Ultrasound-Guided vs. Fluoroscopy-Guided Interventions for Back Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Outcomes
2.3. Data Extraction and Biostatistics Methods
2.4. Methodological Assessment
3. Results
| Author, Citation | Country | Study Design | Study Goals | Age Mean (St Dev) | N of Patients: Total (Intervention/Control) | Patient Characteristics | Injected Agents | Study Conclusion |
|---|---|---|---|---|---|---|---|---|
| Akkaya et al., 2017 [6] | Turkey | RCT | To compare outcomes of FL- or US-guided CESI in post-laminectomy patients | 48.55 (10.66), 45.26 (9.83) | 30: (15/15) | “Patients who had undergone L4-5 or L5-S1 hemilaminectomy within the last 1 year” | “2.5% bupivacaine + dexamethasone 8 mg” | Comparable effectiveness; US more comfortable |
| Evansa et al., 2015 [3] | Latvia | RCT | To assess the technical feasibility of US- and FL-based methods in ESI | 69.2 (10.3), 69.1 (10.2) | 112: (56/56) | “Spinal canal stenosis, disc herniation, spondylolisthesis” | “Corticosteroid (methylprednisolone acetate 80 mg) + 1% lidocaine 4 mL” | Comparable analgesic effect and time to perform |
| Jee et al., 2013 [2] | Republic of Korea | RCT | To compare short-term analgesic effect, functional enhancements, and safety between US-guided SNRB and FL-guided TFEI in the lower cervical spine | 57.76 (9.56), 56.69 (9.32) | 110: (55/55) | “Patients with posterior neck and radicular pain” | “1 mL of 1% lidocaine, then 2 mL dexamethasone (10 mg) and 1 mL 0.5% lidocaine” | Comparable effectiveness in reducing pain |
| Yang et al., 2016 [7] | China | RCT | To assess the precision, impact on pain relief, and safety of US-guided lumbar TFEI | 57 (10), 58 (9) | 80: (40/40) | “Back pain associated with lower limb radiation pain, herniated disk or spinal stenosis” | “1% lidocaine, 4 mL of 0.5% lidocaine + 1 mL of diprospan” | Feasibility and safety of US, success rate of 85% |
| Hazra et al., 2016 [16] | India | RCT | To evaluate the time taken for precise needle positioning and analyze the clinical effectiveness of FL and US guidance in CESI in chronic low back pain | 44.48 (6.48), 41.88 (8.05) | 50: (25/25) | “Chronic low back pain with unilateral or bilateral radiculopathy of more than 3 months duration, not responding to conventional therapy” | “2 mL preservative-free lignocaine (1%) pre-procedure, methylprednisolone 40 mg diluted in 10 mL normal saline” | Comparable pain-sparing effect, better visualization in FL |
| Soneji et al., 2016 [17] | Canada | RCT | To investigate the differences in accuracy and effectiveness between US- and FL-guided SIJI | 50.90 (12.77), 46.85 (11.51) | 40: (20/20) | “Patients with chronic back pain secondary to SIJ arthritis” | “40 mg of methylprednisolone acetate diluted in 3 mL of bupivacaine 0.25% with epinephrine 1:200,000 (total 4 mL injectate)”Fl: “radio-opaque contrast 0.5 mLs followed by fluoroscopy imaging” | Comparable accuracy, efficacy, and overall patient satisfaction |
| Park et al., 2013 [18] | Republic of Korea | RCT | To assess the immediate benefits of US-guided CESI with FL-guided ESI for unilateral radicular pain in the lower lumbar spine | 57.27 (10.11), 58.47 (9.22) | 110: (55/55) | “Patients with unilateral lumbar radicular pain” | “Nonionic contrast medium: 5 mL (Omnipaque 300) + 15 mL (13.0 mL of 0.5% lidocaine + 2 mL of dexamethasone 10 mg)” | Comparable analgesic effect, functional improvement, and patient satisfaction |
| Jee et al., 2014 [19] | Republic of Korea | RCT | To examine the efficacy and outcomes of US-guided and FL-guided SIJI in noninflammatory SIJ dysfunction | 60.98 (8.58), 60.69 (8.02) | 110: (55/55) | “Chronic low back pain (>3 mo) without radiculopathy” | “0.5 mL nonionic contrast media (Omnipaque 300a) + 2 mL (1.0 mL 0.5% lidocaine + dexamethasone 10 mg)” | Comparable efficacy |
3.1. Qualitative Description of the Studies
3.1.1. “US-Guided Selective Nerve Root Block” and “fluoroscopy-Guided Transforaminal Block” for the Treatment of Radicular Pain in the Lower Cervical Spine
3.1.2. US and “FL-Guided Epidural Steroid Injections” in Patients with Degenerative Spinal Diseases
3.1.3. US and “FL-Guided Caudal Epidural Steroid Injection” in Unilateral and Bilateral Radiculopathy
3.1.4. US and “FL-Guided Caudal Steroid Injections” for Degenerative Spinal Diseases
3.1.5. US-Guided and “Fluoroscopy-Controlled Lumbar Transforaminal Epidural Injections”
3.1.6. US-Guided vs. “FL-Guided Caudal Epidural Steroid Injection” in Unilateral Lower Lumbar Radicular Pain
3.1.7. “Fluoroscopic Guidance” vs. “Ultrasound Guidance for Sacroiliac Joint (SIJ) Injection” in Chronic Low Back Pain
3.2. A Meta-Analysis of All Studies Combined
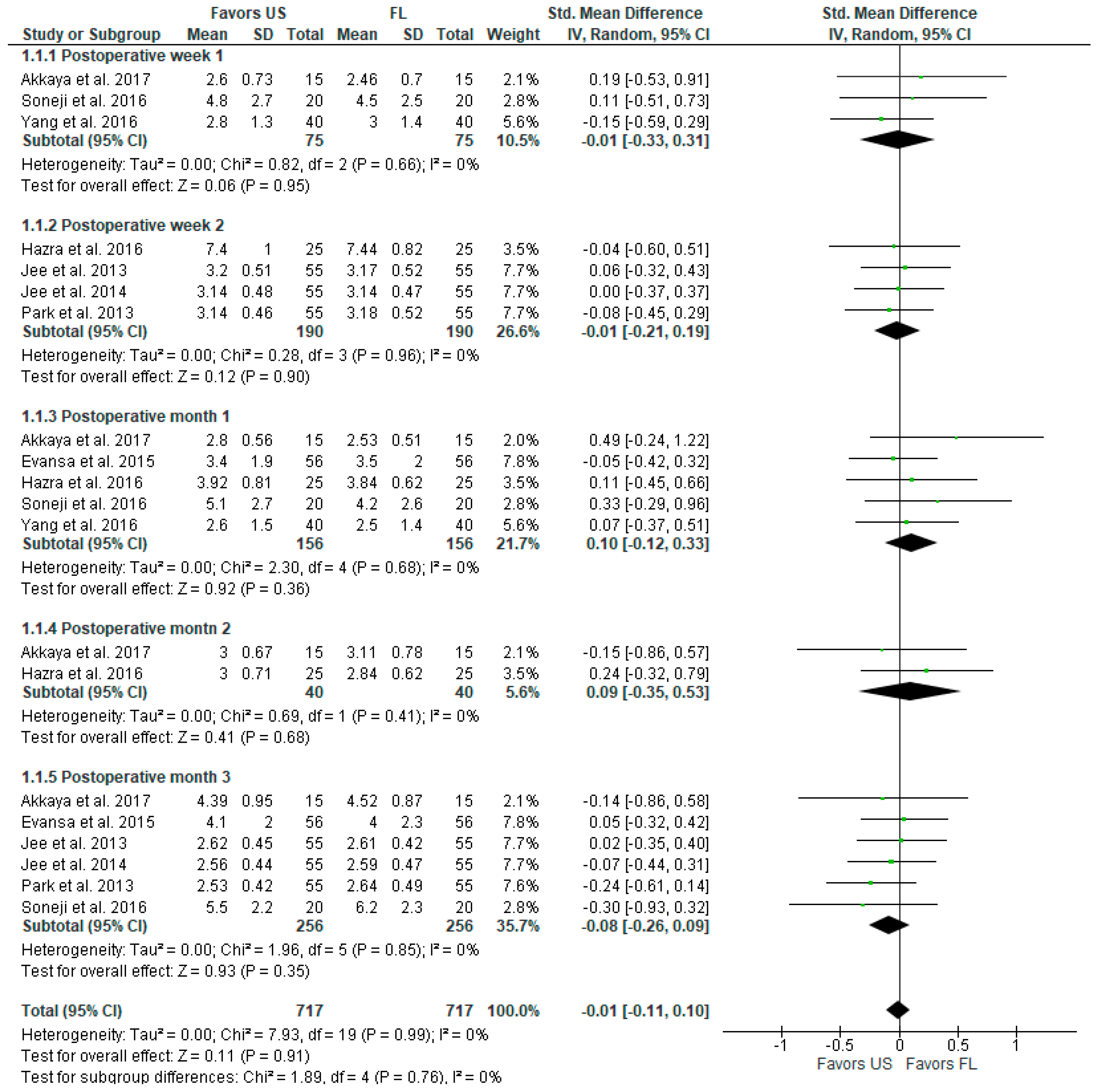
3.2.1. Pain Intensity (VAS, VNS, NRS Scale)
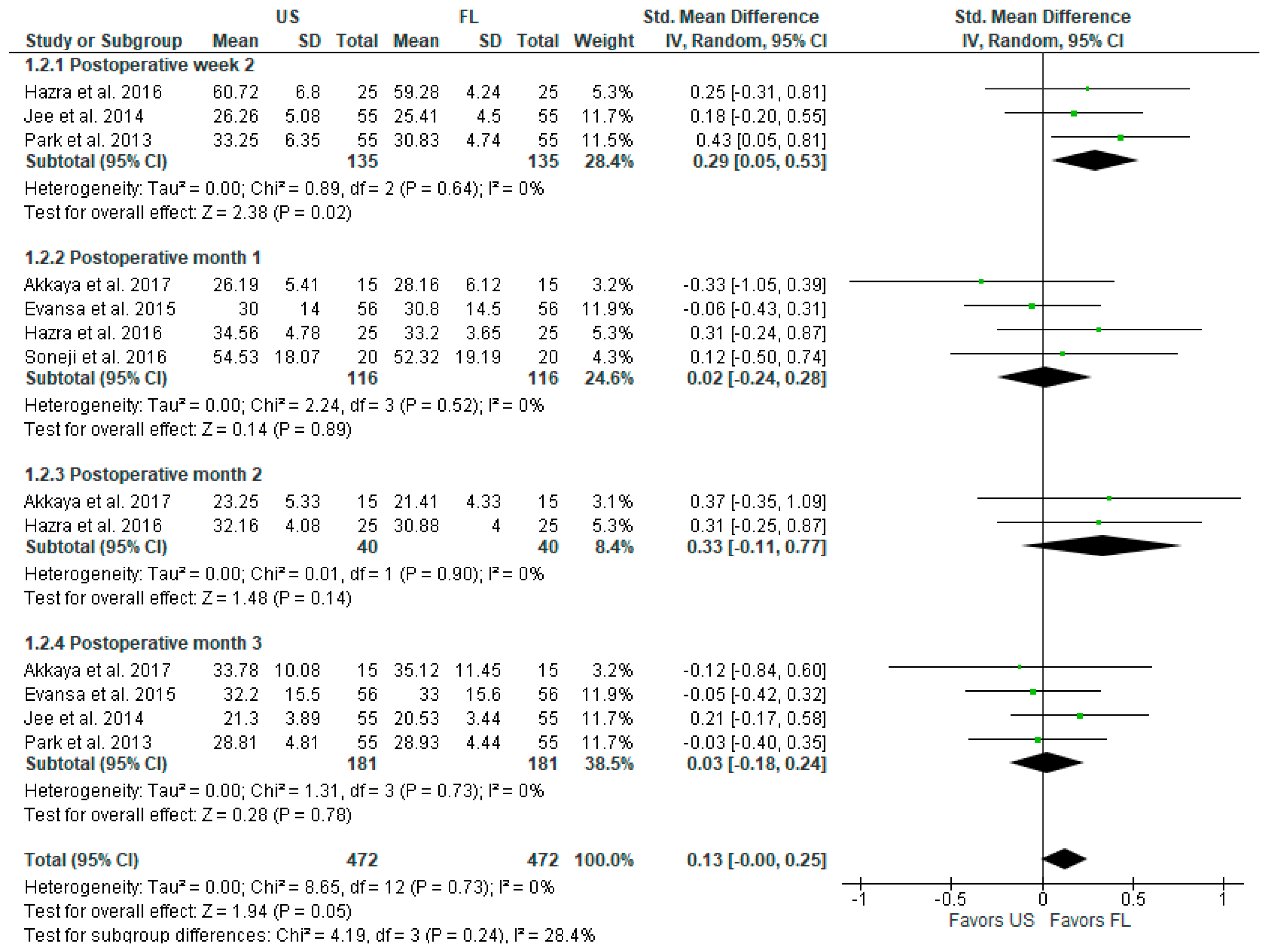
3.2.2. Postoperative Functional Outcomes and Disability (ODI Index)
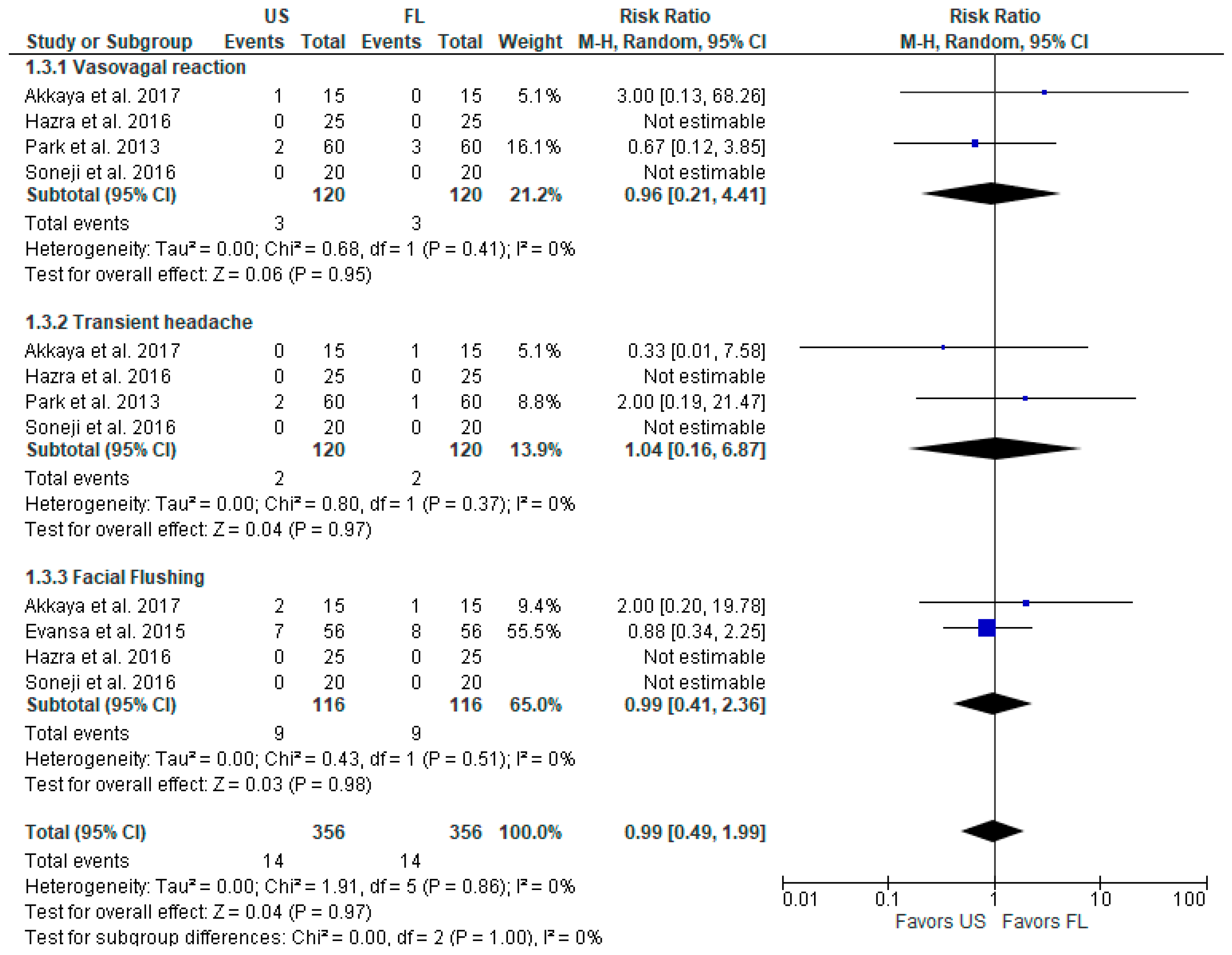
3.2.3. Postoperative Complications
3.3. Assessment of Methodological Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.P.C.; Tang, S.F.T.; Hsu, T.-C.; Tsai, W.-C.; Liu, H.-P.; Chen, M.J.L.; Date, E.; Lew, H.L. Ultrasound Guidance in Caudal Epidural Needle Placement. Anesthesiology 2004, 101, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.; Lee, J.H.; Kim, J.; Park, K.D.; Lee, W.Y.; Park, Y. Ultrasound-Guided Selective Nerve Root Block versus Fluoroscopy-Guided Transforaminal Block for the Treatment of Radicular Pain in the Lower Cervical Spine: A Randomized, Blinded, Controlled Study. Skeletal Radiol. 2013, 42, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Evansa, I.; Logina, I.; Vanags, I.; Borgeat, A. Ultrasound versus Fluoroscopic-Guided Epidural Steroid Injections in Patients with Degenerative Spinal Diseases: A Randomised Study. Eur. J. Anaesthesiol. 2015, 32, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Stitz, M.Y.; Sommer, H.M. Accuracy of Blind versus Fluoroscopically Guided Caudal Epidural Injection. Spine 1999, 24, 1371–1376. [Google Scholar] [CrossRef]
- Manchikanti, L.; Cash, K.A.; Pampati, V.; McManus, C.D.; Damron, K.S. Evaluation of Fluoroscopically Guided Caudal Epidural Injections. Pain Physician 2004, 7, 81–92. [Google Scholar] [CrossRef]
- Akkaya, T.; Ozkan, D.; Kertmen, H.; Sekerci, Z. Caudal Epidural Steroid Injections in Postlaminectomy Patients: Comparison of Ultrasonography and Flouroscopy. Turk. Neurosurg. 2017, 27, 420–425. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Ma, L.; Cai, Z.; Meng, C.; Qi, S.; Zhou, H. Ultrasound-Guided Versus Fluoroscopy-Controlled Lumbar Transforaminal Epidural Injections: A Prospective Randomized Clinical Trial. Clin. J. Pain 2016, 32, 103–108. [Google Scholar] [CrossRef]
- Loizides, A.; Gruber, H.; Peer, S.; Galiano, K.; Bale, R.; Obernauer, J. Ultrasound Guided versus CT-Controlled Pararadicular Injections in the Lumbar Spine: A Prospective Randomized Clinical Trial. AJNR Am. J. Neuroradiol. 2013, 34, 466–470. [Google Scholar] [CrossRef]
- Hashemi, M.; Dadkhah, P.; Taheri, M.; Haji Seyed Abootorabi, S.M.; Naderi-Nabi, B. Ultrasound-Guided Lumbar Transforaminal Epidural Injections; A Single Center Fluoroscopic Validation Study. Bull. Emerg. Trauma 2019, 7, 251–255. [Google Scholar] [CrossRef]
- Blanchais, A.; Le Goff, B.; Guillot, P.; Berthelot, J.-M.; Glemarec, J.; Maugars, Y. Feasibility and Safety of Ultrasound-Guided Caudal Epidural Glucocorticoid Injections. Joint Bone Spine 2010, 77, 440–444. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE Guidelines: A New Series of Articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Hazra, A.K.; Bhattacharya, D.; Mukherjee, S.; Ghosh, S.; Mitra, M.; Mandal, M. Ultrasound versus Fluoroscopy-Guided Caudal Epidural Steroid Injection for the Treatment of Chronic Low Back Pain with Radiculopathy: A Randomised, Controlled Clinical Trial. Indian. J. Anaesth. 2016, 60, 388–392. [Google Scholar] [CrossRef]
- Soneji, N.; Bhatia, A.; Seib, R.; Tumber, P.; Dissanayake, M.; Peng, P.W.H. Comparison of Fluoroscopy and Ultrasound Guidance for Sacroiliac Joint Injection in Patients with Chronic Low Back Pain. Pain Pract. 2016, 16, 537–544. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J.-H.; Park, K.D.; Ahn, J.K.; Park, J.; Jee, H. Ultrasound-Guided vs. Fluoroscopy-Guided Caudal Epidural Steroid Injection for the Treatment of Unilateral Lower Lumbar Radicular Pain: A Prospective, Randomized, Single-Blind Clinical Study. Am. J. Phys. Med. Rehabil. 2013, 92, 575–586. [Google Scholar] [CrossRef]
- Jee, H.; Lee, J.-H.; Park, K.D.; Ahn, J.; Park, Y. Ultrasound-Guided versus Fluoroscopy-Guided Sacroiliac Joint Intra-Articular Injections in the Noninflammatory Sacroiliac Joint Dysfunction: A Prospective, Randomized, Single-Blinded Study. Arch. Phys. Med. Rehabil. 2014, 95, 330–337. [Google Scholar] [CrossRef]
- Kimura, R.; Yamamoto, N.; Watanabe, J.; Ono, Y.; Hongo, M.; Miyakoshi, N. Comparative Efficacy of Ultrasound Guidance and Fluoroscopy or Computed Tomography Guidance in Spinal Nerve Injections: A Systematic Review and Meta-Analysis. Eur. Spine J. 2023. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, W.Y.; Kim, J.W.; Cho, K.R.; Nam, S.H.; Park, Y. Ultrasound-Guided Selective Nerve Root Block versus Fluoroscopy-Guided Interlaminar Epidural Block versus Fluoroscopy-Guided Transforaminal Epidural Block for the Treatment of Radicular Pain in the Lower Cervical Spine: A Retrospective Comparative Study. Pain Res. Manag. 2020, 2020, 9103421. [Google Scholar] [CrossRef] [PubMed]
- Touboul, E.; Salomon-Goëb, S.; Boistelle, M.; Sobhy Danial, J.; Deprez, V.; Goëb, V. Lumbar Zygapophyseal Joints Injections under Ultrasound Guidance an Alternative to Fluoroscopy Guidance in the Management of Low Back Pain. Sci. Rep. 2022, 12, 3615. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Kim, T.K.; Lee, W.Y.; Ahn, J.; Koh, S.H.; Park, Y. Ultrasound-Guided Versus Fluoroscopy-Guided Caudal Epidural Steroid Injection for the Treatment of Unilateral Lower Lumbar Radicular Pain: Case-Controlled, Retrospective, Comparative Study. Medicine 2015, 94, e2261. [Google Scholar] [CrossRef] [PubMed]
- Soto Quijano, D.A.; Otero Loperena, E. Sacroiliac Joint Interventions. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 171–183. [Google Scholar] [CrossRef]
- Pekkafahli, M.Z.; Kiralp, M.Z.; Başekim, C.C.; Silit, E.; Mutlu, H.; Oztürk, E.; Kizilkaya, E.; Dursun, H. Sacroiliac Joint Injections Performed with Sonographic Guidance. J. Ultrasound Med. 2003, 22, 553–559. [Google Scholar] [CrossRef]
- Klauser, A.; De Zordo, T.; Feuchtner, G.; Sögner, P.; Schirmer, M.; Gruber, J.; Sepp, N.; Moriggl, B. Feasibility of Ultrasound-Guided Sacroiliac Joint Injection Considering Sonoanatomic Landmarks at Two Different Levels in Cadavers and Patients. Arthritis Rheum. 2008, 59, 1618–1624. [Google Scholar] [CrossRef]
- Hartung, W.; Ross, C.J.; Straub, R.; Feuerbach, S.; Schölmerich, J.; Fleck, M.; Herold, T. Ultrasound-Guided Sacroiliac Joint Injection in Patients with Established Sacroiliitis: Precise IA Injection Verified by MRI Scanning Does Not Predict Clinical Outcome. Rheumatology 2010, 49, 1479–1482. [Google Scholar] [CrossRef]
- Ashmore, Z.M.; Bies, M.M.; Meiling, J.B.; Moman, R.N.; Hassett, L.C.; Hunt, C.L.; Cohen, S.P.; Hooten, W.M. Ultrasound-Guided Lumbar Medial Branch Blocks and Intra-Articular Facet Joint Injections: A Systematic Review and Meta-Analysis. Pain Rep. 2022, 7, e1008. [Google Scholar] [CrossRef]
- Hofmeister, M.; Dowsett, L.E.; Lorenzetti, D.L.; Clement, F. Ultrasound- versus Fluoroscopy-Guided Injections in the Lower Back for the Management of Pain: A Systematic Review. Eur. Radiol. 2019, 29, 3401–3409. [Google Scholar] [CrossRef]
- Migliore, A.; Sorbino, A.; Bacciu, S.; Bellelli, A.; Frediani, B.; Tormenta, S.; Pirri, C.; Foti, C. The Technique of Intradiscal Injection: A Narrative Review. Ther. Clin. Risk Manag. 2020, 16, 953–968. [Google Scholar] [CrossRef]
- Wang, D. Image Guidance Technologies for Interventional Pain Procedures: Ultrasound, Fluoroscopy, and CT. Curr. Pain Headache Rep. 2018, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.W.H.; Narouze, S. Ultrasound-Guided Interventional Procedures in Pain Medicine: A Review of Anatomy, Sonoanatomy, and Procedures: Part I: Nonaxial Structures. Reg. Anesth. Pain Med. 2009, 34, 458–474. [Google Scholar] [CrossRef] [PubMed]

| Study | Randomization Process | Deviations from the Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Hazra et al., 2016 [16] | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Soneji et al., 2016 [17] | Some concerns | Low risk | Some concerns | Low risk | Low risk | Some concerns |
| Park et al., 2013 [18] | Some concerns | Some concerns | Some concerns | Low risk | Low risk | High risk |
| Akkaya et al., 2017 [6] | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Evansa et al., 2015 [3] | Some concerns | Some concerns | Some concerns | Low risk | Low risk | High risk |
| Jee et al., 2013 [2] | Some concerns | Some concerns | Some concerns | Low risk | Low risk | High risk |
| Yang et al., 2016 [7] | Some concerns | Some concerns | Some concerns | Low risk | Low risk | High risk |
| Jee et al., 2014 [19] | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Outcomes | Risk Ratio [95% CI] | Standardized Mean Difference [95% CI] | Number of Participants (Studies) | Certainty of the Evidence (GRADE) |
|---|---|---|---|---|
| Postoperative pain at 1 month | - | 0.10 [−0.12, 0.33] | 312 (5) | ⊕⊕⊕⊕ High |
| Postoperative functionality (ODI index) at 1 month | - | 0.02 [−0.24, 0.28] | 232 (4) | ⊕⊕⊕⊕ High |
| Vasovagal reaction | 0.96 [0.21, 4.41] | - | 240 (4) | ⊕⊕⊕◯ Moderate a |
| Transient headache | 1.04 [0.16, 6.87] | - | 240 (4) | ⊕⊕⊕◯ Moderate a |
| Facial flushing | 0.99 [0.41, 2.36] | - | 232 (4) | ⊕⊕⊕◯ Moderate a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viderman, D.; Aubakirova, M.; Aryngazin, A.; Yessimova, D.; Kaldybayev, D.; Tankacheyev, R.; Abdildin, Y.G. Ultrasound-Guided vs. Fluoroscopy-Guided Interventions for Back Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics 2023, 13, 3474. https://doi.org/10.3390/diagnostics13223474
Viderman D, Aubakirova M, Aryngazin A, Yessimova D, Kaldybayev D, Tankacheyev R, Abdildin YG. Ultrasound-Guided vs. Fluoroscopy-Guided Interventions for Back Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics. 2023; 13(22):3474. https://doi.org/10.3390/diagnostics13223474
Chicago/Turabian StyleViderman, Dmitriy, Mina Aubakirova, Anuar Aryngazin, Dinara Yessimova, Dastan Kaldybayev, Ramil Tankacheyev, and Yerkin G. Abdildin. 2023. "Ultrasound-Guided vs. Fluoroscopy-Guided Interventions for Back Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Diagnostics 13, no. 22: 3474. https://doi.org/10.3390/diagnostics13223474
APA StyleViderman, D., Aubakirova, M., Aryngazin, A., Yessimova, D., Kaldybayev, D., Tankacheyev, R., & Abdildin, Y. G. (2023). Ultrasound-Guided vs. Fluoroscopy-Guided Interventions for Back Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics, 13(22), 3474. https://doi.org/10.3390/diagnostics13223474