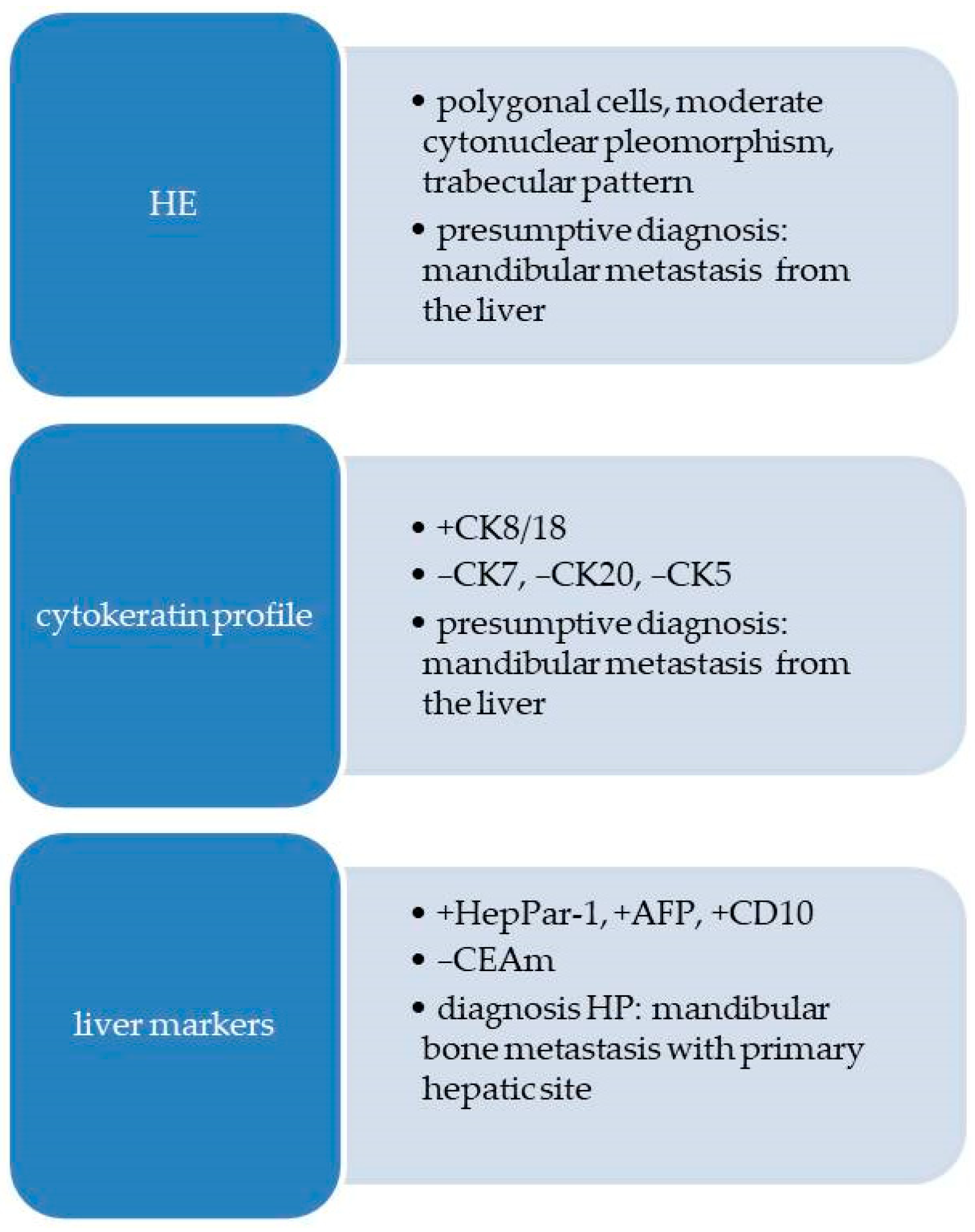
2.2.2. Pathological Findings
2.3. Case 3
2.3.1. Clinical Findings
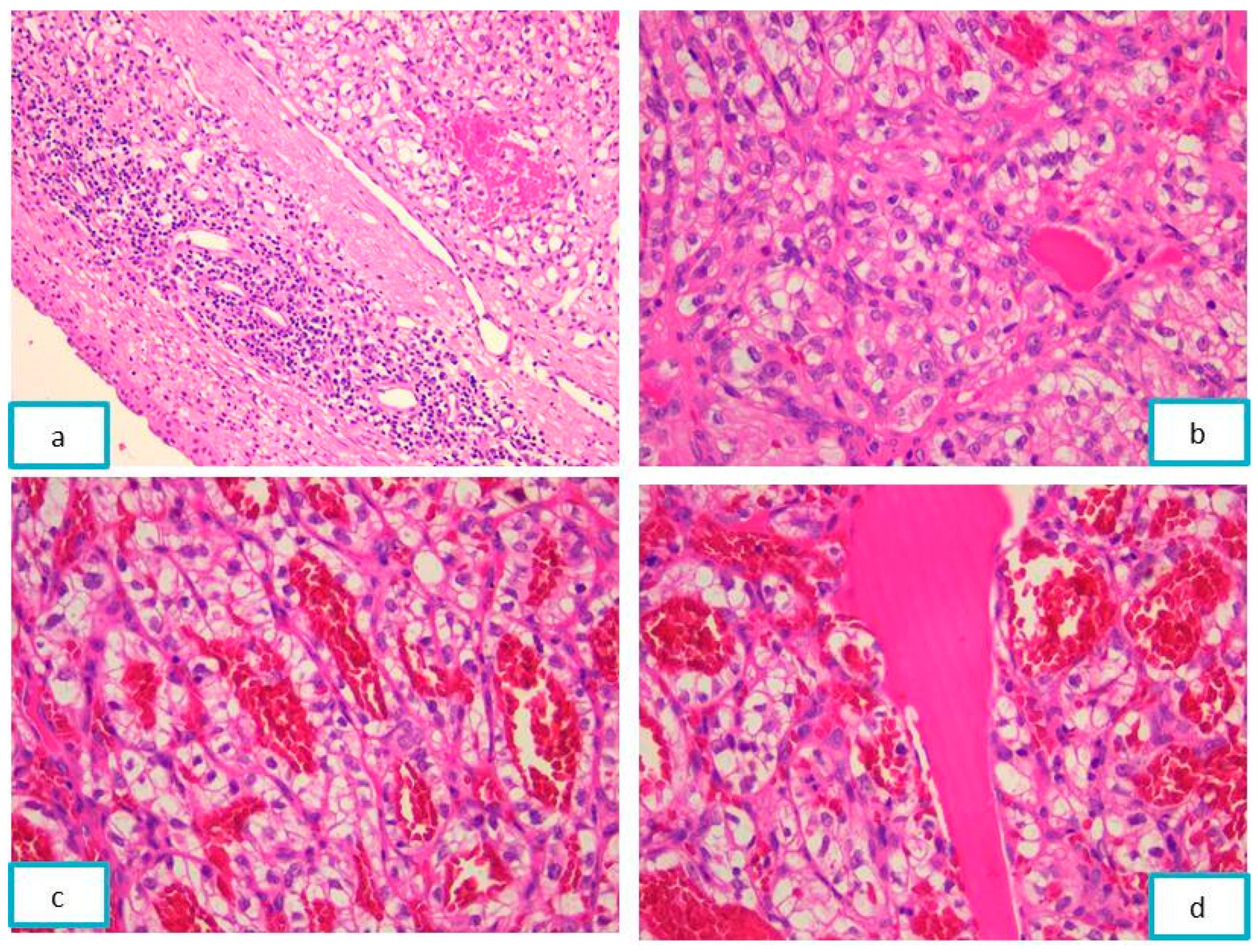
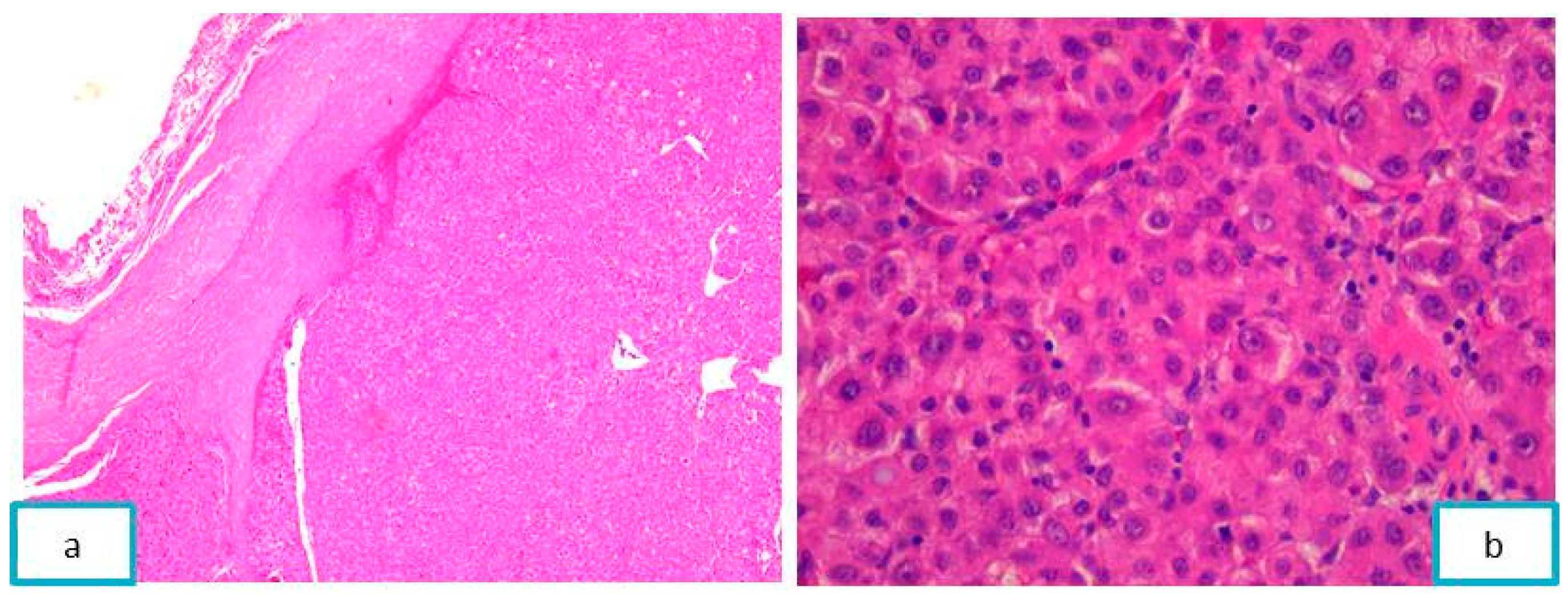
2.3.2. Pathological Findings
3. Discussion
3.1. Metastases of Clear Cell Renal Carcinoma
3.2. Jaw Bone Metastasis of Hepatocellular Carcinoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenkeit, C.; Bank, J.; Shirazi, M. Renal Cell Carcinoma in the Head and Neck: Case Presentation of a Patient with a Rare Metastatic Pattern. Cureus 2020, 12, 11894. [Google Scholar] [CrossRef] [PubMed]
- Lieder, A.; Guenzel, T.; Lebentrau, S.; Schneider, C.; Franzen, A. Diagnostic relevance of metastatic renal cell carcinoma in the head and neck: An evaluation of 22 cases in 671 patients. Int. Braz. J. Urol. 2017, 43, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Remenschneider, A.K.; Sadow, P.M.; Lin, D.T.; Gray, S.T. Metastatic Renal Cell Carcinoma to the Sinonasal Cavity: A Case Series. J. Neurol. Surg. Rep. 2013, 74, 67–72. [Google Scholar]
- Ali, R.A.; Mohamed, K.E. Metastatic Clear Cell Renal Cell Carcinoma Presenting with a Gingival Metastasis. Clin. Pract. 2016, 10, 847. [Google Scholar] [CrossRef][Green Version]
- Morita, Y.; Kashima, K.; Suzuki, M.; Kinosada, H.; Teramoto, A.; Matsumiya, Y.; Uzawa, N. Differential Diagnosis between Oral Metastasis of Renal Cell Carcinoma and Salivary Gland Cancer. Diagnostics 2021, 11, 506. [Google Scholar] [CrossRef]
- Yu, S.; Estess, A.; Harris, W.; Dillon, J. A rare occurrence of hepatocellular carcinoma metastasis to the mandible: Report of a case and review of the literature. J. Oral. Maxillofac. Surg. 2012, 70, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Pesis, M.; Taicher, S.; Greenberg, G.; Hirshberg, A. Metastasis to the jaws as a first manifestation of hepatocellular carcinoma: Report of a case and analysis of 41 cases. J. Craniomaxillofac. Surg. 2014, 42, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Mead, S.G.; Mensh, M.; Schatten, W.E. Primary hepatoma with metastasis to the mandible. Am. J. Surg. 1957, 94, 846–850. [Google Scholar]
- Exposito-Villen, A.; Aranega, E.A.; Franco, D. Functional role of non-coding RNAs during epithelial-To-mesenchymal transition. Non-Coding RNA 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [PubMed]
- Xiong, J.; Liu, Y.; Jiang, L.; Zeng, Y.; Tang, W. High expression of long non-coding RNA lncRNA-ATB is correlated with metastases and promotes cell migration and invasion in renal cell carcinoma. Jpn. J. Clin. Oncol. 2016, 46, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Sarău, C.A.; Poenaru, M.; Balica, N.C.; Baderca, F. Rare sinonasal lesions. Rom. J. Morphol. Embryol. 2017, 58, 1541–1547. [Google Scholar] [PubMed]
- Trandafir, C.M.; Tischer, A.A.; Horhat, I.D.; Balica, N.C.; Sitaru, A.M.; Guran, K.; Morar, R.; Baderca, F.; Jifcu, E.M.; Moţ, I.C.; et al. Fortuitous discovery of melanomas in the ENT Department—A histopathological and immunohistochemical study. Rom. J. Morphol. Embryol. 2020, 6, 1163–1171. [Google Scholar] [CrossRef]
- Baderca, F.; Vincze, D.; Balica, N.; Solovan, C. Mucosal melanomas in the elderly: Challenging cases and review of the literature. Clin. Interv. Aging. 2014, 9, 929–937. [Google Scholar] [CrossRef]
- Rakitovan, M.; Nicoara, A.; Closca, R.M.; Balica, N.C.; Stefanescu, E.H.; Baderca, F. Leiomyoma with Uncommon Localization-Incisive Papilla and Palatal Fibromucosa: A Case Report. Medicina 2023, 59, 1346. [Google Scholar] [CrossRef]
- Zhao, W.; Yangi, L.; Wang, L.; Zuo, W.; Shuanghu Yuan, S.; Yu, J.; Yu, Q.; Xudong Hu, X.; Wang, S.; Liu, N.; et al. Primary clear cell carcinoma of nasal cavity: Report of six cases and review of literature. Int. J. Clin. Exp. Med. 2014, 7, 5469–5476. [Google Scholar]
- Zur, K.B.; Brandwein, M.; Wang, B.; Som, P.; Gordon, R.; Urken, M.L. Primary description of a new entity, renal cell-like carcinoma of the nasal cavity: Van Meegeren in the house of Vermeer. Arch. Otolaryngol. Head. Neck Surg. 2002, 128, 441–447. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 24–26. [Google Scholar]
- Chen, Z.; Wang, Z.; Shi, H.; Liu, Q. Renal cell-like carcinoma of the nasal cavity: A case report and review of the literature. Diagn. Pathol. 2017, 12, 75. [Google Scholar]
- Morvan, J.B.; Veyrières, J.B.; Mimouni, O.; Cathelinaud, O.; Allali, L.; Verdalle, P. Clear-cell renal carcinoma metastasis to the base of the tongue and sphenoid sinus: Two very rare atypical ENT locations. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 91–94. [Google Scholar] [CrossRef]
- Sikka, S.; Sikka, P.; Kaur, G.; Shetty, D.C. A review of histopathological and immunohistochemical parameters in diagnosis of metastatic renal cell carcinoma with a case of gingival metastasis. J. Cancer Res. Ther. 2013, 9, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Feng, Y.; Li, N.; Wang, K.; Wang, S.; Gao, Z. Mandibular metastasis as an initial manifestation of hepatocellular carcinoma: A report of two cases. Oncol. Lett. 2015, 9, 1213–1216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piccirillo, M.; Granata, V.; Albino, V.; Palaia, R.; Setola, S.V.; Petrillo, A.; Izzo, F. Can hepatocellular carcinoma (HCC) produce unconventional metastases? Four cases of extrahepatic HCC. Tumori J. 2013, 99, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Nawale, K.K.; Vyas, M.; Kane, S.; Patil, A. Metastatic tumors in the jaw bones: A retrospective clinicopathological study of 12 cases at Tertiary Cancer Center. J. Oral. Maxillofac. Pathol. 2016, 20, 252–255. [Google Scholar]
- Miller, M.E.; McCall, A.A.; Juillard, G.F.; Nadelman, C.M.; Wang, M.B.; Nabili, V. Hepatocellular carcinoma metastatic to the mandible. Ear Nose Throat J. 2013, 92, 17–19. [Google Scholar]
- Van der Waal, R.I.; Buter, J.; van der Waal, I. Oral metastases: Report of 24 cases. Br. J. Oral. Maxillofac. Surg. 2003, 41, 3–6. [Google Scholar] [CrossRef]
- Irani, S. Metastasis to the Jawbones: A review of 453 cases. J. Int. Soc. Prev. Community Dent. 2017, 7, 71–81. [Google Scholar] [CrossRef]
- Chen, D.; Li, Z.; Song, Q.; Qian, L.; Xie, B.; Zhu, J. Clinicopathological features and differential diagnosis of hepatocellular carcinoma in extrahepatic metastases. Medicine 2018, 97, e13356. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.M.; Dorantes-Heredia, R.; Chable-Montero, F.; Vazquez-Manjarrez, S.; Méndez-Sánchez, N.; Motola-Kuba, D. Bone metastases as the initial presentation of hepatocellular carcinoma. Two case reports and a literature review. Ann. Hepatol. 2014, 13, 838–842. [Google Scholar] [CrossRef]
- Dabbs, D.J. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications, 5th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 576–582. [Google Scholar]
- Hong, J.H.; Lee, K.; Kim, J.; Ahn, K.M. Prognosis of hepatocellular carcinoma metastasizing to the oral cavity. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 9. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Lin, F.; Ma, J. Hepatocellular carcinoma metastasis to the mandibular ramus: A case report. Int. J. Clin. Exp. Pathol. 2019, 12, 1047–1051. [Google Scholar] [PubMed]
| Antibody | Substrate | Clone | Dilution |
|---|---|---|---|
| CK AE1/AE3 1 | Mouse, Monoclonal | AE1/AE3 | 1:100 |
| EMA 2 | Mouse, Monoclonal | GP1.4 | 1:300 |
| CK8/18 3 | Mouse, Monoclonal | 5D3 | 1:100 |
| CK5 4 | Mouse, Monoclonal | XM26 | 1:100 |
| CK7 5 | Mouse, Monoclonal | 307M-94 | 1:100 |
| CK20 6 | Mouse, Monoclonal | L26 | 1:150 |
| CD10 8 | Mouse, Monoclonal | 56C6 | RTU 7 |
| S100 protein | Rabbit, Polyclonal | EP32 | 1:100 |
| HMB45 9 | Mouse, Monoclonal | HMB45 | 1:60 |
| Melan-A | Mouse, Monoclonal | A103 | 1:50 |
| CD34 10 | Mouse, Monoclonal | QBEnd/10 | RTU |
| CD31 11 | Mouse, Monoclonal | 1A10 | 1:75 |
| SMA 12 | Mouse, Monoclonal | asn-1 | 1:50 |
| Desmin | Mouse, Monoclonal | DE-R-11 | 1:75 |
| Vimentin | Mouse, Monoclonal | V9 | 1:800 |
| HHV8 13 | Mouse, Monoclonal | 13B10 | RTU |
| RCC 14 | Mouse, Monoclonal | 66.4.C2 | RTU |
| Antibody | Substrate | Clone | Dilution |
|---|---|---|---|
| EMA | Mouse, Monoclonal | GP1.4 | 1:300 |
| CK8/18 | Mouse, Monoclonal | 5D3 | 1:100 |
| CK5 | Mouse, Monoclonal | XM26 | 1:100 |
| CK7 | Mouse, Monoclonal | 307M-94 | 1:100 |
| CK20 | Mouse, Monoclonal | L26 | 1:150 |
| CD10 | Mouse, Monoclonal | 56C6 | RTU |
| Vimentin | Mouse, Monoclonal | V9 | 1:800 |
| RCC | Mouse, Monoclonal | 66.4.C2 | RTU |
| Antibody | Substrate | Clone | Dilution |
|---|---|---|---|
| CK8/18 | Mouse, Monoclonal | 5D3 | 1:100 |
| CK5 | Mouse, Monoclonal | XM26 | 1:100 |
| CK7 | Mouse, Monoclonal | 307M-94 | 1:100 |
| CK20 | Mouse, Monoclonal | L26 | 1:150 |
| CEA 1 | Mouse, Monoclonal | II-7 | RTU |
| CD10 | Mouse, Monoclonal | 56C6 | RTU |
| AFP 2 | Mouse, Monoclonal | C3 | 1:100 |
| HepPar-1 3 | Mouse, Monoclonal | OCH1ES | RTU |
| Case | Primary Neoplasm | Date | Treatment | Oral Lesion | Date | Treatment |
|---|---|---|---|---|---|---|
| 1 | - | - | - | Alveolar ridge | January 2023 | Radiotherapy and oncological follow-up |
| 2 | Kidney | May 2003 | Resection and oncological follow-up | Nasal fossa | January 2021 | Radiotherapy and oncological follow-up |
| 3 | Liver | December 2018 | Resection, radiotherapy, and oncological follow-up | Mandible | March 2021 | Radiotherapy and oncological follow-up |
| Author | Year of Publication | Journal | Number of Patients |
|---|---|---|---|
| Irani S. [28] | 2017 | J. Int. Soc. Prev. Community Dent. | 453 |
| Lieder A. [2] | 2017 | Int. Braz. J. Urol. | 22 |
| Nawale K. [25] | 2016 | J. Oral Maxillofac. Pathol. | 12 |
| Pesis M. [7] | 2014 | J. Craniomaxillofac. Surg. | 41 |
| Zhao W. [17] | 2014 | Int. J. Clin. Exp. | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Closca, R.-M.; Nicoara, A.; Rakitovan, M.; Mot, I.C.; Baderca, F. Unpredictable Metastasis in the Head and Neck Region: A Diagnostic Immunohistochemical Challenge. Diagnostics 2023, 13, 3513. https://doi.org/10.3390/diagnostics13233513
Closca R-M, Nicoara A, Rakitovan M, Mot IC, Baderca F. Unpredictable Metastasis in the Head and Neck Region: A Diagnostic Immunohistochemical Challenge. Diagnostics. 2023; 13(23):3513. https://doi.org/10.3390/diagnostics13233513
Chicago/Turabian StyleClosca, Raluca-Maria, Adrian Nicoara, Marina Rakitovan, Ion Cristian Mot, and Flavia Baderca. 2023. "Unpredictable Metastasis in the Head and Neck Region: A Diagnostic Immunohistochemical Challenge" Diagnostics 13, no. 23: 3513. https://doi.org/10.3390/diagnostics13233513
APA StyleClosca, R.-M., Nicoara, A., Rakitovan, M., Mot, I. C., & Baderca, F. (2023). Unpredictable Metastasis in the Head and Neck Region: A Diagnostic Immunohistochemical Challenge. Diagnostics, 13(23), 3513. https://doi.org/10.3390/diagnostics13233513







